Jacobson M.Z. Atmospheric Pollution
Подождите немного. Документ загружается.

138 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Table 5.6. Dominant Sources and Components of Nucleation,
Accumulation, and Coarse Mode Particles
Nucleation Fossil-fuel emissions Sea-spray emissions
H
2
O(aq), SO
4
2
, NH
4
BC, OM, SO
4
2
, Fe, Zn H
2
O, Na
, Ca
2
, Mg
2
, K
, Cl
,
SO
4
2
, Br
, OM
Fossil-fuel emissions Biomass-burning emissions Soil-dust emissions
BC, OM, SO
4
2
, Fe, Zn BC, OM, K
, Na
, Ca
2
, Mg
2
, Si, Al, Fe, Ti, P, Mn, Co, Ni, Cr,
SO
4
2
, NO
3
, Cl
, Fe, Mn, Zn, Na
, Ca
2
, Mg
2
, K
, SO
4
2
,
Pb, V, Cd, Cu, Co, Sb, As, Ni, Cl
, CO
3
2
, OM
Cr
Biomass-burning emissions Industrial emission Biomass burning ash, industrial
BC, OM, K
, Na
, Ca
2
, Mg
2
, BC, OM, Fe, Al, S, P, Mn, Zn, Pb, fly-ash, tire-particle emissions
SO
4
2
, NO
3
, Cl
, Fe, Mn, Ba, Sr, V, Cd, Cu, Co, Hg, Sb,
Zn, Pb, V, Cd, Cu, Co, Sb, As, Sn, Ni, Cr, H
2
O, NH
4
, Na
,
As, Ni, Cr Ca
2
, K
, SO
4
2
, NO
3
, Cl
,
CO
3
2
Condensation/dissolution Condensation/dissolution Condensation/dissolution
H
2
O(aq), SO
4
2
, NH
4
, OM H
2
O(aq), SO
4
2
, NH
4
, OM H
2
O(aq), NO
3
Coagulation of all components from Coagulation of all components
nucleation mode from smaller modes
Nucleation Mode Accumulation Mode Coarse Mode
efficient for particles than for gases because particles are heavier than are gases. As
such, particles fall and tend to stay on a surface more readily than do gases, unless
wind speeds are high. Gases, especially if they are chemically unreactive, are more
readily resuspended into the air.
Aerosol particle rainout is a process by which aerosol particles coagulate with
raindrops, which subsequently fall to the ground. Rainout is an important removal
process for aerosol particles. Because rain clouds occur only in the troposphere, rain-
out is not a process by which stratospheric particles are removed. Rainout is an
effective removal process for volcanic particles.
5.4. SUMMARY OF THE COMPOSITION OF AEROSOL PARTICLES
The composition of aerosol particles varies with particle size and location. Some gen-
eralities about composition include the following:
• Newly nucleated particles usually contain sulfate and water, although they may
also contain ammonium.
• Biomass burning and fossil-fuel combustion produce primarily small accumulation
mode particles, but coagulation and gas-to-particle conversion move these particles
to the middle and high accumulation modes. Coagulation also moves some parti-
cles to the coarse mode.
• Metals that evaporate during industrial emissions recondense, primarily onto accu-
mulation mode soot particles and coarse mode fly-ash particles. The metal emitted
in the greatest abundance is usually iron.
• Sea spray and soil particles are primarily in the coarse mode.
• When sulfuric acid condenses, it usually condenses onto accumulation-mode parti-
cles because these particles have more surface area, when averaged over all
particles in the mode, than do nucleation-or coarse-mode particles.
• Once in accumulation-mode particles, sulfuric acid dissociates primarily to sulfate
[SO
4
2
]. To maintain charge balance, ammonia gas [NH
3
(g)], dissolves and disso-
ciates, producing the ammonium ion [NH
4
]; the major-cation in particles. Thus,
ammonium and sulfate often coexist in accumulation-mode particles.
• Because sulfuric acid has a lower SVP and a greater solubility than does nitric
acid, nitric acid is inhibited from entering accumulation-mode particles that
already contain sulfuric acid.
• Nitric acid tends to dissolve in coarse-mode particles and displace chloride in sea-
spray drops and carbonate in soil-dust particles during acidification. Sulfuric acid
also displaces chloride and carbonate during acidification.
Table 5.6 summarizes the predominant components and their sources in each the
nucleation, accumulation, and coarse particle modes.
5.5. AEROSOL PARTICLE MORPHOLOGY AND SHAPE
The morphologies (structures) and shapes of aerosol particles v
ary with composition.
The older an aerosol particle, the greater the number of layers and attachments the par-
ticle is likely to have. If the aerosol particle is hygroscopic, it absorbs liquid water at
high relative humidities and becomes spherical. If
ions are present and the relative humidity decreases,
solid crystals may form within the particle. Some
observed aerosol particles are flat, others are globu-
lar, others contain layers, and still others are fibrous.
Of particular interest is the morphology and
shape of soot particles, which contain BC, OM, O, N,
and H. Soot particles have important optical effects.
The only source of soot is emissions. Globally, about
55 percent of soot originates from fossil-fuel com-
bustion, and the rest originates from biomass burning
(Cooke and Wilson, 1996; Liousse et al., 1996). An
emitted soot particle is irregularly shaped and mostly
solid, containing from 30 to 2000 graphitic spherules
aggregated with random orientation by collision dur-
ing combustion (Katrlnak et al., 1993). An example
of a soot aggregate is shown in Fig. 5.15(b).
Once emitted, soot particles can coagulate or
grow. Because soot particles are porous and have a
large surface area, they serve as sites on which con-
densation occurs. Although BC in soot is
hydrophobic, some organics in soot attract water, in
which inorganics gases dissolve (Andrews and Larson, 1993). Evidence of condensa-
tion and coagulation is abundant because traffic tunnel studies (Venkataraman et al.,
1994) and test vehicle studies (Maricq et al., 1999; ACEA, 1999) indicate that most
fossil-fuel BC is emitted in particles smaller than 0.2 m in diameter, but ambient
AEROSOL PARTICLES IN SMOG AND THE GLOBAL ENVIRONMENT 139
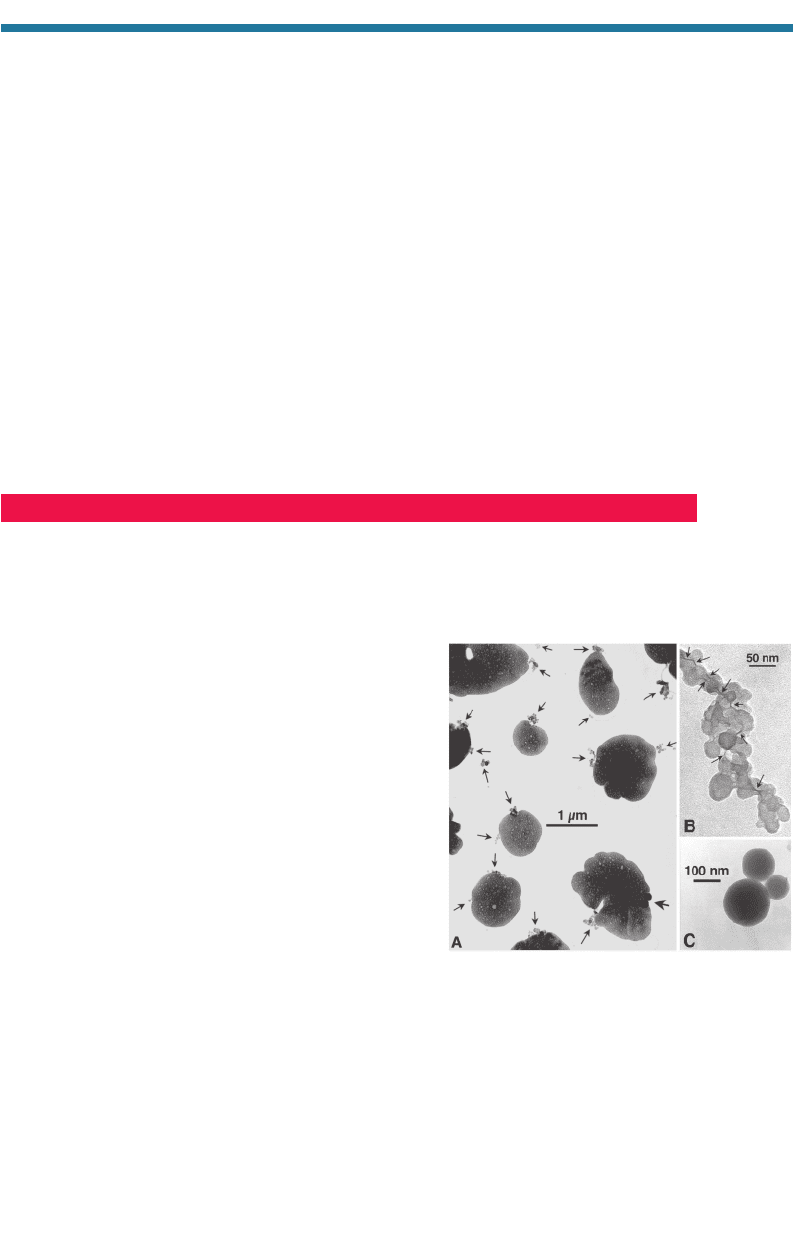
Figure 5.15. Transmission electron microscopy
(TEM) images of (a) ammonium sulfate parti-
cles containing soot (arrows point to soot
inclusions), (b) a chainlike soot aggregate,
and (c) fly-ash spheres consisting of amor-
phous silica collected from a polluted marine
boundary layer in the North Atlantic Ocean by
Pósfai et al. (1999).
measurements in Los Angeles, the Grand Canyon, Glen Canyon, Chicago, Lake
Michigan, Vienna, and the North Sea show that accumulation mode BC often exceeds
emissions mode BC (McMurry and Zhang, 1989; Hitzenberger and Puxbaum, 1993;
Venkataraman and Friedlander, 1994; Berner et al., 1996; Offenberg and Baker, 2000).
The most likely way that ambient BC redistributes so dramatically is by coagulation
and growth. Similarly, the measured mean number diameter of biomass-burning
smoke less than 4 minutes old is 0.10 to 0.13 m (Reid and Hobbs, 1998), yet the
mass of such aerosol particles increases by 20 to 40 percent during aging, with one-
third to one-half the growth occurring within hours after emissions (Reid et al., 1998).
Transmission electron microscopy (TEM) images
support the theory that soot particles can become coat-
ed once emitted. Katrlnak et al. (1992, 1993) show
TEM images of soot from fossil-fuel sources coated
with sulfate or nitrate. Martins et al. (1998) show a
TEM image of a coated biomass-burning soot particle.
Pósfai et al. (1999) show TEM images of North
Atlantic soot particles containing ammonium sulfate.
This image is reproduced in Fig. 5.15(a). They found
that internally mixed soot and sulfate appear to com-
prise a large fraction of aerosol particles in the
troposphere. Almost all soot particles found in the
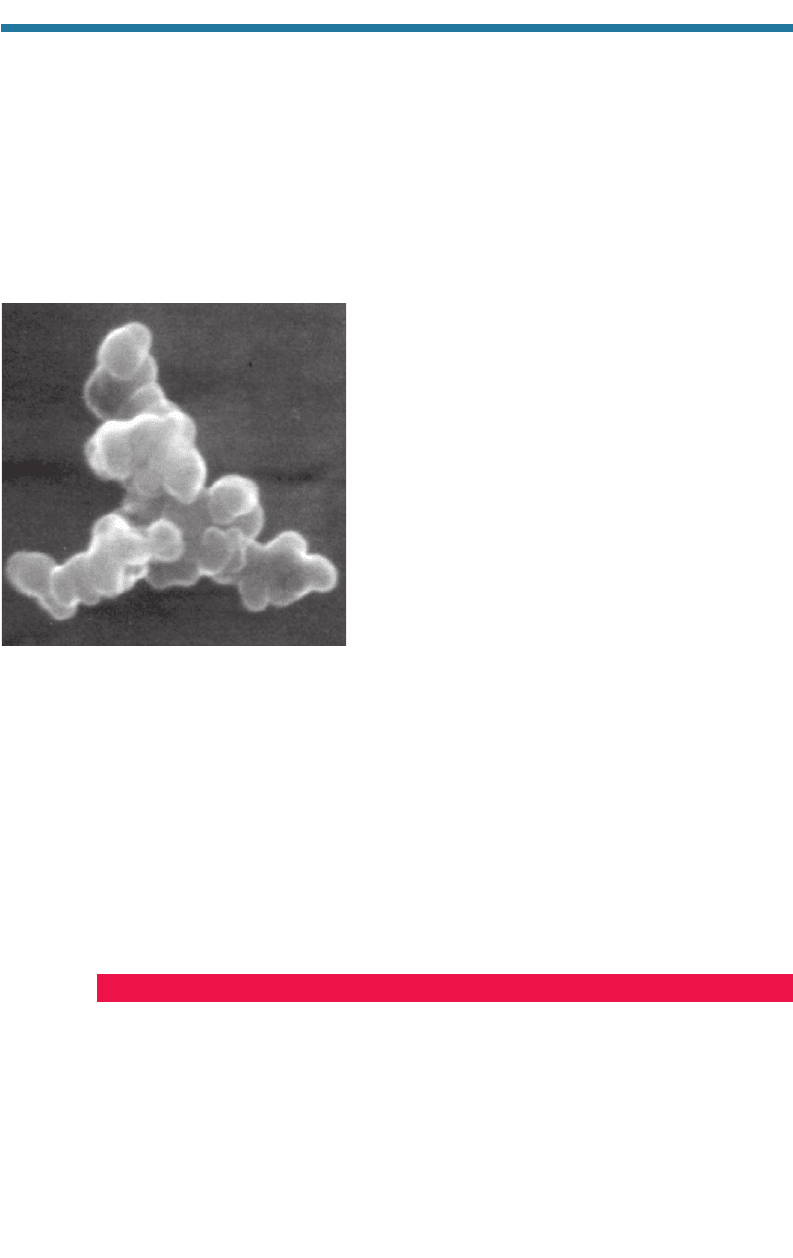
North Atlantic contained sulfate. Strawa et al. (1999)
took scanning electron microscopy (SEM) images of
black carbon particles in the Arctic stratosphere. One
such image is reproduced in Fig. 5.16. The rounded
edges of the particle seems to indicate that the particle
is coated. Katrlnak et al. (1993) report that rounded
grains on black carbon aggregates indicate a coating.
In sum, whereas emitted soot particles are relatively distinct, or externally mixed
from other aerosol particles, soot particles typically coagulate or grow to become
internally mixed with other particle components. Although soot becomes internally
mixed, it does not become “well mixed” (diluted) in an internal mixture because soot
consists of a solid aggregate of many graphite spherules. Thus, soot is a distinct com-
ponent in a mixed particle. Katrlnak et al. (1992) found that coated soot aggregates
have carbon structures similar to uncoated aggregates; thus, individual spherules in BC
structures do not readily break off or compress during coating.
5.6. HEALTH EFFECTS OF AEROSOL PARTICLES
Aerosol particles contain a variety of hazardous inorganic and organic substances.
Some hazardous organic substances include benzene, polychlorinated biphenyls, and
polycyclic aromatic hydrocarbons (PAHs). Hazardous inorganic substances include
metals and sulfur compounds. Metals cause lung injury, bronchioconstriction, and
increased incidence of infection (Ghio and Samet, 1999). Particles smaller than 10 m
in diameter (PM
10
) have been correlated with asthma and chronic obstructive pul-
monary disease (MacNee and Donaldson, 1999).
A major worldwide health problem directly linked to aerosol particles is Coal
Workers’ Pneumoconiosis (CWP), more commonly known as black-lung disease.
140 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Figure 5.16. Scanning electron microscopy
(SEM) image of a coated soot particle from the
Arctic stratosphere by Strawa et al. (1999).

Coal workers develop black-lung disease over many years of exposure to coal dust.
The dust first builds up in air sacs in the lungs, then scars the sacs, making breath-
ing difficult. During the last 30 years alone in the United States, black lung disease,
first identified in 1831, has killed an average of 2000 coal workers per year
(NIOSH, 2001).
A more deadly health hazard linked to aerosol particles is indoor-burning of bio-
mass and coal. Such burning is carried out daily for cooking and home heating by
large segments of the population in many developing countries. The World Health
Organization estimates that, of 2.7 million people who die each year from air pollu-
tion, 1.8 million die in rural areas, where the largest source of mortality is indoor-
burning of biomass and coal (WHO, 2000).
With respect to outdoor air, some studies have found that there may be no low
threshold for PM
10
-related health problems (Pope et al., 1995). Because most mass of
PM
10
is not hazardous, damage from PM
10
may be due primarily to small particles,
particularly ultrafine particles (smaller than 0.1 m in diameter). Such particles may
be toxic to the lungs, ev
en when they contain components that are not toxic when pres-
ent in larger particles (MacNee and Donaldson, 1999).
Studies in the 1970s found a link between cardiopulmonary disease and high
concentrations of aerosol particles and sulfur oxides. Other studies in the 1970s and
1980s found a link between low concentrations of aerosol particles and health. A sum-
mary of several studies by Pope (2000) concluded that short-term (acute) increases of
10 g m
3
PM
10
were associated with a 0.5 to 1.5 percent increase in daily mortality,
higher hospitalization and health-care visits for respiratory and cardiovascular disease,
and enhanced outbreaks of asthma and coughing. Increased death rates usually
occurred within 1 to 5 days following an air pollution episode. Long-term exposures to
5 g m
3
of particles smaller than 2.5 m in diameter (PM
2.5
) above background lev-
els resulted in a variety of cardiopulmonary problems, including increased mortality,
increased disease, and decreased lung function in adults and children (Pope and
Dockery, 1999).
Additional studies have shown that PM
2.5
results in more respiratory illness
and premature death than do larger aerosol particles (Özkatnak and Thurston, 1987;
U.S. EPA, 1996). One six-city, 16-year study concluded that people living in
areas where aerosol particle concentrations were lower than even the federal PM
10
standard had a lifespan two years shorter than people living in cleaner air (Dockery -
et al., 1993). Air pollution was correlated with death from lung cancer and
cardiopulmonary disease. Mortality was correlated with fine particulates, including
sulfates.
Finally, several studies have examined the effect of both gas and particle pollution
on health. A study by Hall et al. (1992) found that 98 percent of the 12 million people
living in the Los Angeles Basin experienced ozone-related symptoms for 17 days each
year, and air pollution in the basin caused 1600 premature deaths per year. Children
and people working outdoors were most likely to experience respiratory problems. A
study by Dr. Kay Kilburn of the University of Southern California found that the lung
function of children raised in Los Angeles was 10 to 15 percent lower than that of
children raised in cleaner environments. A study by Dr. David Abbey of Loma Linda
University found that people living in areas of Los Angeles that violated federal partic-
ulate standards at least 42 days per year had a 33 percent greater risk of bronchitis and
74 percent greater risk of asthma than a control group. Women living in high-particulate
areas had a 37 percent higher risk of developing cancer. A 1987 study by Drs. Russell
AEROSOL PARTICLES IN SMOG AND THE GLOBAL ENVIRONMENT 141

Sherwin and Valda Richters of the University of Southern California found that 75
percent of a group of young people who died accidentally had airspace inflammation,
27 percent of the group had severe damage to their lungs, 39 percent had severe illness
to the bronchial glands, and 29 percent had severe illness in their bronchial linings
(SCAQMD, 2000).
Although epidemiological studies have found an association between short-term
exposure to outdoor particulate air pollution and health problems, people spend most
of their time indoors, and concentrations of aerosol particles are often greater indoors
than they are outdoors. Concentrations of gases and aerosol particles measured in the
vicinity of an individual indoors are even greater than are concentrations measured
from a stationary indoor monitor away from the individual. The relatively high con-
centration of pollution measured near an individual is called the personal cloud
(Rodes et al., 1991; McBride et al., 1999). A personal cloud may arise when a person’s
movement stirs up gases and particles on clothes and nearby surfaces, increasing pol-
lutant concentrations. People also release thermal-IR radiation, which rises, stirring
and lifting pollutants. Personal cloud concentrations can range from 3 to 67 g m
3
for PM
10
and from 6 to 27 g m
3
for PM
2.5
(Wallace, 2000). Personal cloud concen-
trations must be separated from background outdoor or indoor air concentrations when
determining the effects of particles on health.
5.7. SUMMARY
Aerosol particles appear in a variety of shapes and compositions and vary in size from
a few gas molecules to the size of a raindrop. Natural sources of aerosol particles
include sea-spray uplift, soil-dust uplift,
volcanic eruptions, natural biomass burning,
plant material emissions, and meteoric debris. Anthropogenic sources include fugitive
dust emissions, biomass burning, fossil-fuel combustion, and industrial sources.
Aerosol particle size distributions are usually trimodal or quadrimodal, consisting of a
nucleation, one or two subaccumulation, and a coarse particle mode. Homogeneous
nucleation and emissions from combustion and biomass burning dominate the nucle-
ation mode. Emissions of sea-spray, natural soil dust, and fugitive soil dust dominate
the coarse mode. Aerosol particles coagulate and grow by condensation or dissolution
from the nucleation mode to the accumulation mode. Chemistry within aerosol parti-
cles and between gases and aerosol particles affects gro
wth. Growth does not move
accumulation mode particles to the coarse mode, except when water vapor grows onto
aerosol particles to form cloud drops. The main removal processes of aerosol particles
from the atmosphere are rainout, sedimentation, and dry deposition. Aerosol particles
are responsible for a variety of health problems.
5.8. PROBLEMS
5.1. Why do accumulation mode aerosol particles not grow readily into the coarse
mode?
5.2. Why do accumulation mode particles generally contain more sulfate than do
coarse mode particles?
142 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION

5.3. On a global scale, why is most chloride observed in the coarse mode?
5.4. Why is most ammonium found in accumulation mode particles?
5.5. Write an equilibrium reaction showing nitric acid gas reacting with magnesite,
a solid. What particle size mode should this reaction most likely occur in?
5.6. Why is the carbonate ion not abundant in aerosol particles?
5.7. Why might a sea-spray drop over midocean lose all its chloride when it reach-
es the coast?
5.8. Why is more nitrate than sulfate generally observed in particles containing soil
minerals?
5.9. Why is coagulation not an important process for moving particle mass from the
lower to the upper accumulation mode?
5.10. Visibility is affected primarily by particles with diameter close to the wave-
length of visible light, 0.5 m. Which particle mode does this correspond to,
and which three particle components in this mode do you think affect visibility
in the background troposphere the most?
5.11. Particles smaller than 2.5 m in diameter affect human health more than do
larger particles. Identify five chemicals that you might e
xpect to see in high
concentrations in these particles in polluted air. Why did you pick these
chemicals?
AEROSOL PARTICLES IN SMOG AND THE GLOBAL ENVIRONMENT 143

EFFECTS OF
METEOROLOGY ON
AIR POLLUTION
6

I
n this chapter, the effects of meteorology on air pollution are discussed. The
concentrations of gases and aerosol particles are affected by winds, tempera-
tures, vertical temperature profiles, clouds, and the relative humidity. These
meteorological parameters are influenced by large-and small-scale weather systems.
Large-scale weather systems are controlled by large-scale regions of high and low
pressure. Small-scale weather systems are controlled by ground temperatures and
small-scale variations in pressure. The first section of the chapter examines the
forces acting on air. The second section examines how forces combine to form
winds. The third section discusses how radiation, coupled with forces and the rota-
tion of the Earth, generates the global circulation of the atmosphere. Sections 6.4
and 6.5 discuss the two major types of large-scale pressure systems. Section 6.6
discusses the effects of such pressure systems on air pollution. The last section
focuses on the effects of local meteorology on air pollution.
6.1. FORCES
Winds arise due to forces acting on the air. Next, the major forces are described.
6.1.1. Pressure Gradient Force
When high air pressure exists in one location and low pressure exists nearby, air moves
from high to low pressure. The force causing this motion is the pressure gradient
force (PGF). The force is proportional to the difference in pressure di
vided by the
distance between the two locations and always acts from high to low pressure.
6.1.2. Apparent Coriolis Force
When air is in motion over a rotating Earth, it appears to an observer fixed in space to
be deflected to the right in the Northern Hemisphere and to the left in the Southern
Hemisphere by the apparent Coriolis force (ACoF). The ACoF is not a real force; it
is an apparent or fictitious force that, to an observer fixed in space, is really an acceler-
ation of moving air to the right in the Northern Hemisphere and left in the Southern
Hemisphere and arises when the Earth rotates under a body (air in this case) in motion.
The ACoF is zero at the equator, maximum at the poles, zero for bodies at rest, propor-
tional to the speed of the air, and always acts 90 to the right (left) of the moving body
in the Northern (Southern) Hemisphere.
Figure 6.1 illustrates the ACoF. If the Earth did not rotate, an object thrown from
point A directly north would be received at point B, along the same longitude as point A.
Because the Earth rotates, objects thrown to the north have a west-to-east velocity equal
to that of the Earth’s rotation rate at the latitude they originate from. The Earth’s rotation
rate near the equator (low latitude) is greater than that near the poles (high latitudes);
thus, objects thrown from low latitudes have a greater west-to-east velocity than does the
Earth below them when they reach a high latitude. For example, an object thrown from
point A toward point B in the north will end up at point C, instead of at point B by the
time the person at point A reaches point A, and the object will appear as if it has been
deflected to the right (from point B to point C). Similarly, an object thrown from point
B toward point A in the south will end up at point D, instead of at point A by the time
the person at point B reaches point B. The Coriolis effect, therefore, appears to deflect
146
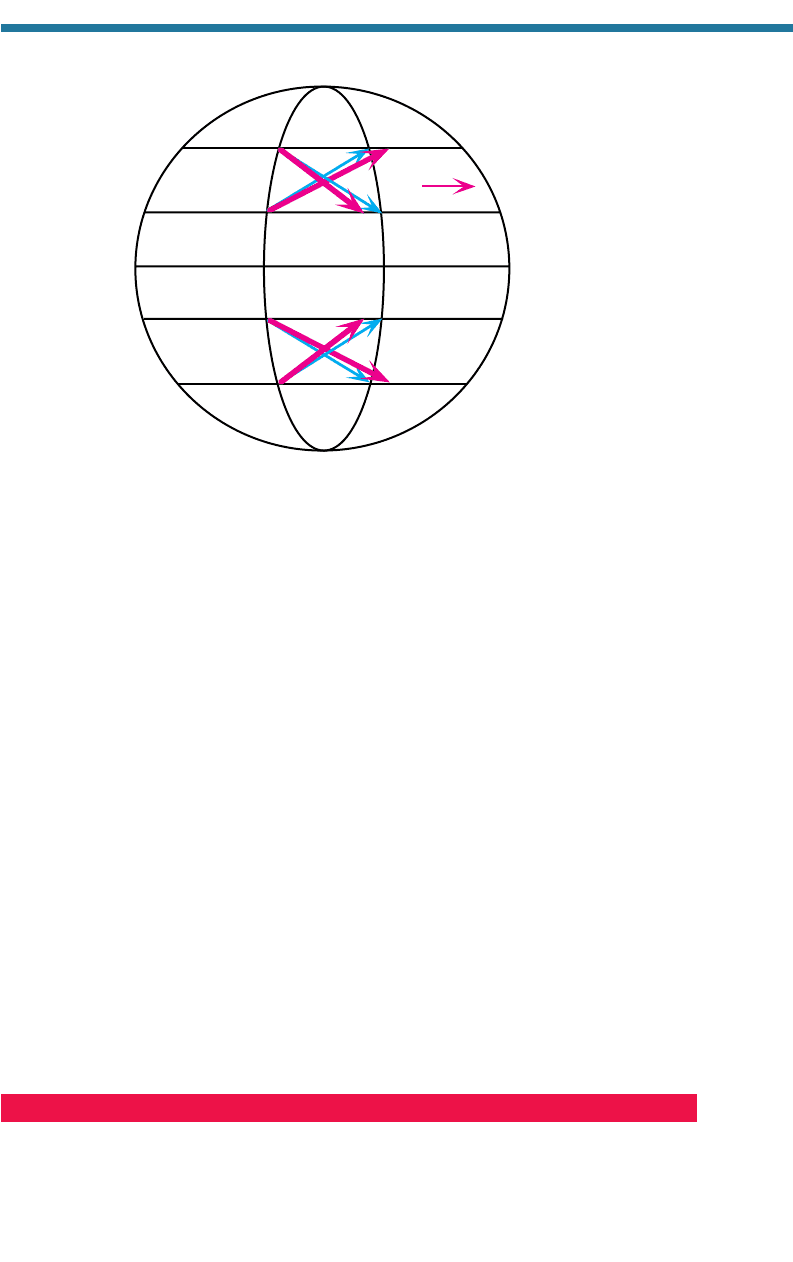
moving bodies to the right in the Northern Hemisphere. Moving bodies in the Southern
Hemisphere appear to be deflected to the left.
6.1.3. Friction Force
A third force that acts on moving air is the friction force (FF). This force is important
near the surface only. The FF slows the wind. Its magnitude is proportional to the wind
speed, and it acts in exactly the opposite direction from the wind. The rougher the sur-
face, the greater the FF. The FF over oceans and deserts is small, whereas the FF over
forests and buildings is large.
6.1.4. Apparent Centrifugal Force
A fourth force, which also acts on moving air, is the apparent centrifugal force
(ACfF). This force is also a fictitious force. The force arises when an object rotates
around an axis. The apparent force is directed outward, away from the axis of rotation.
When a passenger in a car rounds a curve, for example, a viewer travelling with the
passenger sees the passenger being pulled outward, away from the axis of rotation, by
this force. By contrast, a viewer fixed in space sees the passenger accelerating inward
due to a centripetal acceleration, which is equal in magnitude to but opposite in
direction from the apparent centrifugal force.
6.2. WINDS
The major forces acting on the air in the horizontal are the PGF, ACoF, FF, and ACfF.
In the vertical, the major forces are the upward-directed vertical pressure-gradient
force and the downward-directed force of gravity. These forces drive winds. Examples
of horizontal winds arising from force balances are given next.
EFFECTS OF METEOROLOGY ON AIR POLLUTION 147
Equator
North Pole
South Pole
A
A′
B
B′
E E′
F
F′
Direction
of the Earth’s
rotation
C
G
D
H
West East
Figure 6.1. Example of the apparent Coriolis force (ACoF), described in the text. Thin arrows
in the figure are intended paths, thick arrows are actual paths.
