Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.


416 9 Thermal Analysis and Calorimetric Methods
coworkers using calorimetry [39] . Such systems have been studied in an attempt
to enhance the redox properties of ceria by incorporating foreign cations in its
lattice. Besides the redox properties, the acid – base features could be expected to
change as well, since it is well known that the acid – base properties of a metal oxide
can be signifi cantly modifi ed by chemical mixing with another oxide.
It has been shown that the total concentration of the acid sites is the lowest for
pure ceria, and markedly increases upon addition of 20 mol% of zirconia, then
decreases as the ZrO
2
content is further increased up to 80 mol% and fi nally attains
the highest value on pure zirconia. The heat evolved upon NH
3
adsorption increases
with increasing ZrO
2
content in the mixed oxides, while the basic properties are
attenuated as the zirconia content is increased up to 80 mol% and then grow again
for pure zirconia. Hence the inclusion of increasingly high contents of zirconium
into the ceria lattice has a complex infl uence on both the acidity and basicity of
the pure parent oxide.
The co - precipitated ceria – lanthana samples were found to present initial Q
diff
values for ammonia adsorption ranging from 105 to 130 kJ mol
− 1
, the highest value
corresponding to CeO
2
and the lowest one to the La
2
O
3
sample. For all the samples,
an increase in the ammonia uptake is accompanied by a rapid decrease on the
differential heat to a low value ( ≈ 35 kJ mol
− 1
). Concerning basicity, the heterogene-
ity revealed by the Q
diff
versus uptake profi les cannot be ascribed to sites of differ-
ent chemical nature (virtually the only basic sites on the ceria surface are
coordinatively unsaturated O
2 −
ions). The formation of bicarbonates either very
strongly or very weakly held could explain the occurrence of some very high and
very low CO
2
adsorption heats.
One - step partial oxidation of propane to acrylic acid (an essential chemical
widely used for the production of esters, polyesters, amides, anilides, etc.) has been
investigated so far on three types of catalysts, namely, vanadium phosphorus
oxides, heteropolycompounds and, more successfully, on mixed metal oxides. The
active catalysts generally consist of Mo and V elements, which are also found in
catalysts used for the oxidation of propene to acrolein and that of acrolein to acrylic
acid.
Mo – V – Te and Mo – V – Te – Nb mixed - metal oxide catalysts have been character-
ized by means of C
3
H
8
- TPR and NH
3
adsorption calorimetry. All samples were
strongly heterogeneous, with initial adsorption heats of ≈ 100 – 80 kJ mol
− 1
for the
Mo – V – Te samples. Introducing an Nb component into the catalysts slightly
decreased the initial adsorption heats to ≈ 60 kJ mol
− 1
but drastically increased the
surface density of weak acid sites ( < 30 kJ mol
− 1
) [83] .
The oxidative dehydrogenation ( ODH ) of lower alkanes is an attractive process
for the formation of alkenes. The ODH of propane to produce propene has been
particularly studied, given its high demand for the production of polypropene,
acrylonitrile and propene oxide. There is a combined infl uence of the redox and
acid – base properties of the surface of the oxides used for propane ODH. Interme-
diate reducibility, weak Lewis acid centers and oxygen mobility represent the
essential requirements for selective ODH, as they are consistent with the trends
in ODH rates observed in VO
x
, MoO
x
and WO
x
based catalysts.

DSC has been used to determine the heat effects associated with propene
adsorption [84] on Mo/Si : Ti catalysts with different K : Mo molar ratios. The
heats associated with reversible −×
()
−−
3 193 10
42
.mJm
sample
and irreversible
−×
()
−−
0 126 10
42
.mJm
sample
adsorption of propene were found to decrease with the
addition of potassium.
Thermal analysis studies provide insight into the stability of the catalyst and its
chemical evolution. DSC studies can be used to determine not only the tempera-
ture of the transformation if any, but also the corresponding heat evolved. For
example, El Jamal and coworkers [85] studied the stability of the β - phase of
bismuth molybdate (Bi
2
Mo
2
O
9
) by DSC in order to check whether this phase is
stable at the high temperatures required for propene oxidation. It was shown that
the β - phase is metastable at room temperature but stable enough to be used in
catalytic tests. It was also confi rmed that the β - phase is different from an equi-
molar mixture of the α - and γ - phases of bismuth molybdate (Bi
2
Mo
3
O
12
and
Bi
2
MoO
6
respectively). The enthalpy of transformation of the α + γ mixture to β at
833 K was found to be 6330 J mol
− 1
. The endothermic nature of the observed peak
confi rms the evolution of the system to a more organized one, according to the
reaction
Bi Mo O Bi MoO Bi Mo O
2312 2 6 229
2+→
Numerous examples of applications of DSC to the study of the stability of binary,
ternary and quaternary metal oxides can be found in the literature, and we will
not focus on this type of systematic characterization. Fewer studies have been
performed to determine the acid – base character of mixed oxides using adsorption
calorimetry of probe molecules.
Depending on the preparation method, on the SiO
2
/Al
2
O
3
ratio and on the
micro - or mesoporous structure, very different acidic properties have been observed
for silica – alumina mixtures. Some samples contain both Br ø nsted and Lewis sites,
while for others only acidity of the Lewis type was observed [86, 87] .
Thermogravimetric analysis ( TGA ) has been used to collect thermodesorption
curves of 2 - phenylethylamine ( PEA ) from acid surfaces of mixed oxides prepared
by the sol – gel method, with the aim of determining the amount and distribution
of the acid sites of the samples [23] . Thermodesorption curves from silica – alumina,
silica – zirconia and silica – titania samples ( ≈ 87 wt% SiO
2
) were collected at different
heating rates (5 ≤ β (K min
− 1
) ≤ 30) in an inert atmosphere. The activation energies
of PEA desorption from the acid sites were calculated from the dependence upon
the heating rate β of the displacements of the observed desorption peaks ( T
max
) as
determined from the derivative of the TGA profi les. The numbers of acid sites, as
well as the activation energies, determined by this method were in the order
SiO
2
– TiO
2
< SiO
2
– ZrO
2
< SiO
2
– Al
2
O
3
.
However, this order is strongly dependent on the preparation method and
respective amounts of the two oxides. This fact is illustrated in Figure 9.11 , which
shows strengths and numbers of acid sites varying in a different order as deter-
mined by ammonia adsorption calorimetry for sol – gel - prepared silica – alumina,
9.3 Surface Properties of Oxides 417
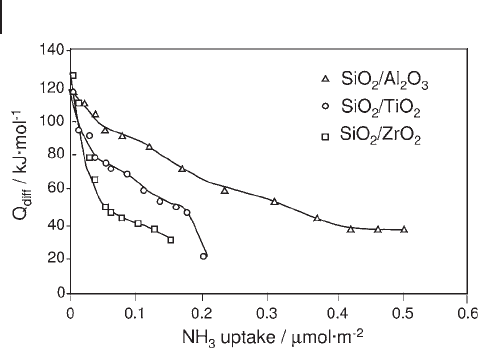
418 9 Thermal Analysis and Calorimetric Methods
silica – titania and silica – zirconia with SiO
2
/Al
2
O
3
, SiO
2
/TiO
2
and SiO
2
/ZrO
2
ratios
of 31, 26 and 26 respectively.
Binary and ternary systems containing copper, zinc oxide and aluminum are
widely used for industrial methanol synthesis. The adsorption of CO on a fully
reduced and clean Cu surface is fully reversible at room temperature, with heats
of adsorption ranging between 70 kJ mol
− 1
at low coverage and 45 kJ mol
− 1
at high
coverage [88] .
Silica – alumina has been used as support for dispersing CuO, Ga
2
O
3
and SnO
2
oxide phases, and the corresponding CuO – Ga
2
O
3
and CuO – SnO
2
binary systems
[89] have been used in NO
x
reduction or methane combustion reactions. The
uptakes and interaction energies of CO adsorbed on the different samples were
determined by adsorption calorimetry at 303 K (Figure 9.12 ). As complementarily
shown by IR CO adsorption measurements, only the copper phase was able to
adsorb CO, so the scale of CO uptake was related to the amounts of dispersed
copper species (Cu
+
and Cu
δ +
) available at the surface. The rather high values of
the initial heats (around 105 – 115 kJ mol
− 1
) confi rmed the abundant presence of
Cu
+
species, strongly interacting with the support.
Calorimetric and spectroscopic studies of NH
3
adsorption have been performed
on the same samples to study the surface acidities of the bare silica – alumina
support and of the CuO – Ga
2
O
3
/SiO
2
– Al
2
O
3
and CuO – SnO
2
/SiO
2
– Al
2
O
3
catalysts
(Figure 9.13 ) from the qualitative (nature of acid sites) and quantitative (number,
strength and strength distribution of acid sites) points of view [90] . The CuO
species increased the capacity to adsorb NH
3
(in particular on medium and weak
acid sites, 100 kJ mol
− 1
< Q
diff
< 150 kJ mol
− 1
), while the strongest fraction of acid
sites ( Q
diff
> 150 kJ mol
− 1
) was enhanced by the addition of Ga
2
O
3
and SnO
2
. In
these papers [89, 90] the authors demonstrated, by means of microcalorimetry
measurements, that it is possible to modulate the acid strength of metal oxide
surfaces by suitable addition of a second oxide component.
Figure 9.11 Differential heats of ammonia adsorption versus
coverage for various mixed oxides.
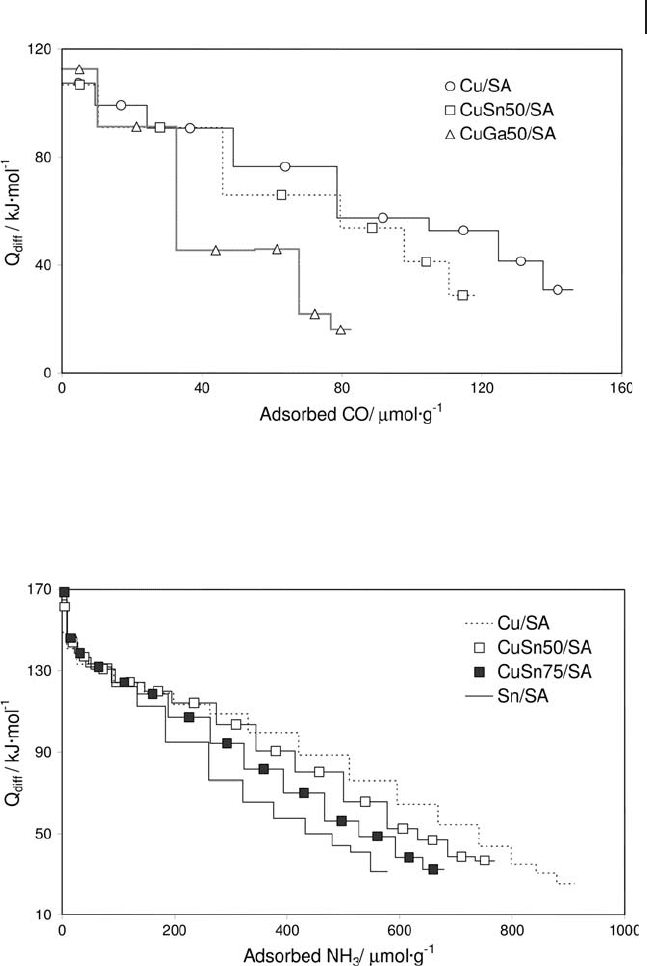
Figure 9.12 Differential heats of carbon monoxide adsorption versus coverage.
Figure 9.13 Differential heats of ammonia adsorption versus coverage.
9.3 Surface Properties of Oxides 419

420 9 Thermal Analysis and Calorimetric Methods
Ceria - based catalysts are intensively used because of their high chemical and
physical stability, high oxygen mobility and high oxygen vacancy concentrations,
which are characteristic of fl uorite - type oxides. The possibility of cycling easily
between reduced and oxidized states (Ce
3+
↔ C e
4+
) permits the reversible addition
and removal of O
2
from CeO
2
. All these features explain the applicability of these
materials as catalysts or catalytic supports in many oxidation reactions or as three -
way catalysts.
The combination of ceria with another oxide from a group III metal (boria,
alumina, gallia and india) using either a co - precipitation method or a sol – gel route
has led to mixed oxide samples with specifi c acid – base and redox features, as evi-
denced by thermal techniques [91] .
The calorimetric study of the adsorption of NH
3
and SO
2
has shown that, among
all the investigated co - precipitated samples, only those containing boria had sig-
nifi cantly enhanced acidity, whereas the basicity has been found to depend on the
nature and amount of group III metal. The amphoteric character of ceria, already
known from the literature, has been confi rmed. The Me
2
O
3
– CeO
2
mixed oxides
have also shown amphoteric behavior, in a manner dependent on the character of
the group III metal. Among the investigated systems, Al
2
O
3
– CeO
2
and In
2
O
3
– CeO
2
express somewhat more pronounced basic than acidic characters. Importantly,
B
2
O
3
– CeO
2
samples present an acidic character that becomes more evident with
an increase in the boron content.
In the case of sol – gel solids [92] , even if the boria – ceria sample had a higher
amount of boria compared to co - precipitated samples, it expressed only insignifi -
cant acidity. The sol – gel method produces an unfavorable distribution of the guest
oxide particles in the mixed oxide, and the acid – base features of the investigated
mixed oxides are quite different from those of the samples obtained by
co - precipitation.
Ammonia adsorption microcalorimetry experiments have been conducted on
various acidic tungsten/zirconia catalysts prepared by different techniques [93] .
The results show that the co - precipitation method produced a tungsten/zirconia
catalyst with a greater number of acidic sites than impregnation of tungsten on
hydrous zirconia, and that the addition of small amounts of iron to the tungsten/
zirconia catalyst increased the acid site strength. The acid site strength of the
tungsten/zirconia materials was similar to or slightly higher than that found in
zeolites or sulfated zirconia, with initial heats ranging from 120 to 170 kJ mol
− 1
depending on the samples.
9.3.5
Hydrotalcites
Hydrotalcites are layered double hydroxides with general formula
MMOH A H
O
1
23
2
2−
++
+
−
()
()()
⋅
xx
x
xm
m
n
, where the divalent ion may be Mg
2+
, Ca
2+
, Zn
2+
or
Ni
2+
, the trivalent ion, Al
3+
, Fe
3+
, Cr
3+
, and so on, and the charge compensating
anions, OH
−
, Cl
−
, NO
3
−
, CO
3
2 −
or SO
4
2 −
. The value of x can be between 0.25 and 0.33.

Thermal treatments induce dehydration, dehydroxylation and loss of the charge -
compensating anions, resulting in mixed oxides with the MgO - type structure.
Hydrotalcites are consequently a class of precursors useful for the preparation of
catalytically active oxides showing basic properties [94] . The acid – base properties
of Mg – Al mixed oxides are governed by the Mg : Al molar ratio, calcination tem-
perature and preparation conditions. The study of the infl uence of the acid – base
properties and chemical composition on the catalytic performance of calcined
hydrotalcites is thus of interest.
Layered double hydroxides with the hydrotalcite structure were synthesized with
varying Mg : Al atomic ratios and with different contents of exchangeable Cl
−
and
CO
3
2 −
anions. The CO
2
adsorption isotherms showed an increase of the uptake and
consequently of the basicity with initial CO
3
2 −
content and calcination temperature
up to 800 K. Increasing the Mg : Al ratio of the hydrotalcites from 2.33 to 3 resulted
in an increase of the total number of basic sites [94] .
In another study, Mg – Al hydrotalcite catalysts with different Mg : Al molar ratios
(0.6, 1.4, 2.2, 3.0) were characterized by microcalorimetry using CO
2
in the gas
phase and benzoic acid in toluene [95] . The calcined Al - rich sample (Mg : Al molar
ratio of 0.6) possesses Lewis acid sites similar in strength to those found on Al
2
O
3
,
but stronger than those found on the Mg - rich hydrotalcites. The liquid - phase
basicity microcalorimetry measurements with benzoic acid in toluene correlated
very well with the catalytic activity for Michael additions.
The strength and accessibility of the basic sites of hydrotalcites with an
Mg : Al ratio of 2, prepared via co - precipitation of the respective nitrates using
carbonate or oxalate as the compensating anions, were assessed by calorimetry of
CO
2
adsorption. Two different methods were used to activate the Mg – Al hydro-
talcites and impart Br ø nsted basicity. The initial enthalpies of CO
2
adsorption at
303 K on the activated hydrotalcites presented very similar values of ≈ 108 kJ mol
− 1
[96] .
The acid – base properties of CuMgAl and NiCuMgAl mixed oxides with hydro-
talcite - like structures containing different proportions of Ni
2+
, Cu
2+
, Mg
2+
and Al
3+
cations have been investigated using adsorption microcalorimetry and XPS with
SO
2
(for basicity) and NH
3
(for acidity) as probe molecules [97, 98] . For the
CuMgAl mixed oxides, the adsorption data indicated an optimum of the concen-
tration and strength of acid sites for the material with Cu : Mg : Al = 1 : 1 : 1, and
suggested that the basicity (number and strength) is the most important for
Mg - rich samples.
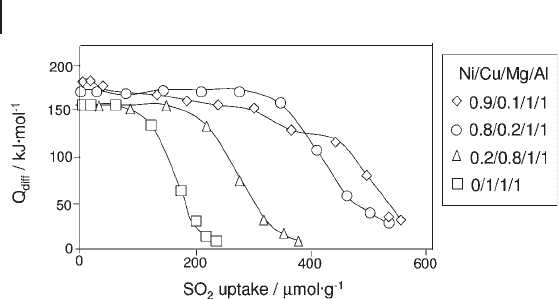
The basicity of the NiCuMgAl materials depends principally on the Ni/Cu
ratio and increases with the proportion of Ni. The basic sites are strong and par-
ticularly homogeneous (plateau around 150 – 170 kJ mol
− 1
depending on the
samples), and they are of both Br ø nsted and Lewis types (the former being pre-
dominant) as confi rmed by XPS. Meanwhile, the acidity (of Lewis type only) is
rather weak, heterogeneous and dependent on the Ni : Cu ratio (as is basicity). The
observed heterogeneity of these acidic sites can be related to the heterogeneity of
the interaction between the nickel atoms and the other elements. The acidity
9.3 Surface Properties of Oxides 421
422 9 Thermal Analysis and Calorimetric Methods
and the basicity both increase with the Ni : Cu ratio, as shown in Figure 9.14 ,
whereas the basicity decreases with increasing Mg : Al ratio [98, 99] .
The decarbonation of carbonated layered double hydroxides containing Mg with
either Al, Fe or Cr trivalent cations or Al with Mg, Ni, Cu or Zn divalent cations
has been studied by thermal analysis. The enthalpy of adsorption of CO
2
on the
resulting calcined mixed oxides was measured by calorimetry, with initial heats of
adsorption close to those reported for MgO (about 100 kJ mol
− 1
) and a relatively
homogeneous strength distribution [100] .
9.3.6
Bulk and Supported Heteropolyacids
Keggin - type heteropoly compounds have attractive and important characteristics
in terms of catalysis. They consist of heteropolyanions and counter - cations such
as H
+
, Cs
+
or NH
4
+
. When the counter - cations are protons, they are called hetero-
polyacid s ( HPA ). An important characteristic of HPAs, such as 12 - tungstophos-
phoric acid (H
3
PW
12
O
40
), is the presence of very strong Br ø nsted acid sites. But
the characteristics of HPAs strongly depend on temperature and relative humidity.
When they are used in heterogeneous catalysis, it is often necessary to support
them on high - surface - area oxides or activated carbons, in order to increase the
surface contact with the reactants.
These materials are used industrially mainly in the reaction of hydration of
alkanes (acid catalysis) and in the synthesis of methacrolein or isobutyric acid
(redox catalysis). Because of the considerable number of available polyanion struc-
tures, it is possible to vary their acidic and redox properties according to the needs
of a specifi c application.
The number and strength of the acid centers of tungstic heteropolyacids have
been determined by ammonia adsorption calorimetry. Ammonia is irreversibly
absorbed, with the formation of the corresponding ammonium salts. An increase
in the number of protons in Keggin heteropolyanions decreases the acidic strength.
Figure 9.14 Differential heats of sulfur dioxide adsorption
versus coverage on NiCuMgAl mixed oxides.
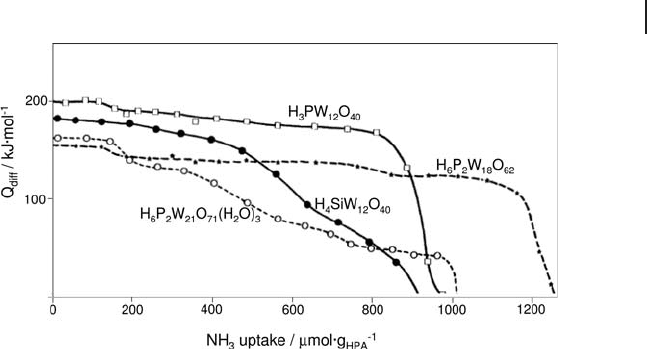
The acidity varies between compounds, but always presents a plateau of sites of
the same strength. The initial heats vary in the order H
3
PW
12
O
40
> H
4
SiW
12
O
40
>
H
6
P
2
W
21
O
71
> H
6
P
2
W
18
O
62
. Diffusion phenomena can be important for some of
the compounds, such as H
6
P
2
W
21
O
71
, depending on the structure [101] , as can be
observed in Figure 9.15 .
It is worth noting that differences in acid strength between anhydrous Keggin
heteropolyacids (H
3
PW
12
O
40
> H
4
SiW
12
O
40
> H
3
PMo
12
O
40
> H
4
SiMo
12
O
40
) mea-
sured by ammonia adsorption calorimetry did not correlate simply with the cata-
lytic activity in the reaction of rapeseed oil transesterifi cation with methanol and
ethanol [102] .
A study of the acidity of carbon - supported and unsupported heteropolyacid cata-
lysts by ammonia sorption calorimetry has been performed at 423 K [103] . The
acidity of the supported Keggin - type heteropolyacids is slightly reduced compared
to that of the corresponding bulk samples, while that of H
21
B
3
W
39
O
132
is enhanced
when supported on carbon. Heterogeneity in acid strength is greatly enhanced
when the HPAs are grafted on the carbon support.
The acidic properties of the alkaline earth salts of 12 - tungstophosphoric acid
have been investigated using ammonia adsorption microcalorimetry [104] . As a
result of the substitution of two protons with one alkaline earth cation, decreases
were observed in both the total number of acid sites and the number of the stron-
gest acid sites (characterized by differential heats higher than 150 kJ mol
− 1
), com-
pared to the values found for 12 - tungstophosphoric acid. In the case of Sr salts,
some steric effects were observed. The acidity of the investigated systems, even if
strong, is uniform only in the case of the parent tungstophosphoric acid. On the
contrary, energetic heterogeneity is evident for the other investigated solids, both
from differential heat versus ammonia uptake profi les and from the results of TPD
of ammonia performed in a TG - DSC - MS experiment.
Figure 9.15 Differential heat of ammonia adsorption versus
ammonia uptake for various HPA samples.
9.3 Surface Properties of Oxides 423

424 9 Thermal Analysis and Calorimetric Methods
Changes in the acidic properties of Cs
2.5
H
0.5
PW
12
O
40
upon heat treatment in
water have been investigated by Nakato and coworkers [105] . Ammonia adsorption
calorimetry showed that the water treatment reduced the acid site number, whereas
the acid strength was little changed, indicating that the decrease observed in cata-
lytic activity was due to that in the number of acid sites. The plateau of heat of
adsorption observed was only slightly affected by the water treatment: 165 kJ mol
− 1
before water treatment and 160 kJ mol
− 1
after treatment.
The acidity of a series of Cs
x
H
3 − x
PW
12
O
40
samples has been studied by NH
3
fl ow
calorimetry linked to mass spectrometry [18] . The incorporation of Cs lowers the
number of titratable acid sites, in quantitative agreement with the theoretical
degree of H
+
exchange. The average heat of NH
3
adsorption falls only slightly with
Cs doping up to x = 2.3, suggesting that the residual acid strength of the Cs - doped
HPW materials is not strongly perturbed during proton exchange. In contrast,
however, the acidity of the heavily substituted ( x > 2.4) samples is signifi cantly
lower.
Ru – HPA metal – acid bifunctional catalysts on various supports (silica, graphite,
KL zeolite) have also been characterized by NH
3
adsorption calorimetry, which
revealed heterogeneous acid site strength distributions varying in the order HPA -
SiO
2
> HPA - graphite > HPA - KL [106] .
9.3.7
Pillared Clays and Layered Silicates
Pillared clay s ( PILC ) have been reported to show catalytic properties comparable
to zeolites in several reactions, and therefore to have comparable acidities [107] .
The preparation and properties of smectites pillared with Al hydroxy oligomers
have been extensively investigated. Most of the work has been performed on
montmorillonite and hectorite, which have been intercalated with a great variety
of pillaring agents and oligocations.
Intercalation of hydroxy silicoaluminum compounds increases the thermal sta-
bility and the acidity of the pillared clays.
The distributions of acid strengths of various pillared clays obtained by calorim-
etry of NH
3
adsorption showed that Al - PILC displays high acidity, with a small
number of sites as strong as those measured on zeolites. Acidity should be directly
related to the environment of the Al ions in the pillars, which could be modifi ed
by the preparation. The adsorption of NH
3
on pillared beidellite showed the pres-
ence of many strong acid sites (160 kJ mol
− 1
) [108] .
A natural clay has been pillared with mixed solutions containing both Al and
Fe, Ti or Cr. The intercalation - generated solids ’ distribution of acid strengths
measured by calorimetric adsorption of ammonia is comparable to that of zeolites.
The surfaces appear as heterogeneous and show initial adsorption heats close to
150 – 160 kJ mol
− 1
if one excludes the fi rst point of the differential heat versus cover-
age curves, which is much higher ( ≈ 190 kJ mol
− 1
) [109] .
The acidity of mesoporous expanded clay catalysts has been studied using
ammonia adsorption microcalorimetry [110] . The inclusion of SiO
2
– TiO
2
clusters

drastically increased the total (Br ø nsted + Lewis) acidity of the clay, in terms of
both the strength and the density of acid sites.
The acidity of pillared clays has been characterized by both microcalorimetric
measurements of the adsorption of aromatic molecules and pyridine and the cata-
lytic ethylbenzene test reaction [111] . The aromatic probe molecules used were a
reactant and a product of the catalytic reaction: ethylbenzene and m - diethylben-
zene, respectively. In this way, only the strongest of the accessible acid sites were
titrated. The heats of adsorption of these molecules indicate that a zirconium oxide
pillared clay had stronger acidity than an aluminum oxide pillared clay, whereas
the pyridine results were equal for both samples.
9.3.8
Zeolites
Aluminosilicates can be divided into two categories, amorphous silica – aluminas
(described in Section 9.3.4 ) and crystalline zeolites or molecular sieves.
In crystalline aluminosilicates, all aluminum and silicon atoms form tetrahedra
which are linked by shared oxygen atoms. These tetrahedra join to form secondary
building units, which can be interconnected to give numerous distinctive zeolite
structures. Each has a regular and well defi ned pore structure together with inner
cavities. The precise control of pore size is one of the greatest distinctions between
zeolites and amorphous silica – aluminas. The morphology, free volume, pore
geometry and electric fi eld gradients determine their acidity and selectivity.
Numerous reviews dealing with the adsorption capacities and acid – base proper-
ties of zeolites have been published [4, 8, 10 – 14, 17] so we will not give a detailed
description of these systems. Only some case studies will be given in order to
assess the possibilities of thermal techniques for characterizing such materials. In
general the total number of acid sites is greater in zeolites than in amorphous
silica – aluminas for a similar Si : Al ratio.
The acidity of high - silica zeolites produced either by direct synthesis or by
chemical dealumination of parent zeolites (either by steaming and acid leaching
or by SiCl
4
vapor treatment), which makes it possible in principle to extract the
aluminum from the network without the structure collapsing, was extensively
studied in the 1990s.
Together with ZSM - 5 zeolites, dealuminated HY (ultra - stable Y or USY) zeolites
are the most widely used in petrochemistry (fl uid catalytic cracking processes)
[112] . The active centers are Br ø nsted acid sites carried by the zeolite framework.
USY is derived from synthetic Y faujasites dealuminated by chemical or, more
commonly, hydrothermal treatments. These treatments partially remove alumi-
num atoms from the crystal framework, improving the thermal stability, as well
as decreasing the number of Br ø nsted sites, which is a function of the concentra-
tion of Al atoms, but generating extra - framework aluminum ( EFAL ) species and
framework defects, which are generally associated with Lewis acidity. The Lewis
sites may have their own catalytic activity and also may interact with Br ø nsted
sites, increasing their strength [62] .
9.3 Surface Properties of Oxides 425
