Jacobson M.Z. Atmospheric Pollution
Подождите немного. Документ загружается.

108 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
The benzylperoxy radical, formed from the abstraction pathway, converts NO(g) to
NO
2
(g). It also results in the formation of benzaldehyde [C
6
H
5
CHO(g)], which, like
formaldehyde and acetaldehyde, decomposes to form ozone. The toluene-hydroxyl
radical adduct, which is also a peroxy radical, converts NO(g) to NO
2
(g). Cresol reacts
with OH(g) to form the methylphenylperoxy radical [C
6
H
5
CH
3
O
2
(g)], which converts
NO(g) to NO
2
(g), resulting in O
3
(g) formation.
The most important organic gas producing ozone in urban air is xylene
[C
6
H
5
CH
3
(g)] (Table 4.4). Xylene is present in gasoline, lacquers, and glues. Its mix-
ing ratios in polluted air range from 1 to 30 ppbv (Table 3.3). As with toluene
oxidation, xylene oxidation is primarily through reaction with OH(g). Oxidation of
xylene by OH(g) produces peroxy radicals, which convert NO(g) to NO
2
(g), resulting
in ozone formation.
4.3.6. Ozone Production from Terpenes
The free troposphere and urban areas are affected by biogenic emissions of isoprene
and other terpenes. Biogenic emissions are emissions produced from biological
sources, such as plants, trees, algae, bacteria, and animals. Strictly speaking, terpenes
are hydrocarbons that have the formula C
10
H
16
. Loosely speaking, they are a class of
compounds that include hemiterpenes [C
5
H
8
(g)], such as isoprene; monoterpenes
[C
10
H
16
(g)], such as -pinene, -pinene, and d-limonene; sesquiterpenes [C
15
H
24
(g)];
and diterpenes [C
20
H
32
(g)]. Isoprene is emitted by sycamore, oak, aspen spruce, wil-
low, balsam, and poplar trees; -pinene is emitted by pines, firs, cypress, spruce, and
hemlock trees; -pinene is emitted by loblolly pine, spruce, redwood, and California
black sage trees; and d-limonene is emitted by loblolly pine,
eucalyptus, and
California black sage trees, and by lemon fruit.
Table 4.3 shows that OH(g), O
3
(g), and NO
3
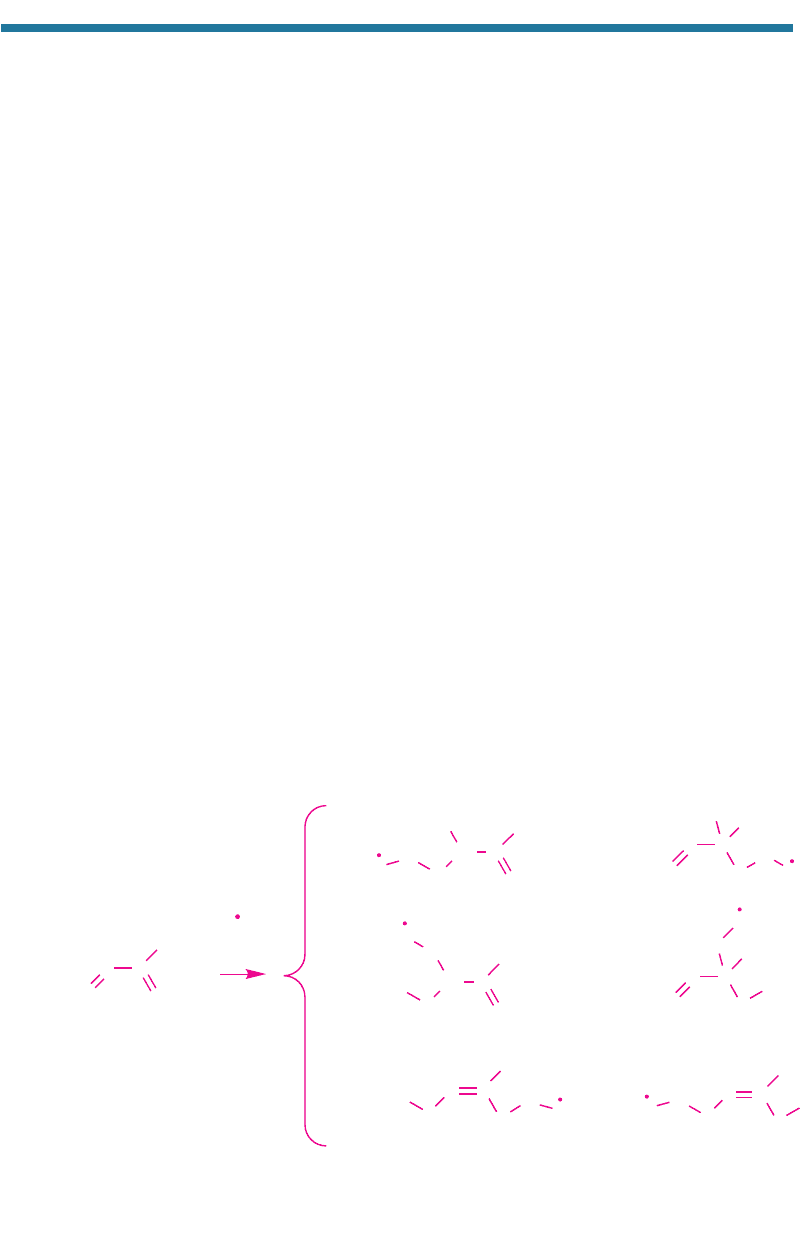
(g) decompose isoprene. The reac-
tion pathways of isoprene with OH(g) produce at least six peroxy radicals. The
pathways are
(4.54)
Isoprene
H
CC
H
2
C
CH
2
CH
3
CH C
C
H
2
CH
2
CH
3
HO
O
O
CH C
C
H
2
CH
2
CH
3
O
HO
O
H
CC
C
H
2
C
H
2
CH
3
HO
O
O
H
CC
H
2
C
C
H
2
CH
3
O
O
HO
H
CC
H
2
C
C
H
2
CH
3
OH
O
O
H
CC
C
H
2
C
H
2
CH
3
O
O
OH
(1)
16.4%
(2)
12.3%
(3)
12.3%
(4)
23.6%
(5)
21.2%
(6)
14.1%
Isoprene peroxy radicals
+ OH(g),
O
2
(g)
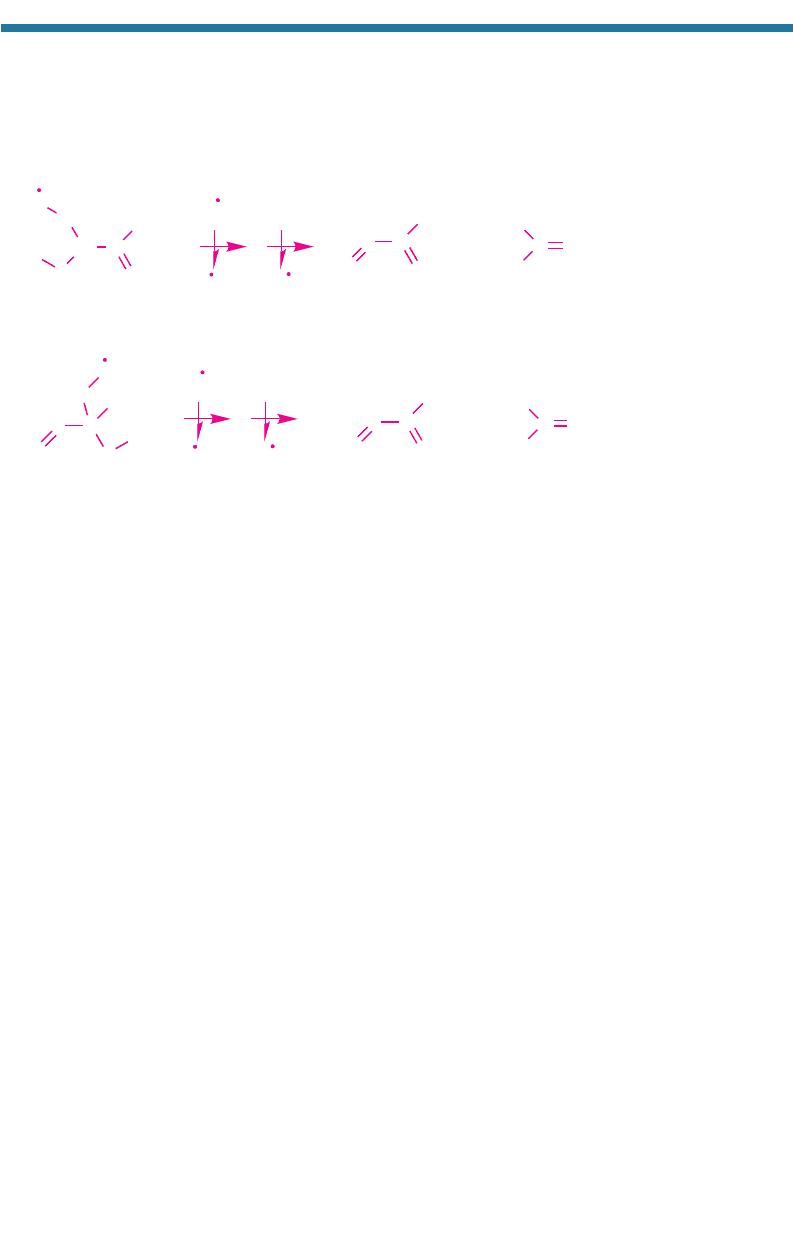
URBAN AIR POLLUTION 109
(Paulson and Seinfeld, 1992). The e-folding lifetime of isoprene against reaction with
OH(g) is about 30 minutes when [OH] 5.0 10
6
molecules cm
3
. All six peroxy
radicals convert NO(g) to NO
2
(g). The second and fifth radicals also create
methacrolein and methylvinylketone by
(4.55)
(4.56)
respectively. The NO
2
(g) from these reactions produces ozone. Methacrolein and
methylvinylketone react with OH(g) and O
3
(g) to form additional products that con-
vert NO(g) to NO
2
(g), resulting in more ozone.
The isoprene–ozone reaction is slower than is the isoprene–hydroxyl–radical reac-
tion. Products of the isoprene–ozone reaction include methacrolein, methylvinylketone,
the criegee biradical, and formaldehyde, all of which reproduce ozone.
In cities near forests, such as Atlanta, Georgia, terpenes can account for up to 40
percent of ozone above background levels. In less vegetated areas and in areas where
anthropogenic emissions are large, such as in Los Angeles, they may account for only
3 to 8 percent of ozone above background levels.
4.3.7. Ozone Production from Alcohols
Alcohols, which can be distilled from corn, grapes, potatoes, sugarcane, molasses, and
artichokes, among other farm products, have been used as an engine fuel since April 1,
1826, when Orford, New Hampshire, native Samuel Morey (1762–1843) patented the
first internal combustion engine. His engine ran on ethanol [C
2
H
5
OH(g)] and turpen-
tine. In September 1829, his engine was used to power a boat 5.8 m long up the
Connecticut River at seven to eight miles per hour
. Although alcohols were used in
later prototype engines, they became relatively expensive in the United States due to a
federal tax placed on alcohol following the Civil War of 1860 to 1865.
On August 27, 1859, Edwin Laurentine Drake (1819–1880) discovered oil after
using a steam engine to power a drill through 21 m of rock in Titusville,
Pennsylvania. This discovery is considered the beginning of the oil industry. Drake
later died in poverty because of his poor business sense. Oil was soon refined to pro-
duce gasoline. The lower cost of gasoline in comparison with that of ethanol resulted
in the comparatively greater use of gasoline than ethanol in early United States and
European automobiles. The first practical gasoline-powered engine was constructed
NO
2
(g)
+ NO(g) + O
2
(g)
HO
2
(g)
CO
H
H
H
CC
H
2
C
O
CH
3
Iso
p
rene
p
erox
y
radical Meth
y
lvin
y
lketone
+
Formaldeh
y
de
H
CC
H
2
C
C
H
2
CH
3
OH
O
O
CH C
C
H
2
CH
2
CH
3
O
HO
O
H
CC
O
CH
2
CH
3
Isoprene peroxy radical
Methacrolein
+
Formaldehyde
CO
H
H
NO
2
(g)
+ NO(g) + O
2
(g)
HO
2
(g)
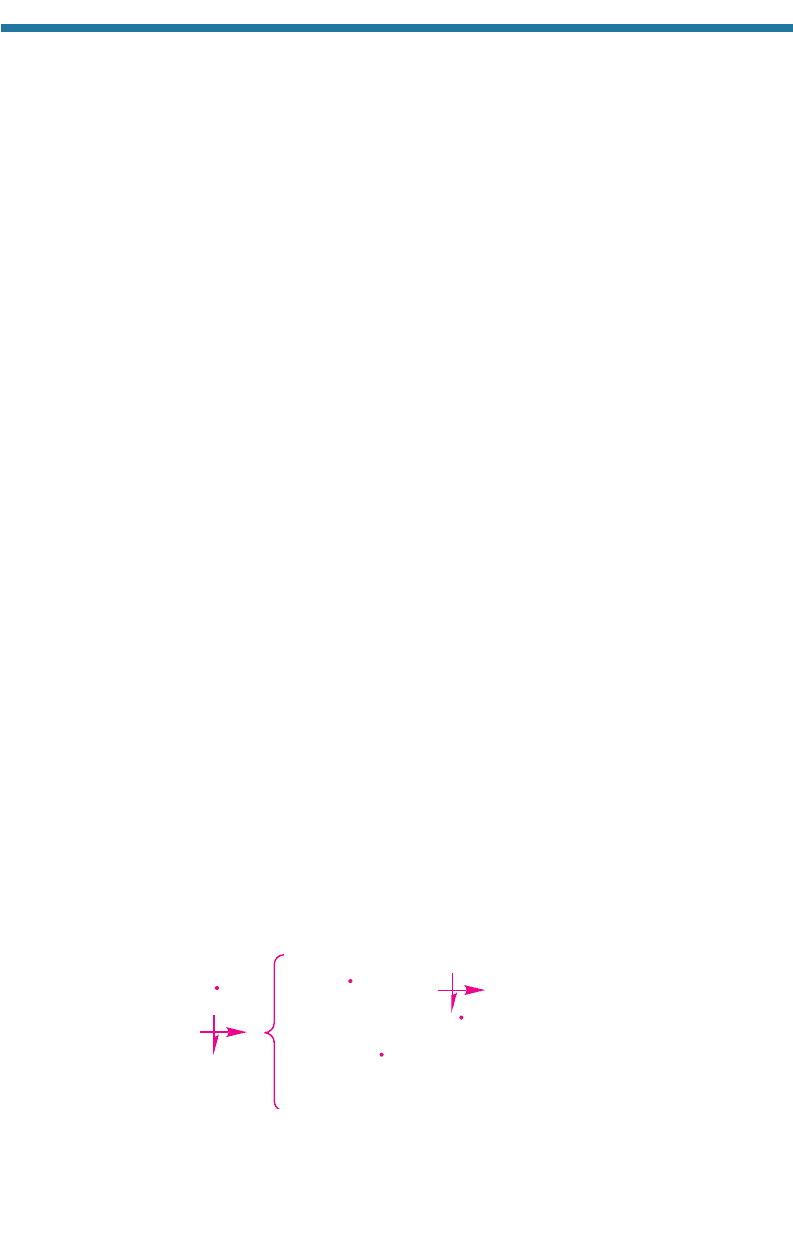
110 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
by Étienne Lenoir (1822–1900) of France in 1860 and ran on illuminating gas. In
1862, he built an automobile powered by this engine. In 1864, Austrian Siegfried
Marcus (1831–1898) built the first of four four-wheeled vehicles he developed that
were powered by internal combustion engines. In 1876, German Nikolaus Otto
(1832–1891) developed the first four-stroke internal-combustion engine. In 1885, Karl
Benz (1844–1929) of Germany designed and built the first practical automobile pow-
ered by an internal-combustion engine. The same year, Gottlieb Daimler (1834–1900)
of Germany patented the first successful high-speed internal-combustion engine and
developed a carburetor that allowed the use of gasoline as a fuel. In 1893, J. Frank
Duryea (1869–1967) and Charles E. Duryea (1861–1938) produced the first success-
ful gasoline-powered vehicle in the United States. In 1896, Henry Ford (1863–1947)
completed his first successful automobile in Detroit, Michigan.
Whereas the United States had large oil reserves to draw on, France and Germany
had few oil reserves and used ethanol as a fuel in automobiles to a greater extent. In
1906, 10 percent of engines in the Otto Gas Engine Works company in Germany ran
on ethanol (Kovarik, 1998).
The same year, the United States repealed the federal tax
on ethanol, making it more competitive with gasoline. Soon after, however, oil fields in
Texas were discovered, leading to a reduction in gasoline prices and the near-death of
the alcohol-fuel industry.
Yet the alcohol-fuel industry continued to survive. Since the 1920s, every industri-
alized country except the United States has marketed blends of ethyl alcohol with
gasoline in greater than nontrivial quantities. In the 1920s, I. G. Farben, a German
firm, discovered a process to make synthetic methanol [CH
3
OH(g)] from coal.
Production of alcohol as a fuel in Germany increased to about 52 million gallons per
year in 1937 as Hitler prepared for war (Egloff, 1940). Nevertheless, alcohol may
never have represented more than 5 percent of the total fuel use in Europe in the 1930s
(Egloff, 1940).
More recently, Brazil began a national effort in the 1970s to ensure that all gaso-
line sold contained ethanol (Section 8.2.3). In the United States, gasoline prices have
always been much lower than alcohol-fuel prices, inhibiting the popularity of alcohol
as an alternative to gasoline.
The chemical products of methanol oxidation are formaldehyde and ozone, and
those of ethanol are acetaldehyde (a precursor to PAN) and ozone. Table 4.3 indicates
that the only important loss process of alcohol is reaction with OH(g).
The reaction of methanol with OH(g) is
(4.57)
Methanol lost from these reactions has an e-folding lifetime of 71 days when [OH]
5.0 10
6
molecules cm
3
; thus, the reaction is not rapid. The organic product of the
first reaction is formaldehyde, and that of the second reaction is the methoxy radical,
which produces formaldehyde by Reaction 4.21. Formaldehyde is an ozone precursor.
+ O
2
(g)
Methanol
Formaldehyd
e
Methoxy radical
85%
15%
+ OH(g)
H
2
O(g)
HO
2
(g)CH
3
OH(g)
CH
2
OH(g)
CH
3
O(g)
HCHO(g)

URBAN AIR POLLUTION 111
Ethanol oxidation by OH(g) produces three sets of possible products
(4.58)
Ethanol lost from the most-probable (middle) reaction has an e-folding lifetime of
about 19 hours when [OH] 5.0 10
6
molecules cm
3
. Acetaldehyde, formed from
the middle reaction, produces PAN and ozone. Cities in Brazil have experienced high
PAN mixing ratios since the introduction of their alcohol-fuel program.
4.4. POLLUTANT REMOVAL
Severe air pollution episodes generally last from a few days to more than a week,
depending on the meteorology. During a pollution episode, air is usually confined so
that pollution concentrations build up over successive days. The major loss processes
of pollutants during an episode are chemical reaction and deposition to the ground.
When meteorological conditions change,
pollutants may diffuse upward, be swept
away by winds to the background troposphere, or be rained out to the ground.
Organic gases emitted in polluted air are ultimately broken down to carbon dioxide
and water. In the case of aromatics and other heavy organics, the initial breakdown
steps are relatively fast. In the case of many simpler, lighter organic gases, the initial
breakdown steps are often slower. Organic gases are also deposited to the ground and
oceans, converted to aerosol particle constituents, and transported to the background
troposphere. Oxides of nitrogen often evolve chemically to nitric acid, which converts
to particulate matter or deposits to the soil or ocean water. Oxides of nitrogen also
react with organic gases to form organic nitrate gases. Such gases decompose,
convert
to particulate matter, or deposit to the ground.
4.5. SUMMARY
Anthropogenic urban air pollution has been a problem for centuries. Before the twenti-
eth century, most air pollution problems arose from the burning of wood, coal, and
other raw materials without emission controls. Such burning resulted not only in
smoky cities, but also in health problems. In the early-and mid-twentieth century,
severe London-type smog events, during which emissions coupled with fog or a strong
temperature inversion, were responsible for fatalities. Increased use of the automobile
in the 1900s increased emissions of nitrogen oxides and reactive organic gases. In the
presence of sunlight, these chemicals produce ozone, PAN, and a host of other
products, giving rise to photochemical smog. Smog initiates when reactive organic
gases photolyze or are oxidized by OH(g), HO
2
(g), NO
3
(g), O
3
(g), or O(g) to produce
+ O
2
(g)
Ethanol
+ OH(g)
H
2
O(g)
HO
2
(g)
C
2
H
5
OH(g)
CH
2
CH
2
OH(g)
Acetaldehyde
Ethoxy radical
5%
90%
5%
CH
3
CHOH(g)
CH
3
CH
2
O(g)
CH
3
CHO(g)

112 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
organic radicals. The radicals convert NO(g) to NO
2
(g), which photolyzes to O(g),
which reacts with O
2
(g) to form O
3
(g). The most important reactive organic gases in
urban air are aromatics, alkenes, and aldehydes. Although alkanes are emitted in
greater abundance than are the other organics, alkanes are less reactive and longer
lived than are the others. Most organic gases are destroyed in urban air, but long-lived
organics, particularly methane, ethane, and propane, are transported to the background
troposphere, where they decay and produce ozone. Carbon monoxide is another gas
emitted in abundance in urban air that escapes to the background troposphere because
of its long lifetime. CO(g) not only produces ozone in the background troposphere, but
it is also a chemical source of CO
2
(g), a greenhouse gas. In the background tropo-
sphere, the concentrations of ozone, nitric oxide, and nitrogen dioxide are strongly
coupled through the photostationary-state relationship. This relationship usually does
not hold in urban air.
4.6. PROBLEMS
4.1. Calculate the photostationary-state mixing ratio of ozone when p
d
1013 mb,
T 298 K, J ≈ 0.01 s
1
, k
1
≈ 1.8 10
14
cm
3
molecule
1
s
1
,
χ
NO(g) 180
ppbv, and
χ
NO
2
(g) 76 ppbv. Perform the same calculation under the same
conditions, except,
χ
NO(g) 9 ppbv and
χ
NO
2
(g) 37 ppbv.
(a) Ignoring the constant temperature and photolysis coefficient, which of the
two cases do you think represents afternoon conditions? Why?
(b) If the NO(g) and NO
2
(g) mixing ratios were measured in urban air, do you
think the morning or afternoon ozone mixing ratio calculated by the pho-
tostationary-state relationship would be closer to the actual mixing ratio
of ozone? Why?
4.2. Explain why the photostationary-state relationship is a useful relationship for
background-tropospheric air but less useful for urban air.
4.3. Why does ozone not form at night?
4.4. Why does the hydroxyl radical not form at night?
4.5. Why are nighttime ozone mixing ratios always nonzero in the background tro-
posphere but sometimes zero in urban areas?
4.6. If nighttime ozone mixing ratios in one location are zero and in another nearby
location are nonzero, what do you think is the reason for the difference?
4.7. If ozone mixing ratios are 0.16 ppmv and ROG mixing ratios are 1.5 ppmC,
what is the best regulatory method of reducing ozone, if only the effects of
NO
x
(g) and ROGs on ozone are considered?
4.8. If NO
x
(g) mixing ratios are 0.2 ppmv and ROG mixing ratios are 0.3 ppmC,
what is the best regulatory method of reducing ozone, if only the effects of
NO
x
and ROGs on ozone are considered?

URBAN AIR POLLUTION 113
4.9. If NO
x
(g) mixing ratios are 0.05 ppmv and ROG mixing ratios are 1 ppmC,
what would be the resulting ozone mixing ratio if ROGs were increased by 1
ppmC and NO
x
(g) were increased by 0.1 ppmv?
4.10. In Fig. 4.10, why are ozone mixing ratios high and nitric oxide mixing ratios
low in San Bernardino, whereas the reverse is true in central Los Angeles?
4.11. Why don’t aromatic gases, emitted in urban air, reach the stratosphere to pro-
duce ozone?
4.12. What are the two fundamental differences between ozone production in the
background troposphere and in urban air?
4.13. If the hydroxyl radical did not break down ROGs, would aromatics still be
important smog producers? What about aldehydes? Explain.
4.14. Why is CO(g), the most abundantly emitted gas in urban air, not an important
smog producer?
4.15. Why should PAN mixing ratios peak at about the same time as ozone mixing
ratios during the day?
4.16. In terms of chemical lifetimes and by-products, what are some of the costs and
benefits of methanol and ethanol as alternative fuels?
4.17. Write out a chemical mechanism for the production of ozone from propane
[C
3
H
8
(g)] oxidation by OH(g).
4.18. Write out a chemical mechanism showing how benzaldehyde [C
6
H
5
CHO(g)]
could form ozone.

AEROSOL PARTICLES IN
SMOG AND THE GLOBAL
ENVIRONMENT
5
A
lthough most regulations of air pollution focus on gases, aerosol particles
cause more visibility degradation and possibly more health problems than do
gases. Particles smaller than 2.5 m in diameter cause the most severe health
problems. Particles enter the atmosphere by emissions and nucleation. In the air, their
number concentrations and sizes change by coagulation, condensation, chemistry,
water uptake, rainout, sedimentation, dry deposition, and transport. Particle concentra-
tion, size, and morphology affect the radiative energy balance in urban air and in the
global atmosphere. In this chapter, compositions, concentrations, sources, transforma-
tion processes, sinks, and health effects of aerosol particles are discussed. The effects
of aerosol particles on visibility are described in Chapter 7. Regulations relating to
particles are given in Chapter 8.
5.1. SIZE DISTRIBUTIONS
Aerosol and hydrometeor particles are characterized by their size distribution and
composition. A size distribution is the variation of concentration (i.e., number, sur-
face area, volume, or mass of particles per unit volume of air) with size.
Table 5.1
compares typical diameters, number concentrations, and mass concentrations of gases,
aerosol particles, and hydrometeor particles under lower tropospheric conditions. The
table indicates that the number and mass concentrations of gas molecules are much
greater than are those of particles. The number concentration of aerosol particles
decreases with increasing particle size. The number concentrations of hydrometeor
particles are typically less than are those of aerosol particles, but the mass concentra-
tions of hydrometeor particles are always greater than are those of aerosol particles.
Aerosol particle size distributions can be divided into modes, which are region of
the size spectrum (in diameter space) in which distinct peaks in concentration occur.
Usually, each mode can be described analytically with a lognormal function, which is
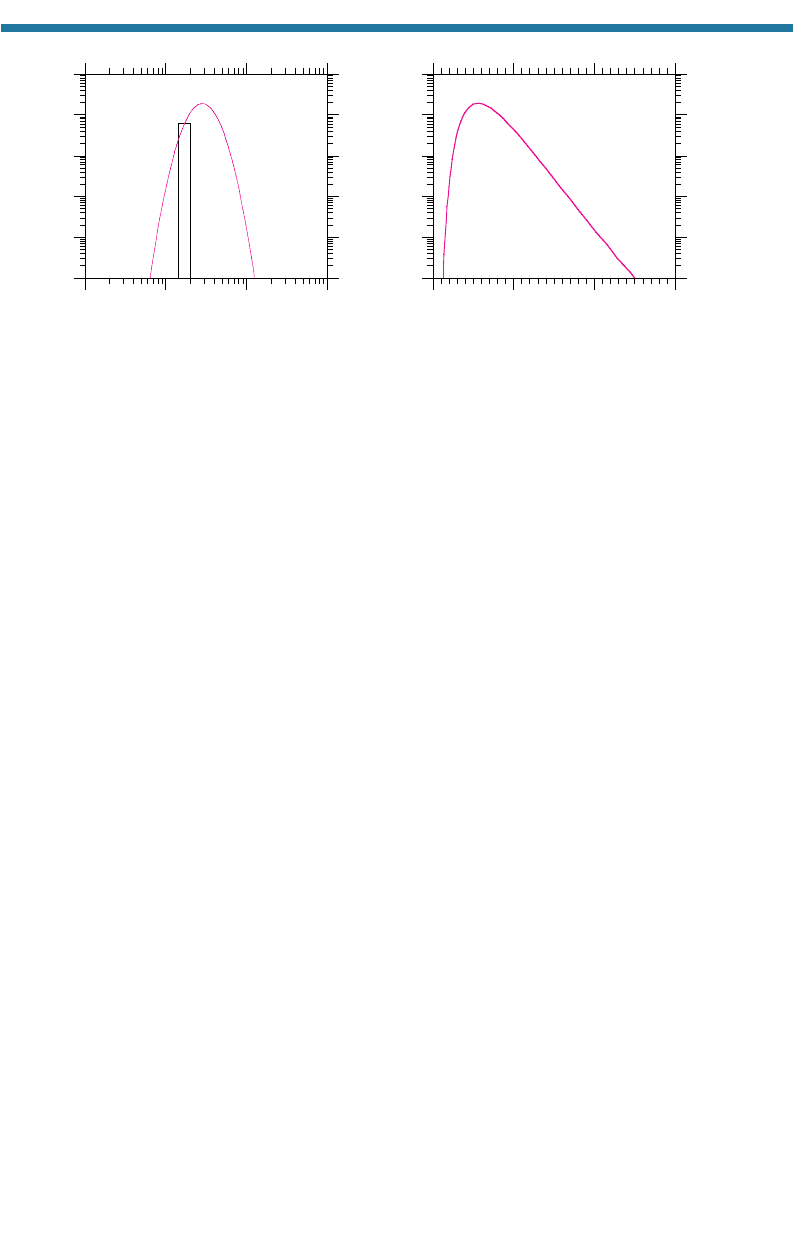
a bell-curve distribution on a log–log scale, as shown in Fig. 5.1(a). Figure 5.1(b)
shows the same distribution on a log-linear scale.
Aerosol particle distributions with 1, 2, 3, or 4 modes are called unimodal, bimodal,
trimodal, or quadrimodal, respectively. Such modes may include a nucleation mode,
116
Table 5.1. Characteristics of Gases, Aerosol Particles,
and Hydrometeor Particles
Gas molecules 0.0005 2.45 10
19
1.2 10
9
Aerosol particles
Small 0.2 10
3
–10
6
1
Medium 0.2–2.0 1–10
4
250
Large 2.0 1–10 250
Hydrometeor particles
Fog drops 10–20 1–500 10
4
–10
6
Cloud drops 10–200 1–1000 10
4
–10
7
Drizzle 200–1,000 0.01–110
5
–10
7
Raindrops 1,000–8,000 0.001–0.01 10
5
–10
7
Number Concentration
(Molecules or Particles Mass Concentration
Typical Diameter (
m) cm
3
)(g m
3
)
Data are for typical lower tropospheric conditions.
two subaccumulation modes, and a coarse mode. The nucleation mode (mean diame-
ters less than 0.1 m) contains small emitted particles or newly nucleated particles
(particles formed directly from the gas phase). Small nucleated or emitted particles
increase in size by coagulation (collision and coalescence of particles) and growth (con-
densation of gases onto particles). Only a few gases, such as sulfuric acid, water, and
some heavy organic gases, among others, condense onto particles. Molecular oxygen
and nitrogen, which make up the bulk of the gas in the air, do not.
Growth and coagulation move nucleation mode particles into the accumulation
mode, where diameters are 0.1 to 2 m. Some of these particles are removed by rain,
but they are too light to fall out of the air by sedimentation (dropping by their own
weight against the force of drag). The accumulation mode sometimes consists of two
submodes with mean diameters near 0.2 m and 0.5 to 0.7 m (Hering and
Friedlander, 1982; John et al., 1989), possibly corresponding to newer and aged parti-
cles, respectively. The accumulation mode is important for two reasons. First,
accumulation mode particles are likely to affect health by penetrating deep into the
lungs. Second, accumulation mode particles are close in size to the peak wavelengths
of visible light and, as a result, affect visibility (Chapter 7). Particles in the nucleation
and accumulation modes together are fine particles.
The coarse mode consists of particles larger than 2 m in diameter. These parti-
cles originate from windblown dust, sea spray, volcanos, plants, and other sources.
Coarse mode particles are generally heavy enough to sediment out rapidly within
hours to days. The emission sources and deposition sinks of fine particles differ from
those of coarse mode particles. Fine particles usually do not grow by condensation to
much larger than 1 m, indicating that coarse mode particles originate primarily from
emissions.
In general, the nucleation mode has the highest number concentration, the accumu-
lation mode has the highest surface area concentration, and the coarse mode has the
highest volume (or mass) concentration of aerosol particles. Figure 5.2 shows a quad-
rimodal distribution, fitted from data at Claremont, California, for the morning of
AEROSOL PARTICLES IN SMOG AND THE GLOBAL ENVIRONMENT 117
10
-3
10
-2
10
-1
10
0
10
1
10
2
0.001 0.01 0.1 1
dv (μm
3
cm
-3
)/d log
10
D (μm)
Particle diameter (D, μm)
D
2
D
1
10
-3
10
-2
10
-1
10
0
10
1
10
2
0 0.05 0.1 0.15
dv (μm
3
cm
-3
)/d log
10
D (μm)
Particle diameter (D, μm)
(a) (b)
Figure 5.1. (a) A lognormal particle volume distribution on a log–log scale. The incremental
volume concentration (dv, m
3
cm
3
-air) of material between any two diameters (D
1
and D
2
,
m) is estimated by multiplying the average value from the curve between the two diameters
by d log
10
D log
10
D
2
-
log
10
D
1
. Thus, for example, the volume concentration between diame-
ters D
1
and D
2
is approximately 6 m
3
cm
3
m
1
0.15 m 0.9 m
3
cm
3
. (b) The
lognormal curve shown in (a), drawn on a log-linear scale.
