Jacobson M.Z. Atmospheric Pollution
Подождите немного. Документ загружается.

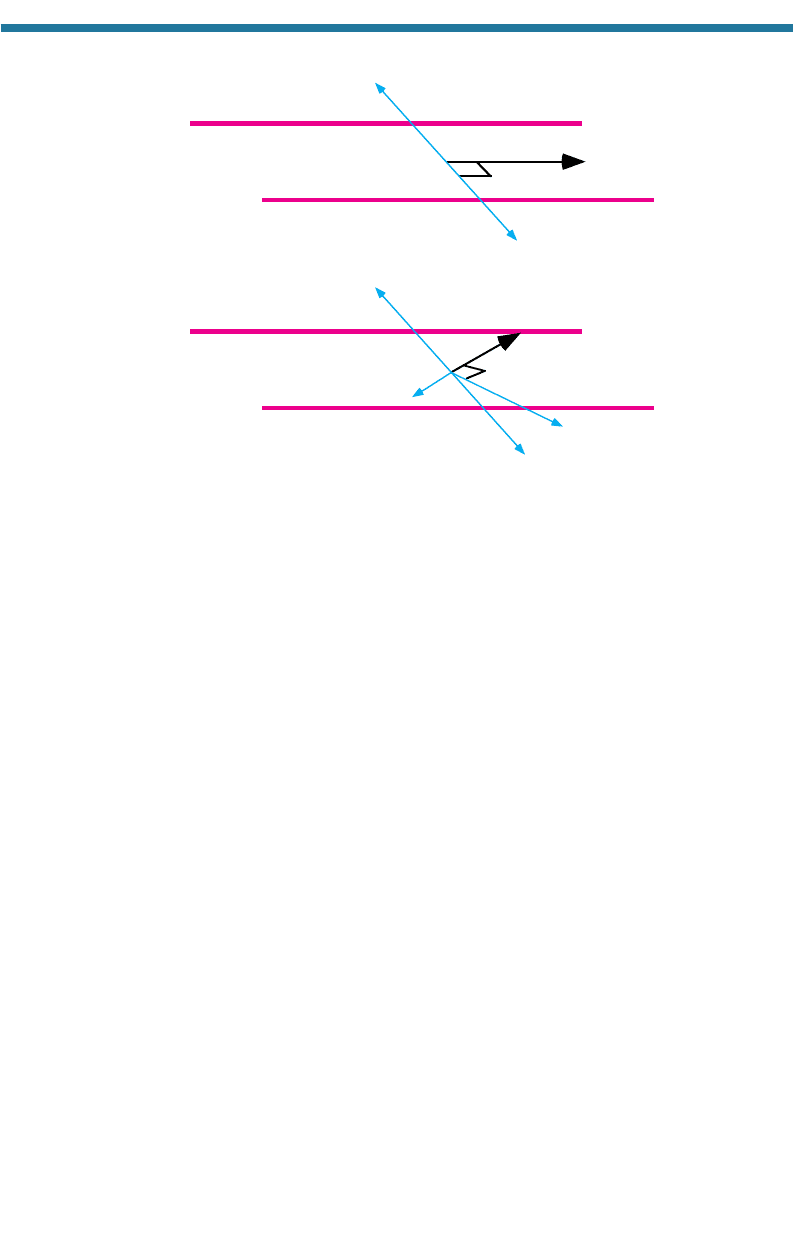
6.2.1. Geostrophic Wind
The type of wind involving the least number of forces is the geostrophic (“Earth-
turning”) wind. This wind involves only the PGF and ACoF. It arises above the
boundary layer, where surface friction is negligible, and along straight isobars. An
isobar is a line of constant pressure. Suppose a horizontal pressure gradient, repre-
sented by two parallel isobars, exists, such as in the top diagram of Fig. 6.2. The
PGF causes still air to move from high to low pressure. As the air moves, the ACoF
deflects the air to the right. The ACoF continues deflecting the air until the ACoF
exactly balances the magnitude and direction of the PGF (geostrophic balance).
Figure 6.2 shows that the resulting geostrophic wind flows parallel to isobars. The
closer the isobars are together
, the faster the geostrophic wind. In reality, geostroph-
ic balance occurs following a process called geostrophic adjustment, during which
the wind overshoots then undershoots its ultimate path in an oscillatory fashion. In
the Southern Hemisphere, the geostrophic wind flows in the opposite direction from
that shown in the top of Fig. 6.2.
6.2.2. Surface Winds along Straight Isobars
When isobars are straight near the surface, the FF affects the equilibrium wind speed
and direction. The bottom of Fig. 6.2 shows the wind direction that results from a
balance among the PGF, ACoF, and FF at the surface in the Northern Hemisphere.
Friction, which acts in the opposite direction from the wind, slows the wind.
Because the magnitude of the ACoF is proportional to the wind speed, a reduction in
wind speed reduces the magnitude of the ACoF. Because the sum of the FF and the
ACoF must balance the PGF, the equilibrium wind direction shifts toward low pres-
sure. On average, surface friction turns winds 15 to 45 toward low pressure, with
lower values corresponding to smooth surfaces and higher values corresponding to
rough surfaces.
148 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
H
L
Surface
H
L
PGF
Aloft
ACoF
FF+ACoF
Geostrophic wind
Surface wind
PGF
ACoF
FF
Figure 6.2. Forces acting to give winds aloft and at the surface in the Northern Hemisphere.
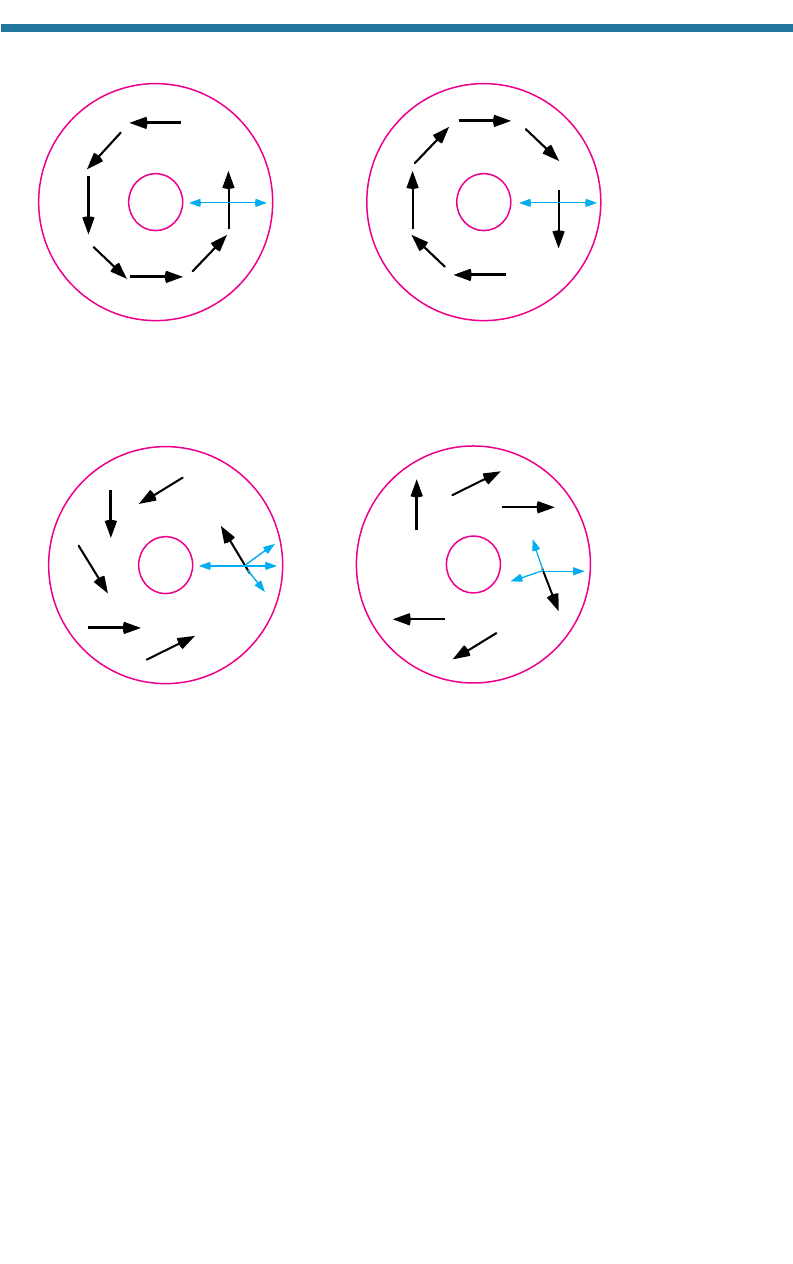
6.2.3. Gradient Wind
When centers of low and high pressure exist aloft, the wind is controlled by the PGF,
ACoF, and ACfF. The resulting wind is the gradient wind. For a low-pressure center,
the PGF acts toward and the ACfF acts away from the center of the low. Balance
requires that the ACoF also oppose the PGF. Figure 6.3(a) shows the resulting
counterclockwise (cyclonic) gradient wind in the Northern Hemisphere. Around a high-
pressure center aloft in the Northern Hemisphere, the PGF and ACfF act away from and
the ACoF acts toward the center of the high. Figure 6.3(b) shows the resulting clock-
wise (anticyclonic) wind. In the Southern Hemisphere, gradient winds flow clockwise
around low-pressure centers and counterclockwise around high-pressure centers.
6.2.4. Surface Winds along Curved Isobars
Near the surface, the FF affects the flow around centers of low and high pressure.
A surface low-pressure center is a cyclone, and a surface high-pressure center is an
anticyclone. Figure 6.4 shows the force balances and resulting winds in the presence
of a surface (a) low-pressure system and (b) high-pressure system in the Northern
Hemisphere. In the low-pressure case, surface winds rotate counterclockwise
EFFECTS OF METEOROLOGY ON AIR POLLUTION 149
L ACoF +
ACfFPGF
Gradient
wind
834 mb
830 mb
PGF +
ACfF
H
ACoF
834 mb
830 mb
Gradient
wind
(a) (b)
Figure 6.3. Gradient winds around a center of (a) low and (b) high pressure in the Northern
Hemisphere, and the forces affecting them.
Surface
wind
L
1,000 mb
996 mb
ACoF
ACfF
PGF
FF
PGF +
ACfF
H
ACoF
1,016 mb
1,012 mb
Surface
wind
FF
(a) (b)
Figure 6.4. Surface winds around centers of (a) low and (b) high pressure in the Northern
Hemisphere and the forces affecting them.
(cyclonically), but the FF causes them to converge toward the center of the low. In the
high-pressure case, surface winds rotate clockwise (anticylonically), but the FF caus-
es them to diverge away from the center of the high. In the Southern Hemisphere,
winds converge clockwise around a surface low-pressure center and diverge counter-
clockwise around a surface high-pressure center.
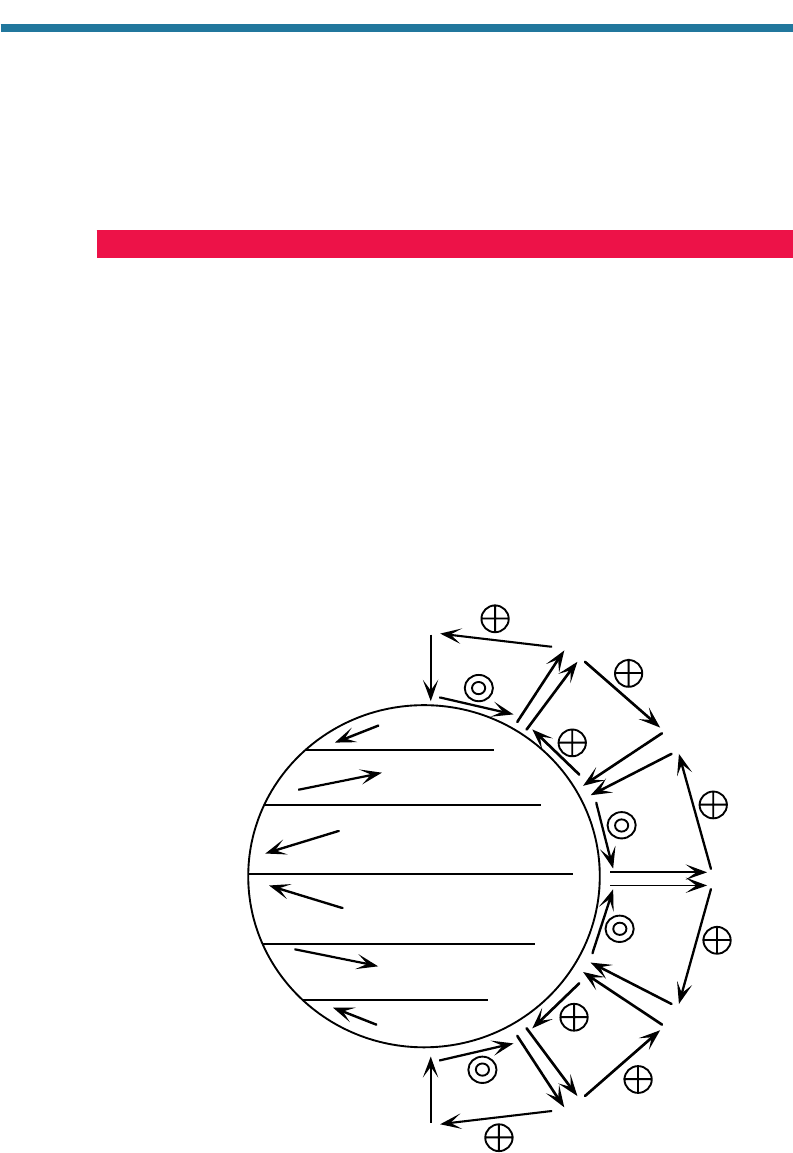
6.3. GLOBAL CIRCULATION OF THE ATMOSPHERE
Air pollution is affected by winds, winds are affected by large-scale pressure systems,
and large-scale pressure systems are affected by the global circulation of the atmosphere.
Figure 6.5 shows features of the global circulation, including the major circulation cells,
the belts of low and high pressure, and the predominant wind directions.
Winds have a west-east (zonal), south-north (meridianal), and vertical compo-
nent. The three circulation cells in each hemisphere shown in Fig. 6.5 represent the
meridianal and vertical components of the Earth’s winds, averaged zonally (over all
longitudes) and over a long time period. The cells are symmetric about the equator and
extend up to the tropopause (Section 3.3.1.2), which is near 18 km altitude over the
equator and near 8 km altitude over the poles.
Two cells, called Hadley cells, extend from 0 to 30N and S latitude, respectively.
These cells were named after George Hadley (1685–1768), an English physicist and
150 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Figure 6.5. Diagram of the three major circulation cells in the atmosphere, the predominant surface pressure
systems, and the predominant surface wind systems on the Earth; H, high pressure; L, low pressure.
Elevated high and low pressures are relative to pressures at the same altitude. The circled X’s denote winds
going into the page (west to east). The circled dots denote winds coming out of the page (east to west).
Polar cell
Ferrel cell
Hadley
cell
H
Equatorial low - 0 °N
(Doldrums), ITCZ
Subtropical high - 30
°N
(Horse latitudes)
Subpolar low - 60
°N
Polar high - 90
°N
Polar high - 90
°S
Subtropical high - 30
°S
Subpolar low 60
°S
Northeast trade winds
Southwesterlies
Southeast trade winds
Northwesterlies
Polar easterlie
s
Polar easterlies
H
H
H
L
L
L
L
Polar cell
Ferrel cell
Hadley
cell
H
H
H
L
L
L
West
East

meteorologist who, in 1735, first proposed the cells in a paper called, “Concerning the
Cause of the General Trade Winds,” presented to the Royal Society of London.
Hadley’s original cells, however, extended between the equator and poles.
In 1855, William Ferrel (1817–1891; Fig. 6.6)
an American school teacher, meteorologist, and
oceanographer, published an article in the Nashville
Journal of Medicine pointing out that Hadley’s one-
cell model did not fit observations so well as did the
three-cell model shown in Fig. 6.5. In 1860, Ferrel
went on to publish a collection of papers showing
the first application of mathema-tical theory to fluid
motions on a rotating Earth. Today, the middle cell
in the three-cell model is named the Ferrel cell.
Ferrel cells extend from 30 to 60 in the Northern
and Southern Hemispheres. Two Polar cells extend
from 60 to 90N and S latitude, respecti
vely.
6.3.1. Equatorial Low-Pressure Belt
Circulation in the three cells is controlled by heating
at the equator, cooling at the poles, and the rotation
of the Earth. Air rises over the equator because
the sun heats this region intensely. Much of the
heating occurs over water, some of which evapo-
rates. As air containing water vapor rises, the air expands and cools, and the water
vapor recondenses to form clouds of great v
ertical extent. Condensation of water
vapor releases latent heat, providing the air with more buoyancy. Over the equator,
the air can rise up to about 18 km before it is decelerated by the stratospheric inver-
sion. Once the air reaches the tropopause, it cannot rise much farther, so it diverges to
the north and south. At the surface, air is drawn in horizontally to replace the rising
air. So long as divergence aloft exceeds convergence at the surface, surface air pres-
sure decreases and air pressure aloft increases (relative to pressures at the same
altitude but other latitudes). The surface low-pressure belt at the equator is called the
equatorial low-pressure belt. Because pressure gradients are weak, winds are light,
and the weather is often rainy over equatorial wa
ters, this region is also called the
doldrums.
6.3.2. Winds Aloft in the Hadley Cells
As air diverges toward the north in the elevated part of the Northern Hemisphere
Hadley cell, the ACoF deflects much of it to the right (to the east), giving rise to
westerly winds aloft (winds are generally named after the direction that they originate
from). Westerly winds aloft in the Northern Hemisphere Hadley cell increase in
magnitude with increasing distance from the equator until they meet equatorward-
moving air from the Ferrel cell at 30N, the subtropical front. The front is a region of
sharp temperature contrast. The winds at the front are strongest at the tropopause,
where they are called the subtropical jet stream. Winds aloft in the Southern
Hemisphere Hadley cell are also westerly and culminate in a tropopause subtropical
jet stream at 30S.
EFFECTS OF METEOROLOGY ON AIR POLLUTION 151
Figure 6.6. William Ferrel (1817–1891).

6.3.3. Subtropical High-Pressure Belts
As air converges at the subtropical fronts at 30N and S, much of it descends. Air is
drawn in horizontally aloft to replace the descending air. So long as inflow aloft
exceeds outflow at the surface, surface air pressure builds up. The surface high-
pressure belts at 30N and S are called subtropical high-pressure belts. Because
descending air compresses and warms, evaporating clouds, and because pressure gra-
dients are relatively weak around high-pressure centers, surface high-pressure systems
are characterized by sunny skies and light winds. Sunny skies and the lack of rainfall
at 30N and S are two reasons why most deserts of the world are located at these lati-
tudes. The light winds forced many ships sailing at 30N to lighten their cargo, the
heaviest and most dispensable component of which was often horses. Thus the 30N
latitude band is also called the horse latitudes.
6.3.4. The Trade Winds
At the surface at 30N and S, descending air diverges both equatorward and poleward.
Most of the air moving equatorward is deflected by the ACoF to the right (toward the
west) in the Northern Hemisphere and to the left (toward the west) in the Southern
Hemisphere, except that friction reduces the extent of ACoF turning. The resulting
winds in the Northern Hemisphere are called the northeast trade winds because they
originate from the northeast. Those in the Southern Hemisphere are called the south-
east trade winds because they originate from the southeast. Sailors from Europe have
used the northeast trades to speed their voyages westward since the fifteenth century.
The trade winds are consistent winds. As seen in Fig. 6.5, the northeast and southeast
trade winds converge at the Intertropical Convergence Zone (ITCZ), which moves
north of the equator in the Northern Hemisphere summer and south of the equator in
the Southern Hemisphere summer, generally following the direction of the sun. At the
ITCZ, air convergence and surface heating lead to the rising arm of the Hadley cells.
6.3.5. Subpolar Low-Pressure Belts
As surface air moves poleward in the Ferrel cells, the ACoF turns it toward the east in
both hemispheres, but surface friction reduces the extent of turning, so that near-surface
winds at midlatitudes (30 to 60N and S) are generally westerly to southwesterly (from
the west or southwest) in the Northern Hemisphere and westerly to northwesterly in the
Southern Hemisphere. In both hemispheres, poleward-moving near-surface air in the
Ferrel cell meets equatorward-moving air from the Polar cell at the polar front, which
is a region of sharp temperature contrast between these two cells. Converging air at the
surface front rises and diverges aloft, reducing surface air pressure and increasing air
pressure aloft relative to pressures at other latitudes. The surface low-pressure regions at
60N and S are called subpolar low-pressure belts. Regions of rising air and surface
low pressure are associated with storms. Thus, the intersection of the Ferrell and Polar
cells is associated with stormy weather. Unlike at the equator, surface pressure gradients
and winds at the polar front are relatively strong. West–east wind speeds also increase
with increasing height at the polar fronts. At the tropopause in each hemisphere, they
culminate in the polar front jet streams. Whereas the subtropical jet streams do not
meander to the north or south over great distances, the polar front jet streams do. Their
predominant direction is still from west to east.
152 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
EFFECTS OF METEOROLOGY ON AIR POLLUTION 153
1018
1014
1006
1022
1018
1022
1018
1018
1010
1006
1022
1026
10
20
30
40
50
60
70
80
-180 -170 -160 -150 -140 -130 -120 -110
Latitude (degrees)
Longitude (degrees)
HH
L
L
= 17.67 m/s
(a)
5450
5600
5550
5800
5900
5550
5500
5450
5400
5600
5600
5900
5850
10
20
30
40
50
60
70
80
-180 -170 -160 -150 -140 -130 -120 -110
Latitude (degrees)
Longitude (degrees)
H
H
L
L
= 32.15 m/s
(b)
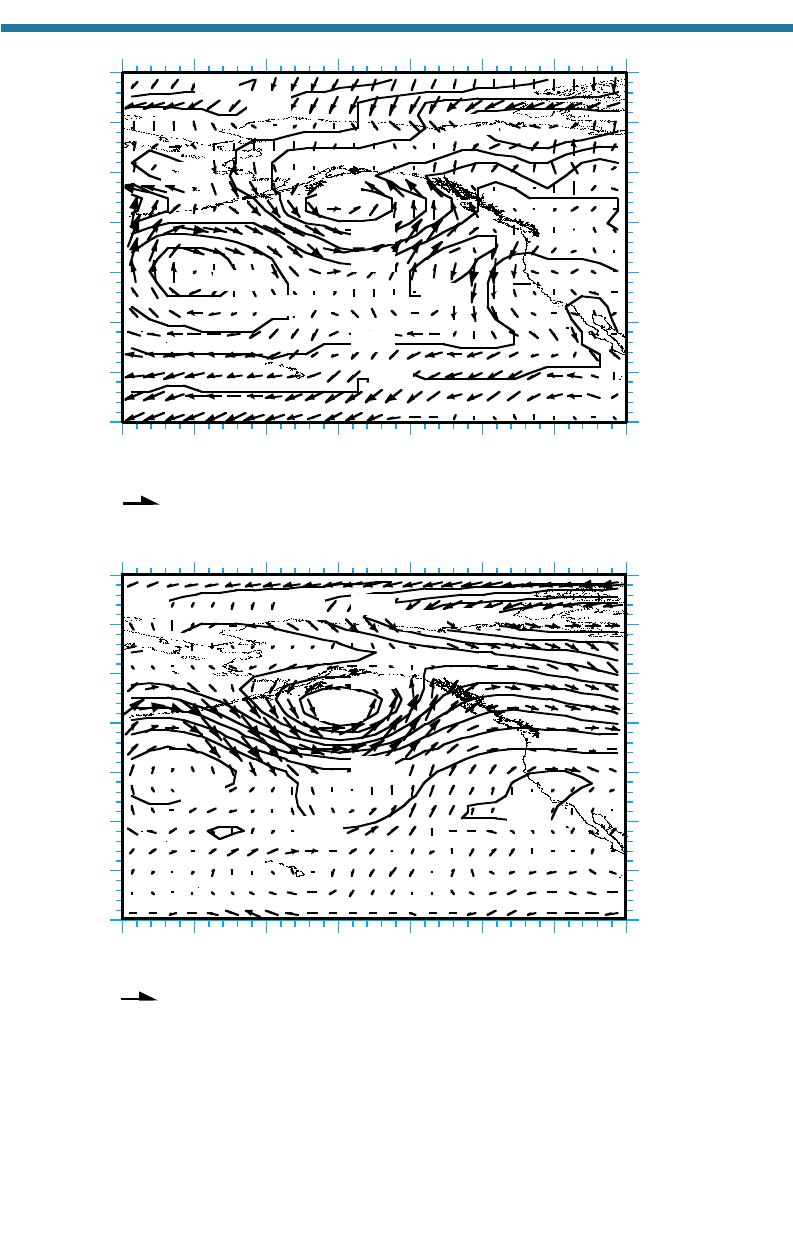
Figure 6.7. Map of (a) sea-level pressures (mb) and near-surface winds (m/s) and (b) 500-mb
heights (m) and winds (m/s) obtained from NCEP (2000) for August 3, 1990, at 12 GMT for
the northern Pacific Ocean. Height contours on a constant pressure chart are analogous to
isobars on a constant height chart; thus, high (low) heights in map (b) correspond to high
(low) pressures on a constant height chart. The surface low-pressure system at 148W,
53N in (a) is the Aleutian low. The sur face high-pressure system at 134W, 42N in (a) is
the Pacific high.

6.3.6. Westerly Winds Aloft at Midlatitudes
One would expect the ACoF to deflect air moving equatorward aloft in the Ferrel cells
to the west, creating easterly winds (from east to west) aloft at midlatitudes in both
hemispheres. In fact, the Coriolis force does act on the winds in this way, but the
winds aloft in these regions are accelerated in the opposite direction (from west to
east) by the movement of air around pressure centers.
Descending air at 30N and S creates centers of surface high pressure, and rising
air at 60N and S creates bands or centers of surface low pressure. Figure 6.7(a)
shows an example of surface high- and low-pressure centers formed over the Pacific
Ocean in the Northern Hemisphere at these respective latitudes. Surface winds mov-
ing around a Northern Hemisphere surface high-pressure center travel clockwise
(diverging away from the center of the high), and surface winds moving around a sur-
face low-pressure center travel counterclockwise (converging into the center of the
low). Indeed, these characteristics can be seen in Fig. 6.7(a),
which shows winds trav-
eling clockwise around the highs and counterclockwise around the lows. The reason
winds move the way they do around pressure centers was described in Sections 6.2.3
and 6.2.4. The positions of the highs and lows shown in Fig. 6.7(a) create a near-
surface west-to-east flow that meanders sinusoidally around the globe. The flow
created by these highs and lows is consistent with the e
xpectation that near-surface
winds in the Ferrel cell are predominantly westerly.
Figure 6.7(b) shows an elevated map corresponding to the surface map in
Fig. 6.7(a). The elevated map shows height contours on a surface of constant pressure
(500 mb) and winds traveling around centers of low and high heights. Height contours
on a constant pressure chart are analogous to isobars on a constant altitude chart; thus,
high (low) heights in Fig. 6.7(b) correspond to high (low) pressures on a constant
height chart. The lows aloft lie slightly to the west of the surface lows. The figure indi-
cates that winds traveling around the highs and lows aloft connect, as they do at the
surface, resulting in sinusoidal west-to-east flow around the globe. Thus, high-and
low-pressure systems aloft are responsible for westerly winds aloft in the Ferrel cell.
6.3.7. Polar Easterlies
Air moving poleward aloft in the Polar cells is turned toward the east in both
hemispheres by the ACoF, causing elevated winds in the Polar cells to be westerly. At
the poles, air aloft descends, increasing surface air pressures. The surface high-
pressure regions are called Polar highs. Air at the polar surface diverges equatorward.
The ACoF turns this air toward the west. Friction is weak because polar surfaces are
either snow or ice, and the resulting surface winds in the Polar cells are easterly and
called polar easterlies.
6.4. SEMIPERMANENT PRESSURE SYSTEMS
The subtropical high-pressure belts in the Northern and Southern Hemispheres are
dominated by surface high-pressure centers over the oceans. These high-pressure cen-
ters are called semipermanent surface high-pressure centers because they are
usually visible on a sea level isobar map most of the year. These pressure systems tend
to move northward in the Northern Hemisphere summer and southward in the winter.
On average, they are centered near 30N or S. In the Northern Hemisphere, the two
154 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION

semipermanent surface high-pressure systems are the Pacific high (in the Pacific
Ocean) and the Bermuda–Azores high (in the Atlantic Ocean). In Fig. 6.7(a), the sur-
face high-pressure center at 134W, 42N is the Pacific high. In the Southern
Hemisphere, semipermanent high-pressure systems are located at 30S in the south
Pacific, south Atlantic, and Indian Oceans.
The subpolar low-pressure belt in the Northern Hemisphere is dominated by semi-
permanent surface low-pressure centers. The subpolar low-pressure belt in the
Southern Hemisphere is dominated by a band of low pressure. In the Northern
Hemisphere, the two semipermanent low-pressure centers are the Aleutian low (in the
Pacific Ocean) and the Icelandic low (in the Atlantic Ocean). These pressure systems
tend to move northward in the Northern Hemisphere summer and southw
ard in the
winter, but generally stay between 40 and 65N. In Fig. 6.7(a), the surface low-pressure
center at 148W, 53N is the Aleutian low.
6.5. THERMAL PRESSURE SYSTEMS
Whereas semipermanent surface high- and low-pressure centers exist over the oceans
all year, thermal surface high- and low-pressure systems form over land seasonally.
Thermal pressure systems form in response to surface heating and cooling, which
depend on properties of soil and water, such as specific heat. Specific heat (J kg
1
K
1
)
is the energy required to increase the temperature of 1 g of a substance 1 K. Soil has a
lower specific heat than does water, as shown in Table 6.1. During the day, the addition
of the same amount of sunlight increases the temperature of soil more than it does that
of water. During the night, the release of the same amount of thermal-IR energy
decreases soil temperature more than it does that of water. As such, land heats during
the day and cools during the night more than does water. Similarly, land heats during
the summer and cools during the winter more than does water.
Specific heats vary not only between land and water, but also between different
soil types, as shown in Table 6.1. Because sand has a lower specific heat than does
clay, sandy soil heats to a greater extent than does clayey soil during the day and sum-
mer. The preferential heating of sand in comparison with that of clay and the
preferential heating of land in comparison with that of water is an important factor that
results in thermal low-pressure centers.
When a region of soil heats, air above the soil warms, rises, and diverges aloft,
creating low pressure at the surface. Because the low-pressure system forms by heat-
ing, it is called a thermal low-pressure system. Thermal low-pressure centers form in
the summer over deserts and other sunny areas (e.g., the Mojave Desert in southern
California, the plateau of Iran, the north of India). These areas are all located near
30N, the same latitude as the semipermanent highs. Descending air at 30N due to the
highs helps to form the thermal lows by evaporating clouds, clearing the skies. The
descending air also keeps the thermal lows shallow (air rising in the thermal lows
EFFECTS OF METEOROLOGY ON AIR POLLUTION 155
Table 6.1. Specific Heats of Four Media at 298.15 K
Dry air at constant pressure 1004.67
Liquid water 4185.5
Clay 1360
Dry sand 827
Substance Specific Heat (J kg
1
K
1
)
diverges horizontally at a low altitude). In some cases, such as over the Mojave Desert,
the thermal lows do not produce clouds due to the lack of water vapor and shallowness
of the low. In other cases, such as over the Indian continent in the summer, rising air in
a low sucks warm, moist surface air in horizontally from the ocean, ultimately produc-
ing heavy rainfall. The strong sea breeze due to this thermal low is the summer
monsoon, where a monsoon is a seasonal wind caused by a strong seasonal variation
in temperature between land and water.
During the winter, when temperatures cool over land, air densities increase, caus-
ing air to descend. Aloft, air is drawn in horizontally to replace the descending air,
building up surface air pressure. The resulting surface high-pressure system is called a
thermal high-pressure system. In the Northern Hemisphere, two thermal high-
pressure systems are the Siberian high (over Siberia) and the Canadian high (over
the Rocky Mountains between Canada and the United States). As with thermal low-
pressure systems, thermal high-pressure systems are often shallow.
6.6. EFFECTS OF LARGE-SCALE PRESSURE SYSTEMS
ON AIR POLLUTION
Semipermanent and thermal pressure systems affect air pollution. Table 6.2 compares
characteristics of such pressure systems, including their effects on pollution. Semi-
permanent low-pressure systems are associated with cloudy skies, stormy weather, and
fast surface winds. Thermal low-pressure systems, which are often shallow, may or may
not produce clouds. Air rises in both types of low-pressure systems, dispersing near-
surface pollution upward. When clouds form in low-pressure systems, they block
sunlight that would otherwise dri
ve photochemical reactions, reducing pollution further.
Surface high-pressure systems are characterized by relatively slow surface winds,
sinking air, and cloud-free skies. In such pressure systems, air sinks, confining near-
surface pollution. In addition, the slow near-surface winds associated with high-
pressure systems prevent horizontal dispersion of pollutants, and the cloud-free skies
caused by the pressure systems maximize the sunlight available to drive photochemi-
cal smog formation. In sum, the major effects of pressure systems on pollution are
through vertical pollutant transfer, horizontal pollutant transfer, and cloud cover. Each
of these effects is discussed in turn.
156 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Table 6.2. Summary of Characteristics of Northern Hemisphere Surface Low- and
High-Pressure Systems
Latitude range 45–65N25–45N25–45N45–65N
Surface pressure gradients Strong Varying Weak Varying
Surface wind speeds Fast Varying Slow Varying
Surface wind directions Converging, Converging, Diverging, Diverging,
counterclockwise counterclockwise clockwise clockwise
Vertical air motions Upward Upward Downward Downward
Cloud cover Cloudy Cloud-free or cloudy Cloud-free, sunny Cloud free
Storm formation? Yes Sometimes No No
Effect on air pollution Reduces Reduces Enhances Enhances
Surface Low-Pressure Systems Surface High-Pressure Systems
Characteristic Semipermanent Thermal Semipermanent Thermal

6.6.1. Vertical Pollutant Transport
Pressure systems affect vertical air motions and, therefore, pollutant dispersion by
forced and free convection (Section 3.2.2). In a semipermanent low-pressure system,
for example, near-surface winds converge and rise, dispersing near-surface pollutants
upward. In a semipermanent high-pressure system, winds aloft converge and sink, con-
fining near-surface pollutants. Both cases illustrate forced convection. In thermal
low-pressure systems, surface warming causes near-surface air to become buoyant and
rise. In thermal high-pressure systems, surface cooling causes near-surface air to
become negatively buoyant and sink or stagnate. Both cases illustrate free convection.
To understand better how free convection in thermal pressure systems and forced con-
vection in semipermanent pressure systems af
fect pollutant dispersion, it is necessary
to discuss adiabatic processes and atmospheric stability.
6.6.1.1. Adiabatic and Environmental Lapse Rates
Whether air rises or sinks buoyantly in a thermal pressure system depends on
atmospheric stability, which depends on adiabatic and environmental lapse rates.
These terms are discussed next.
Imagine a balloon filled with air. The air pressure inside the balloon exactly equals
that outside the balloon. Imagine also that no energy (such as solar or thermal-IR ener-
gy or latent heat energy created by condensation of water vapor) can enter or leave the
balloon, but that the balloon’s membrane is flexible enough for it to expand and con-
tract due to changes in air pressure outside the balloon. Suppose now that the balloon
rises. Because air pressure always decreases with increasing altitude, the balloon must
rise into decreasing air pressure. For the air pressure inside the balloon to decrease to
the air pressure outside the balloon, the balloon must expand in volume. This type of
expansion, caused by a change in air pressure alone, is called an adiabatic expansion.
Solar heating and latent heat release are diabatic heating processes and do not con-
tribute to an adiabatic expansion.
During an adiabatic expansion, kinetic energy of air molecules is converted to
work to expand the air. Because temperature is proportional to the kinetic energy of air
molecules (Equation 3.1), an adiabatic expansion cools the air. In sum, rising air
expands and expanding air cools; thus, rising air cools during an adiabatic expansion.
The rate of cooling during an adiabatic expansion near the surface of the Earth is
approximately 9.8 K or C per kilometer increase in altitude. This rate is called the
dry or unsaturated adiabatic lapse rate (
d
). Lapse rates are opposite in sign to
changes in temperature with height; thus, a positive lapse rate indicates that tempera-
ture decreases with increasing height.
If the balloon in our example rises in air saturated with water vapor, the resulting
adiabatic expansion cools the air and decreases the saturation vapor pressure of water
in the balloon, causing the relative humidity to increase to more than 100 percent and
water vapor to condense to form cloud drops, releasing latent heat. The rate of temper-
ature increase with increasing height due to this latent heat release is typically 4 K
km
1
, but it increases to 8 K km
1
in the tropics. Subtracting this latent-heat release
rate from the dry adiabatic lapse rate gives a net lapse rate during cloud formation of
between 6 and 2 K km
1
. This rate is called the wet-, saturated-, or pseudoadiabatic
lapse rate (
w
). It is the negative rate of change of temperature with increasing alti-
tude during an adiabatic expansion in which condensation also occurs. The wet
adiabatic lapse rate is applicable only in clouds.
EFFECTS OF METEOROLOGY ON AIR POLLUTION 157
