Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

396 9 Thermal Analysis and Calorimetric Methods
the sample) and a secondary isotherm obtained after desorption under vacuum
and re - adsorption of the gaseous probe at the same temperature. This difference
can be roughly interpreted as the amount of strong sites (often associated with
adsorption heats of the order of at least 100 – 120 kJ mol
− 1
, depending on the probe
molecule and the experimental conditions).
For each dose or expansion, the equilibrium data such as the pressure P
i
and
the integral evolved heat ∆ Q
int, i
, are measured. Kinetic results for each dose are
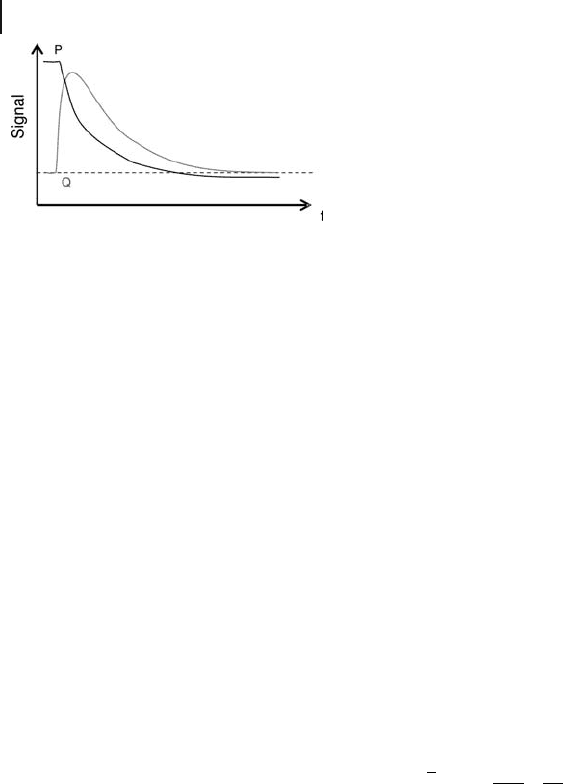
also measured, such as the heat fl ow and the evolution of the gas pressure as a
function of time. (Figure 9.2 ).
From these results we can also obtain the equilibrium pressure P , the adsorbed
amount up to the dose i ( Σ ∆ n
a, i
= n
a
), and the corresponding evolved heat ( Σ ∆ Q
int, i
= Q
int
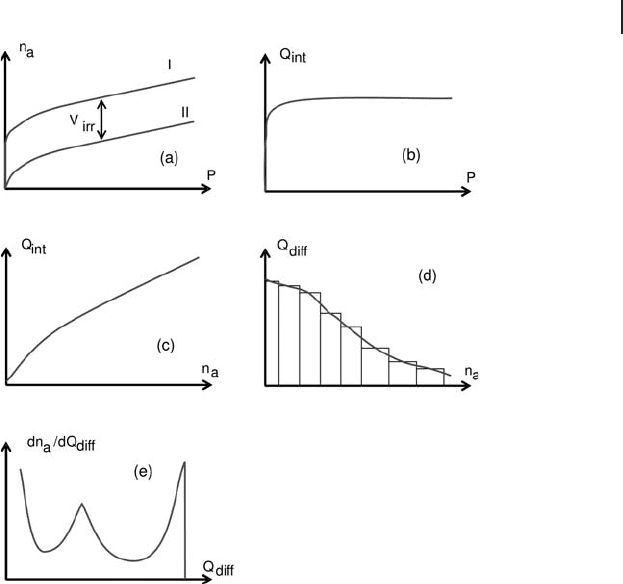
). These data can be expressed in fi ve ways, as illustrated in Figure 9.3 .
1. The volumetric isotherms ( n
a
, P ) for a cycle consisting of adsorption I,
desorption, and re - adsorption II (Figure 9.3 a)
2. The corresponding calorimetric isotherms ( Q
int
, P ) (Figure 9.3 b).
From these two types of isotherm (calorimetric and volumetric) the internal
energy and the molar entropy of adsorption
SS
Q
Tn
R
n
nd
P
ag
aa
a
n
a
=+ +
∫
int
ln
0
can be evaluated as functions of the degree of coverage, using the pairs of values
( n
a
, Q
int
) measured at the same equilibrium pressure.
When there are several possible adsorption mechanisms (e.g. reversible and
irreversible adsorption), these isotherms can be used to evaluate the sequence
and the importance (number of active sites and bond energy) of the different
processes.
3. Another representation of calorimetric data is that of integral heats as a function
of the adsorbed quantities ( Q
int
= f( n
a
)) (Figure 9.3 c). This representation leads
to the detection of coverage ranges with constant heat of adsorption, for which
the evolved heat is a linear function of the coverage.
4. Complementary information is given by the variation of the differential heats
Q
diff
as a function of n
a
(Figure 9.3 d).
Figure 9.2 Recorded data (pressure and heat fl ow signals) for
each dose of probe molecule as a function of time.
The variation of the differential heat of adsorption ( Q
diff
= ∆ Q
int, i
/ ∆ n
a, i
) as a
function of coverage is an indication of the homogeneity or heterogeneity of the
adsorption sites.
The ratio of the amount of heat evolved for each increment to the number of
moles adsorbed is equal to the average value of the differential enthalpy of
adsorption for the adsorbed quantity considered. The curve showing the
differential heat variation as a function of the adsorbed amount is traditionally
represented by histograms. However, for simplifi cation, the histogram steps
are often replaced by a continuous curve connecting the midpoints of the
histograms.
A decrease in the differential heat values as a function of coverage is
generally attributed to heterogeneity of the adsorption sites on the surface of the
solids.
A classical calorimetric curve of differential heat of adsorption against probe
uptake is presented in Figure 9.4 .
This curve can present:
(a) An initial region of high adsorption heat, mainly ascribed to adsorption on
Lewis sites, which falls abruptly.
Figure 9.3 Data obtained from volumetric – calorimetric adsorption experiments.
9.2 Techniques and Procedures 397
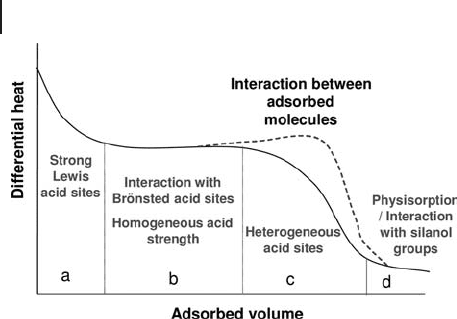
398 9 Thermal Analysis and Calorimetric Methods
(b) One or more plateaux of intermediate strength sites with a nearly constant
heat, attributed preferentially to homogeneous Br ø nsted sites.
(c) A region where heats decrease more or less steeply depending on the
heterogeneity of the sites (probably Lewis sites).
A bump in the curve can also be observed sometimes, indicating interactions
between adsorbed molecules.
(d) A reversible adsorption domain, characteristic of physisorption of the probe,
or hydrogen bonding between the probe and the sample, or very weak Lewis
acidity. The curve reaches a value close to that of the heat of condensation
of the adsorbate in the liquid phase [14] .
Such a heat (enthalpy) diagram can be used to assess the uniformity – non - uni-
formity of the surface of the adsorbent with respect to energy, the energy of the
lateral (adsorbate – adsorbate) interactions, and the structural or textural changes
that the adsorbent often undergoes as a result of interaction with the adsorbate
[17] .
5. It is possible to obtain a distribution of the energies of the adsorption sites by
plotting d n
a
/d Q
diff
as a function of Q
diff
. The area under the curve is representative
of the number of molecules that are adsorbed with a given evolved heat and
thus the number of sites corresponding to a given strength (Figure 9.3 e).
However, this type of representation is somewhat less accurate than the
previous one.
Finally, the systematic determination of the time constants (or thermokinetic
parameters) of the calorimetric signal peaks gives information on the diffusion
Figure 9.4 Regions in a typical curve of differential heats of
adsorption versus adsorbed amount. All regions (a, b, c, d)
can be observed for zeolite samples presenting both Lewis
and Br ø nsted acid sites, as probed by ammonia adsorption.
For oxides presenting only Lewis acid sites, the regions a, c
and d are observed.

rate of the probe molecule on the solid framework. The change of gas pressure is
related to the kinetics of the adsorption process; however, concerning the calori-
metric curve, the kinetic data necessary to detect reaction mechanisms can some-
times be distorted by the inertia of the calorimeter. The limitations and advantages
of the technique are fully described in Ref. [15] .
Micro - calorimetric adsorption measurements require a proper in situ vacuum
activation at a higher temperature than the adsorption process. A fi rst pre - treat-
ment under oxygen is performed in the calorimetric cell in order to eliminate the
impurities present on the sample (essentially carbonates, nitrates, carbonaceous
residues and water present from the preparation, calcination and exposure to
atmosphere) and to avoid the partial reduction of the surface of an oxide that is
easily reduced under vacuum.
9.2.1.2 Gas Flow Methods
An alternative method is fl ow adsorption microcalorimetry, which involves the use
of a carrier gas passing continuously through the adsorption cell. The catalyst is
placed on a glass frit in a gas circulation cell in the calorimeter. In order to deter-
mine the amounts of gas adsorbed, fl ow calorimetry must be used in combination
with another technique, most frequently TG, MS or GC [8, 18] .
When the system is used in pulse mode, it allows the measurement of heats of
adsorption of a gaseous reactant on a solid or interaction heats between a gaseous
reactant and pre - adsorbed species. When used as a fl ow reactor, it allows the
kinetic study of catalytic reactions as well as the study of the activation or the aging
of the catalyst. This is also a suitable system to perform calorimetric temperature
programmed reduction ( TPR ), temperature programmed oxidation ( TPO ) or tem-
perature programmed desorption ( TPD ) experiments. In addition to calorimetry,
temperature programmed desorption ( TPD ) of adsorbed probe molecules can in
principle also be used to estimate heats of adsorption [19] .
A comparison between pulsed fl ow and conventional pulsed static calorimetry
techniques for characterizing surface acidity using base probe molecule adsorp-
tion has been performed by Brown and coworkers [20, 21] . In a fl ow experiment,
both reversible and irreversible probe adsorption occurring for each dose can be
measured, and the composition of the gas fl ow gas can be easily modifi ed. The
∆ H
ads
versus coverage profi les obtained from the two techniques were found to
be comparable. The results were interpreted in terms of the extent to which NH
3
adsorption on the catalyst surface is under thermodynamic control in the two
methods.
Thermal desorption techniques have gained great popularity owing to their
comparatively easy operation. Various different mathematical analyses of the ther-
modesorption profi les have been proposed by different authors to provide kinetic
information about the distribution of the surface acid strength [22] . A simple
approach is based on the analysis of thermodesorption curves collected at different
heating rates ( β ). The shift of the temperature of the maximum rate of desorption
( T
max
) as a function of β can be exploited to derive activation parameters (i.e. E
a
)
9.2 Techniques and Procedures 399

400 9 Thermal Analysis and Calorimetric Methods
of the desorption reaction by means of different model equations. When hetero-
geneous solids are concerned, in order to derive the activation energy distribution
of the solid surface, the shift of T
max
with β has to be considered separately for
each peak. However, for this approach to provide a reliable determination of the
activation parameters, the thermodesorption curves have to be well resolved, with
clearly detectable T
max
values [23] . It has been shown that the results obtained by
this method are in good agreement with those derived from ammonia adsorption
calorimetry when the comparison is confi ned to the strong fraction of acid sites.
In general, when the experiments are carefully conducted and the samples are
pre - treated identically, similar values of numbers of acid sites and heats of adsorp-
tion can be obtained from both calorimetric and TPD measurements when using
NH
3
as probe. However, the results for pyridine adsorption can be quite different
for the two techniques [24] . The difference has been attributed to the fact that
desorption of pyridine can be severely limited by restricted diffusion, for example
within zeolite crystallites. The limitations and advantages of the TPD techniques
are fully described in Ref. [19] .
9.2.1.3 Calorimetry in Liquid Phase
Finally, it should be noted that calorimetric measurements can also be used to
monitor adsorption phenomena at the solid – liquid interface (in a solvent). This
method has been used to measure the adsorption heats evolved upon injection of
dilute solutions of pyridine in alkanes ( n - hexane, cyclohexane) onto an acidic solid
itself in a slurry with n - hexane. The amount of free base in solution is measured
separately with a UV - Vis spectrometer, leading to an adsorption isotherm that is
measured over the range of base addition used in the calorimetric titrations. The
combined data from the calorimetric titration and adsorption measurements are
analyzed simultaneously to determine equilibrium constants, quantities of sites
per gram and acid site strengths for different acid sites on the solid.
The measurements are performed in a non - interacting hydrocarbon solvent (e.g.
cyclohexane) whose molecular mass is close to that of the donor (e.g. pyridine) in
order to cancel out contributions from a dispersion component to the measured
enthalpy [25] . As an example, the acid strength of tungsten oxide supported on a
silica gel has been determined by this method [26] .
A similar technique has been used to determine the acidic character of niobium
oxide and niobyl phosphate catalysts in different solvents (decane, cyclohexane,
toluene, methanol and isopropanol) using aniline and 2 - phenyl - ethylamine as
probe molecules [27, 28] . The heat evolved from the adsorption reaction derives
from two different contributions: the exothermic enthalpy of adsorption and the
endothermic enthalpy of displacement of the solvent, while the enthalpy effects
describing dilution and mixing phenomena can be neglected owing to the differ-
ential design and pre - heating of the probe solution.
The titration of acid sites in liquids of different polarities and proticities (decane,
cyclohexane, toluene, methanol and isopropanol) makes it possible to discriminate
the acid site strength distribution more accurately than from the more conven-
tional gas – solid phase titration with ammonia.

9.2.2
Temperature Dependence of Adsorption – Desorption Heats
In adsorption microcalorimetry, surface equilibration depends not only on the
chosen probe molecule but also on the adsorption temperature. It is worth men-
tioning that the literature contains some controversial articles on this subject
[29] .
Heats of adsorption of probe molecules have frequently been measured at room
temperature [8] ; however, the results obtained from such measurements can
sometimes be of questionable accuracy as a result of non - equilibrium conditions
and non - specifi c adsorption.
In fact, the adsorption temperature should not be too low, in order to allow
the detection of differences among the sites; otherwise under certain circum-
stances the measured evolved heat can be just an average value. Another impor-
tant issue is that one must ensure that chemisorption predominates over
physisorption.
Cardona - Martinez and Dumesic [30] have analyzed the problem of surface
mobility of the adsorbates for the particular case of adsorption of basic probe
molecules on acid sites of oxides. Without equilibration of adsorbate with surface
sites, the measured differential heat would only be an average value of the sites
that the molecules adsorb on, and differences among sites would not be detected.
Thus, ideally, measurements should be made at as a high a temperature as feasible
without desorbing or decomposing the adsorbate.
In addition to isothermal measurements, temperature programmed calorimetry
experiments can also be performed using DSC. For such measurements, the
sample is equilibrated with the adsorbate at low temperatures. The temperature
is then increased in a controlled manner, and the heat absorbed (or evolved) by
the sample over a certain temperature region is related to the heat of the event
occurring at that point.
The data obtained from the temperature programmed method must be inter-
preted with care in order to properly determine the distribution of surface sites of
different heats of adsorption. If adsorption is carried out at room or slightly ele-
vated temperatures at the beginning of the experiment, suffi cient time must be
provided to ensure coverage of all the sites. Otherwise non - discriminating adsorp-
tion would occur. It has been shown that, for porous samples, re - adsorption along
the pore as a desorbed molecule diffuses out of a pore is practically unavoidable.
Therefore, although a temperature programmed experiment might appear to be
simpler to perform, there are more potential artifacts that complicate quantitative
interpretation of the results than in the case of isothermal experiments [4, 19] .
9.2.3
Probe Molecules
Appropriate probe molecules to be selected for adsorption microcalorimetry
should be stable with time and with temperature. Furthermore, in the case of
9.2 Techniques and Procedures 401

402 9 Thermal Analysis and Calorimetric Methods
zeolites and other microporous materials, they should be small enough to readily
penetrate into the intra - crystalline space. The adsorbed probe at a given tempera-
ture should also have suffi cient mobility to equilibrate with active sites. As
calorimetry gives the total number of adsorption sites, and possibly catalytically
active centers, the values obtained depend on the nature and size of the probe
molecule [14] .
9.2.3.1 Probing Surface Basicity
The number of acidic probes able to cover a wide range of strength is rather small.
The ideal probe molecule should be specifi c to basic sites and should not be
amphoteric. For example CO
2
(p K
a
= 6.37) is commonly chosen to characterize the
basicity of solids, but it may be either adsorbed on cations or physisorbed, or may
react with hydroxyls and oxide ions to form carbonated species.
CO
2
is a poor donor but a good electron acceptor. Owing to its acidic character,
it is frequently used to probe the basic properties of solid surfaces. IR evidence
concerning the formation of carbonate - like species of different confi gurations has
been reported for metal oxides [31] , which accounts for the heterogeneity of the
surface revealed by micro - calorimetric measurements. The possibility that CO
2
could behave as a base and interact with Lewis acid sites should also be considered.
However, these sites would have to be very strong Lewis acid sites and this particu-
lar adsorption mode of the CO
2
molecule should be very weak and can usually be
neglected [32] .
The same problems may arise when using SO
2
as an acidic probe, despite the
fact that SO
2
(p K
a
= 1.89) is more acidic than CO
2
and, thus, more likely to probe
the total basicity of the surface.
9.2.3.2 Probing Surface Acidity
Ammonia (p K
a
= 9.24, proton affi nity in gas phase = 857.7 kJ mol
− 1
) and pyridine
(p K
a
= 5.19, proton affi nity in gas phase = 922.2 kJ mol
− 1
) are the favored molecules
for probing the overall solid acidity, since both Lewis and Br ø nsted acid sites retain
these molecules. However the use of IR spectroscopy or XPS is necessary to dis-
tinguish qualitatively and unambiguously between these two types of sites. In
addition to NH
3
and pyridine, trimethylamine and triethylamine have also been
used to probe the acidity of supported oxides. However, it has been mentioned
[33] that these two molecules might not be able to equilibrate completely with the
surface under typical experimental conditions. The use of substituted pyridines
(2,6 - dimethylpyridine) has also been considered in order to probe specifi cally the
Br ø nsted sites [34] .
Ammonia is among the smallest strongly basic molecules, and its diffusion
is hardly affected by a porous structure. In the light of the different possible
modes of interaction of this molecule with the oxide surface, it has been found
that NH
3
mostly coordinates to Lewis sites, but in a few cases the results were
interpreted by considering the simultaneous occurrence (to a low extent) of
dissociative adsorption leading to NH
2
−
and OH
−
surface species. Dissociative

adsorption of ammonia is related to the presence of very strong Lewis - type acid
sites.
Reviews by Gorte and coworkers [35, 36] deal with the adsorption complexes
formed by strong and weak bases with acid sites in zeolites. They examine the
adsorption enthalpies of a series of strongly basic molecules such as alkylamines,
pyridines and imines. These workers also performed studies of the adsorption
properties of weak bases, including water, alcohols, thiols, olefi ns, aldehydes,
ketones and nitriles. They report a poor correlation between the differential heats
of adsorption on H - MFI zeolites and the enthalpies of protonation in aqueous
solutions, but a much better correlation with gas - phase proton affi nities [37] .
Acetonitrile is also an interesting molecule for probing acid sites in catalysts.
It is a weak base, so no protons are abstracted and actual hydroxyl groups can
be observed. It also allows the investigation of both Lewis and Br ø nsted acidities
[15] .
NO and CO can also be employed as probes to identify Lewis acid sites and
characterize their density and strength [38] .
9.2.3.3 Probing Redox Properties
The heats of reduction of oxide samples can be determined by studying the adsorp-
tion of hydrogen, CO and various hydrocarbons on the fully oxidized catalysts. The
extent of reduction of the catalyst surface can be evaluated in particular using H
2
.
The measurement of hydrocarbon (e.g. propene, propane, acrolein, etc.) adsorp-
tion heats is complicated by the subsequent reaction of the adsorbed species or by
incomplete desorption of the products.
In the case of CO reduction, the catalyst – oxygen bond energy has to be calculated
by subtracting the heat of formation of CO
2
.
However, it is known that, in the absence of processes other than plain surface
coordination, CO acts as a weak Lewis base and can interact with the strongest
surface Lewis acid sites. NO can also be employed either as a probe to identify
Lewis acid sites (as mentioned in Section 9.2.3.2 ) or as a reducing agent. However,
NO may disproportionate into N
2
O and oxygen and it is also very likely to form
nitrosyl complexes in the presence of transition metal ions.
The heats of oxidation of the reduced oxides can be further measured using O
2
adsorption. Large variations of the re - oxidation heat can be sometimes observed
when any further oxidation is limited by the diffusion of oxygen into the reduced
portion of the particle.
9.3
Surface Properties of Oxides
On one hand, both acidity and basicity of catalysts are known to be important
factors for partial oxidation reactions. Moreover, strong acidity can reduce the
selectivity by carbon – carbon bond breaking and by promoting the production of
9.3 Surface Properties of Oxides 403

404 9 Thermal Analysis and Calorimetric Methods
CO
2
. Acid sites are cations that exhibit either a high oxidation state or an unsatu-
rated coordination. On the other hand, redox properties are also known to play an
important role and to be related to M
n +
→ M
( n − 1)+
equilibrium constant and to lattice
O
2 −
ion lability.
Since various supported and unsupported metal oxides are oxidation catalysts
for hydrocarbons, there has been interest in determining the relationship between
the reduction/re - oxidation properties of the oxides and their catalytic properties.
A common oxidation mechanism involves a redox cycle in which lattice oxygen is
removed in the reaction and then replenished by oxygen from the gas phase. Thus
there are studies to determine the energetics of removal of oxygen from the cata-
lysts and replenishment by various routes. These energetics have been determined
as a function of catalyst formulation or modifi cation, and as a function of the oxi-
dation state of the catalyst [4] .
In order to try to clarify the different types of mechanism involving either redox
cycles and/or acid – base properties, a study of the surface chemistry of single,
doped and mixed oxides is of much interest. The calorimetric technique, by allow-
ing heat transfer measurements, can provide very informative data on the ther-
modynamics of solid – gas interactions and for the study of the surface and reactivity
of these metal oxides.
9.3.1
Bulk Oxide Catalysts
The surface acid – base properties of bulk oxides can be conveniently investigated
by studying the adsorption of suitably chosen basic – acidic probe molecules on
the solid. Acidic and basic sites are often present simultaneously on solid surfaces.
The two centers may work independently or in a concerted way, and the occur-
rence of bifunctional reaction pathways requiring a cooperative action of acidic
and basic centers has also received considerable attention [39] . The acid – base
properties of numerous amorphous metal oxides investigated by microcalorime-
try have been summarized in an extensive review by Cardona - Martinez and
Dumesic [11] .
The infl uence of the pre - treatment temperature on the acidic properties is a very
important factor. For Br ø nsted sites, the differential heat is the difference between
the enthalpy of dissociation of the acidic hydroxyl and the enthalpy of protonation
of the probe molecule. For Lewis sites, the differential heat of adsorption repre-
sents the energy associated with the transfer of electron density towards an elec-
tron - defi cient, coordinatively unsaturated site, and probably an energy term related
to the relaxation of the strained surface [40] .
Increasing the pre - treatment temperature modifi es the surface acidity of the
solids. For γ - alumina, there are numerous surface models, and various acid sites
having different strengths are formed on the surface during dehydration. The
infl uence of the pre - treatment temperature, between 573 and 1073 K, on the
surface acidity of a transition alumina has been studied by ammonia adsorption
microcalorimetry. The number and strength of the strong sites, which should be
mainly Lewis sites, have been found to increase when the temperature increases.
Moreover the pre - treatment temperature affects the whole spectrum of adsorption
heats at various coverages, and not only the initial heat [13] .
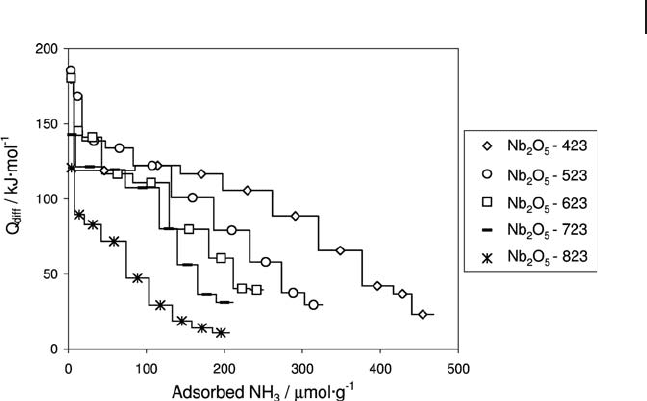
Niobium oxide surfaces are also very dependent on the dehydration tempera-
ture. An illustration is given in Figure 9.5 , which represents the differential heats
of NH
3
adsorption versus ammonia coverage for a niobium oxide from CBMM
pre - treated at 423, 523, 623, 723 and 823 K [41] .
As can be seen, a regular decrease in the adsorption heats occurs when the
evacuation temperature increases from 423 to 823 K. The number of strong sites
is less affected by dehydroxylation of the surface than that of weak sites, and tends
to a limit.
Figure 9.6 represents the differential heats of NH
3
and SO
2
adsorption as a
function of coverage for silica, magnesia and γ - alumina samples [15] .
Silica presents low heats of adsorption for both basic and acidic molecules,
indicating that the surface sites on silica are either weakly acidic or weakly basic.
The adsorption is mainly due to hydrogen bonding and van der Waals interaction.
The differential heats of adsorption of ammonia on silica show a decrease as the
adsorption temperature increases [12] . The differential heats of adsorption of
ammonia and sulfur dioxide on the surface of alumina show the presence of
strong acid and strong basic sites, respectively. The heats of adsorption for alumina
are typical of a strong acidic surface, but also indicate the amphotericity of the
surface. The initial heat increases and the adsorption capacity decreases with
increasing pre - treatment temperature [13] . By contrast, magnesia displays only
basic sites adsorbing SO
2
molecules preferentially, and very few acidic sites.
The differential heats of adsorption of NH
3
and CO
2
over 18 bulk oxides
have been determined [42, 43] . These oxides were classifi ed, according to their
9.3 Surface Properties of Oxides 405
Figure 9.5 Variation with the activation temperature of the
differential heats of adsorption versus ammonia coverage.
