Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

406 9 Thermal Analysis and Calorimetric Methods
properties, as acidic oxides: Cr
2
O
3
, WO
3
, Nb
2
O
5
, V
2
O
5
and MoO
3
; amphoteric
oxides: BeO, TiO
2
, Al
2
O
3
, ZrO
2
and ZnO; and basic oxides: ThO
2
, Nd
2
O
3
, MgO,
CaO and La
2
O
3
. It was found that many oxides in the amphoteric group adsorbed
more NH
3
and with higher heats ( > 200 kJ mol
− 1
) than some of those in the acidic
group. Also the ZrO
2
sample adsorbed NH
3
with a heat of 150 kJ mol
− 1
, comparable
to Nb
2
O
5
and WO
3
. The group of basic oxides adsorbed NH
3
very weakly
( < 20 kJ mol
− 1
) and essentially by physisorption. The oxides of La, Nd and Th
adsorbed CO
2
in larger abundance and with higher heats, for instance 200 kJ mol
− 1
for Nd
2
O
3
, than other oxides. It is worth noting that, on the alkaline earth oxides,
the heats of adsorption of CO
2
were only 120 – 160 kJ mol
− 1
, perhaps because of
bidentate adsorption of CO
2
. Some of the oxides of the amphoteric group such
as TiO
2
, ZrO
2
and Al
2
O
3
also adsorbed CO
2
with heats higher than 100 kJ mol
− 1
,
but with an amount adsorbed smaller than on the basic oxides. Except for the
adsorption of NH
3
on Cr
2
O
3
and ZnO, and CO
2
on the alkaline earth oxides, the
other data all indicate heterogeneous site distributions. A fair correlation was
found between the average adsorption heats and the percentage of ionic character
of the oxides.
The surface acid – base properties of polycrystalline MgO surfaces have been
assessed by means of thermogravimetry and DSC of desorption of pyridine and
CO
2
in the room temperature to 400 ° C temperature range [44] . The endotherms
and corresponding ∆ H of desorption were discussed in relation with results deter-
mined previously using differential adsorption calorimetry and taking into account
the structure, surface area and defects of the studied surfaces.
Differential and integral heats of chemisorption of H
2
O on nanocrystalline α -
Al
2
O
3
and γ - Al
2
O
3
as a function of hydroxyl coverage have been reported by McHale
and coworkers [45] . A greater number of high - energy sites was evidenced on α -
Al
2
O
3
per unit surface area.
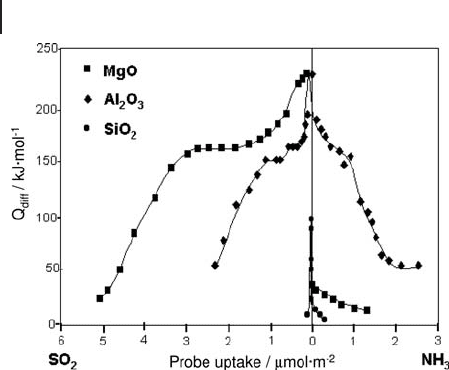
Figure 9.6 Differential heats of adsorption of ammonia and
sulfur dioxide on SiO
2
, γ - Al
2
O
3
and MgO.

Another study examined the NH
3
and CO
2
adsorption heats on several zirconia
catalysts, differing in their preparation procedure and/or in the addition of dopants
[46] . The differential heats of NH
3
and CO
2
adsorption show a wide range of vari-
ability, displaying either a plateau of constant heat or a continuous decrease indica-
tive of adsorption heterogeneity [12] . The ratio between the number of the basic
and acidic sites, n
B
/ n
A
, was calculated for each catalyst from the microcalorimetry
results, by dividing the amount of adsorbed CO
2
by the amount of adsorbed NH
3
.
These catalysts were used to produce alk - 1 - ene from 4 - methylpentan - 2 - ol. Alk - 1 -
ene selectivity was found to fi rst increase with the n
B
/ n
A
ratio, reach a maximum
and then decrease, whereas ketone formation continuously increased, being neg-
ligible for low n
B
/ n
A
values.
Calorimetric measurements of adsorption of CO
2
at 303 K on different titania
samples have provided evidence of their surface heterogeneity, as expected for
oxides, with heats of adsorption ranging from ∼ 100 to 30 kJ mol
− 1
. Acidity measure-
ments by ammonia adsorption microcalorimetry on the same samples gave rise
to adsorption heats ranging between 150 and 60 kJ mol
− 1
[47] .
Information on the acid – base properties of lanthanum and cerium oxides
has been obtained by adsorption microcalorimetry of ammonia and carbon dioxide
at 353 K. The initial heats of NH
3
adsorption ranged from 125 to 95 kJ mol
− 1
and
the differential heats smoothly decreased with increasing coverage. The results
for CO
2
adsorption showed high initial heats (180 – 225 kJ mol
− 1
, depending on
the samples). A plateau can be seen around 145 – 160 kJ mol
− 1
, followed by a con-
tinuous decrease of the differential heat. The interaction of CO
2
is more pro-
nounced for La
2
O
3
than for CeO
2
, in terms of both number and strength of the
adsorbing sites [32] .
The acidity and basicity of bulk Ga
2
O
3
and SnO
2
were determined by microcalo-
rimetry of NH
3
and SO
2
adsorptions performed at 353 K. Both are amphoteric,
with initial heats of NH
3
adsorption of 110 and 180 kJ mol
− 1
and initial heats of
SO
2
adsorption of 195 and 180 kJ mol
− 1
for Ga
2
O
3
and SO
2
respectively [48] . The
Lewis acidity of a phase - pure γ - Ga
2
O
3
was also studied by the adsorption of CO at
ambient temperature. The Q
diff
values at low coverage are in the range 40 – 45 kJ mol
− 1
and rather heterogeneous, and are followed by a weak and reversible adsorption
process in the 25 – 30 kJ mol
− 1
range [49] .
The interactions of ammonia, methanol, water and dimethyl ether with amor-
phous Nb
2
O
5
and NbOPO
4
samples have been investigated by means of adsorption
microcalorimetry in order to determine the number and strength of the active sites
for dimethyl ether ( DME ) synthesis by dehydration of methanol [50] . NbOPO
4
is
more acidic than Nb
2
O
5
, and both present Br ø nsted and Lewis sites on their
surface. They both strongly chemisorb a small amount of water, while most of the
adsorbed water corresponds to reversible physical adsorption. The results of micro-
calorimetry experiments associated with IR spectroscopy suggest that methanol
was, for the most part, strongly dissociatively adsorbed on Nb
2
O
5
and NbOPO
4
to
form methoxy species, and that DME was mainly molecularly chemically adsorbed.
The four probe molecules used in this work were adsorbed more strongly on
NbOPO
4
than on Nb
2
O
5
because of the stronger acidity of NbOPO
4
.
9.3 Surface Properties of Oxides 407

408 9 Thermal Analysis and Calorimetric Methods
9.3.2
Doped and Modifi ed Oxides
Doped metal oxide catalysts are widely used in various catalytic processes. In many
cases, the catalytic activity and selectivity of these catalysts may be related to their
acidity or basicity.
Microcalorimetry of ammonia and sulfur dioxide adsorption and the catalytic
reaction of 2 - propanol conversion have been used to study the effects on the
acid – base properties of adding small amounts of various ions (Ca
2+
, Li
2+
, Nd
3+
, Ni
2+
,
Zn
4+
, SO
4
2 −
) to γ - alumina, silica or magnesia surfaces [51] .
It was found that the modifi cation of γ - Al
2
O
3
surface properties with small
amounts of the above ions changed its amphoteric properties only moderately.
More substantial changes, consisting of the formation of centers of moderate and
weak basic strength, were observed on magnesia. The number of acid – base centers
on silica was strongly affected by the introduction of doping ions. The acidity of
the catalysts correlated with the charge/radius ratio and with the electronegativity
of the doping ions. The basicity correlated with the partial oxygen charge of the
corresponding oxides.
The effect of Ca loading on the acid – base and redox properties of chromia
catalysts supported on alumina has been investigated by microcalorimetry of NH
3
adsorption and TPR. This alkaline promoter strongly decreases the acidity of
the chromia catalyst, particularly suppressing the medium and strong acid sites.
No clear correlations were found between the surface acidic properties and the
catalytic behavior of the investigated samples in the oxidative dehydrogenation of
isobutene, while clear trends were observed between reducibility and catalytic
activity [52] .
Sulfated zirconias and sulfated titanias are interesting solids that were fi rst
reported to be superacids. The acidity of such samples has been determined by
NH
3
adsorption calorimetry [47, 53 – 56] . Depending on the preparation method,
the quantity of sulfate ions, calcination temperature and hydroxylation degree, the
initial heats of adsorption of NH
3
observed can vary from ∼ 120 up to 200 kJ mol
− 1
,
but most of these materials display heats of adsorption close to those of H - ZSM - 5
zeolite.
The very low initial enthalpy of adsorption observed by calorimetry on
sulfated titanias suggests the occurrence of an endothermic process (the dissocia-
tion of NH
3
) counterbalancing the exothermic process of adsorption. A plateau of
heats around 150 kJ mol
− 1
is then observed, followed by a regular decrease of the
heats.
9.3.3
Supported Metal Oxide or Metal Catalysts
The even spreading of an oxide over another oxide as support has been widely
investigated. Depending on the coverage, different types of species are deposited,

for instance monomeric, polymeric or bulk - type species. These three main types
of species often exhibit different catalytic and acidic properties.
Gervasini and coworkers [57] have studied the modifi cations of the acid – base
properties of materials obtained by depositing variable amounts (from 1 up to 50%
of the support surface coverage) of Li
+
, Ni
2+
or SO
4
2 −
on supports such as alumina,
magnesia and silica, using NH
3
and SO
2
adsorption microcalorimetry experiments
to probe the surfaces. It has been demonstrated that the addition of lithium, nickel
or sulfate ions to γ - Al
2
O
3,
SiO
2
or MgO results in non - linear changes in the number
and character of acid – base sites. However the effects of modifi cation are more
pronounced on silica owing to its very weak acidity. The observed acid – base site
strengths and numbers have been correlated with an ion - specifi c effect and the
charge difference between the host and guest oxides.
Supported boria catalysts have been prepared using two different methods, a
classical impregnation method and chemical vapor deposition ( CVD ) on porous
and non - porous γ - aluminas, and studied using ammonia adsorption microcalo-
rimetry [58] . The acid sites, at least for the weaker sites of the samples, have been
shown to increase in number but not in strength with boron oxide content. A large
number of weak acid sites were created on the catalyst surface when the boria
amount was greater than the theoretical monolayer. However the number of acid
sites determined when using pyridine as probe molecule was lower than when
using ammonia. Ammonia was shown to cover all types of sites from strong to
weak acid sites, while pyridine only titrated the stronger sites of the samples,
perhaps because of steric hindrance.
At low loadings, new acid sites of medium strength were generated by coverage
of the strong acid sites of alumina. At high boron oxide loadings, weak acid sites
were generated by formation of oxide agglomerates. The basicity of the system,
measured by sulfur dioxide adsorption, decreased progressively with the increase
in boron oxide content. It was also shown that the basic sites of the amphoteric
alumina support are neutralized by 10 wt% of boron oxide on a non - porous
alumina support and 20 wt% of B
2
O
3
on a porous alumina. Moreover, the catalytic
activity for partial oxidation of ethane increased with acidity and reached a
maximum constant value for the monolayer [58] .
The atomic layer deposition ( ALD ) method based on surface - saturating gas –
solid reactions was applied to obtain highly dispersed titania species on a silica
support [59] . The surface - controlled gas - phase deposition of titania on silica pro-
gressed at a growth rate of
1
2
atomnm
support
−
per ALD cycle. The acidic properties
of the catalysts and pure TiO
2
(DT51 Rh ô ne - Poulenc anatase) were probed by NH
3
adsorption microcalorimetry at 353 K. The acid site strength distributions of the
support and ALD samples are represented in Figure 9.7 [60] .
The deposition of sub - monolayer and super - monolayer (monolayer = 4 Ti
atoms nm
supp
−
)
2
amounts of titania by ALD greatly changed the neutral character of
the silica support. Even at the low titania coverage of
32 3
2
.,Ti atoms nm TiSi
supp
−
()
the highly dispersed titania species on silica contributed to the creation of strong
surface acid sites by the formation of strong Ti – O – Si bonds. Those sites exhibited
9.3 Surface Properties of Oxides 409
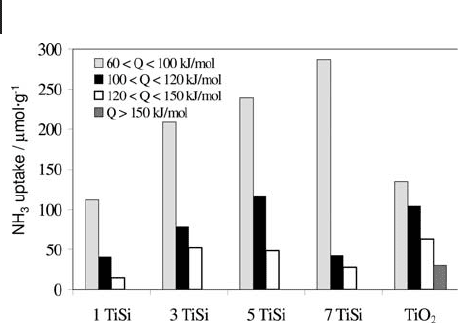
410 9 Thermal Analysis and Calorimetric Methods
differential heat values from 120 to 150 kJ mol
− 1
. A further increase of the titania
content to above monolayer level
71
2
.Tiatomsnm
supp
−
in the 7 TiSi sample mainly
produced additional weak and medium strength acid sites at Q
diff
values from 60
to 120 kJ mol
− 1
.
Alumina - supported tin and gallium oxides with varying amounts of tin and
gallium were prepared by wet impregnation, and characterized by NH
3
, SO
2
, NO
2
and NO adsorption microcalorimetry [48] . NO showed only physisorption proper-
ties whatever the amount of tin or gallium oxide. On varying the concentration of
tin dioxide deposited on Al
2
O
3
, the amount of chemisorbed ammonia passed
through a maximum around monolayer coverage, while the amount of SO
2
adsorbed was found to decrease. The authors concluded that either tin oxide is
preferentially bonded to the basic sites of the amphoteric alumina, or that SnO
2
creates new acid sites and weakens existing basic sites. NO
2
appeared to behave
as an acidic probe, showing the same behavior as SO
2
on the tin dioxide
samples.
The number of sites titrated by SO
2
on the supported gallium oxide samples
increased with increasing amounts of Ga
2
O
3
. This behavior suggested that gallium
oxide is bonded more to the acid sites than to the basic sites of alumina, creating
a loss of acid sites in alumina, although it may also refl ect the presence of new
basic sites provided by Ga
2
O
3
.
Among various other properties, the hydrophilic/hydrophobic character of gallia
supported on zirconia, titania or alumina has been studied using calorimetry
[61] .
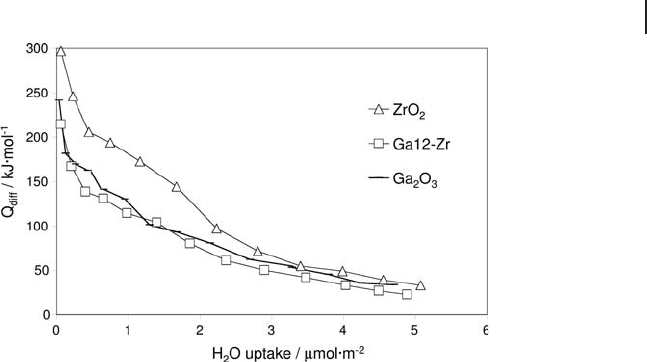
Figure 9.8 represents the differential heats of water adsorption versus coverage
at 353 K on pure zirconia, pure gallia and a sample with 12.7 wt% of Ga
2
O
3
(close
to monolayer coverage) on zirconia. The deposition of Ga
2
O
3
on zirconia decreased
considerably the strength of water interaction with the surface and confi rmed the
higher hydrophobicity of the gallia surface [62] .
Figure 9.7 Acid site distributions of ALD silica - supported TiO
2
determined by ammonia adsorption at 353 K. The labels 1, 3,
5, 7 represent the precursor - oxygen cycle number for titania
growth
number of Ti atom nm
support
−
()
2
.
The differential heats as a function of adsorbate coverage for the adsorption of
NH
3
and CO
2
at 423 K on γ - Al
2
O
3
and SnO
2
/ γ - Al
2
O
3
pre - treated at 753 K have been
reported by Shen and coworkers [63] . The initial heat of ammonia adsorption on
γ - Al
2
O
3
was close to 160 kJ mol
− 1
, while the addition of SnO
2
decreased the initial
heat of NH
3
adsorption to ∼ 145 kJ mol
− 1
. Moreover, the authors observed that
the addition of SnO
2
decreased the strength of stronger acid sites and increased
the strength of intermediate and weak acid sites. Similarly they observed that the
addition of SnO
2
decreased the heat of CO
2
adsorption on essentially all the basic
sites and decreased the saturation CO
2
coverage. These data are in accordance with
those of Ref. [48] .
Samples with various amounts of tin oxide were prepared by impregnation of
γ - Al
2
O
3
, TiO
2
, SiO
2
, ZrO
2
and MgO with tin tetrachloride solutions and studied by
ammonia adsorption microcalorimetry [64, 65] . The infl uence of the adsorption
temperature, evacuation temperature, amount of SnO
2
deposited and nature of
the support on the adsorption properties were studied. The properties of the result-
ing samples were strongly determined by the support. Supporting SnO
2
on TiO
2
or ZrO
2
did not result in an appreciable change in the acidity, whereas remarkable
increases were observed for SiO
2
and MgO, and alumina was mainly modifi ed in
the medium acid strength domain. The amount of deposited tin dioxide had less
infl uence; an increase from 3 to 20 wt% Sn did not reinforce signifi cantly the
acidity, except for the magnesia - supported samples. For the 20 wt% Sn series,
based on the temperature at the maximum of the reduction peak observed by DSC
under a fl ow of mixed H
2
and He, the reducibility scale for Sn
IV
to Sn
II
was found
to be in the order SnSi > SnTi > SnAl > SnZr.
The infl uence of the oxide support (i.e. Al
2
O
3
, Nb
2
O
5
, SiO
2
and TiO
2
) on the
surface properties, reduction and oxidation properties and acid – base properties of
supported indium oxide catalysts has been investigated by temperature pro-
Figure 9.8 Differential heats of water adsorption versus coverage
for bulk zirconia and gallia, and zirconia - supported gallia.
9.3 Surface Properties of Oxides 411
412 9 Thermal Analysis and Calorimetric Methods
grammed reduction/oxidation using thermogravimetry coupled to DSC, and
ammonia and sulfur dioxide adsorption calorimetry [66] .
Two series of In
2
O
3
- containing catalysts with low ( ∼ 3 wt%) and theoretical geo-
metric monolayer (from 20 to 40 wt%) In
2
O
3
contents were prepared, and their
surface properties were compared with those of bulk In
2
O
3
material. Moreover, in
the case of the γ - alumina support, the infl uence of the In
2
O
3
loading was studied
in detail [67] .
As general rule, indium oxide can be considered as more basic than acidic: the
overall acidity was slightly decreased and the basicity signifi cantly increased upon
deposition of indium oxide on the support. The irreversible amounts of SO
2
adsorbed were greater than the irreversible amounts of NH
3
adsorbed, suggesting
the presence of stronger basic sites on the indium - loaded samples.
Indium oxide is easily reduced, at a temperature related to particle size, whereas
oxidation is more strongly infl uenced by the nature of the support. The oxidation
of indium oxide takes place at a lower temperature than the reduction, but the
measured oxidation heats are higher than the reduction heats.
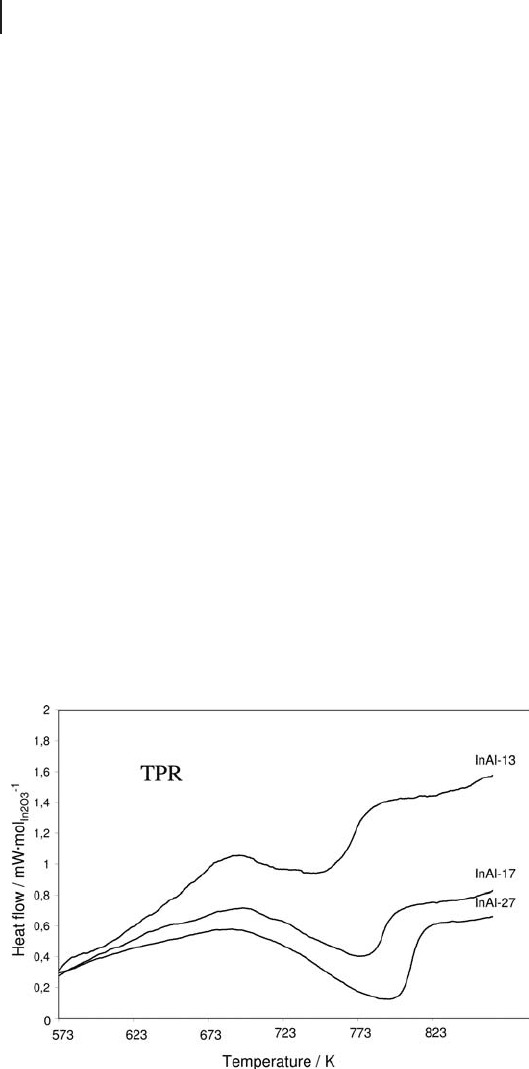
The redox properties of some alumina - supported indium oxide samples, as well
as bulk indium oxide, have been studied by TG - DSC (Figure 9.9 ). Only one endo-
thermic peak is observed, at a reduction temperature that increases with increas-
ing indium oxide loading. Moreover, the measured heat increases with the amount
of indium oxide deposited, varying from 48.5 up to 171.4 kJ mol
− 1
171 4
3
1
.kJmol
In O
2
−
for InAl with 13 wt% In
2
O
3
and bulk In
2
O
3
, respectively. As the measured reduc-
tion heats are given per mole of indium oxide, a constant value could be expected
a priori . This variation of the measured heat with the loading is attributed to
varying interactions with the support. At low loadings, strong interactions with
the support lower the reduction heats.
Figure 9.9 DSC TPR profi les of alumina - supported india samples.

Niobium oxide has been extensively studied as a potential catalyst. However, it
has seldom been used as a support for metallic or oxide catalysts. With the aim to
involve Mo as an active cation for partial oxidation of olefi ns or for oxidative dehy-
drogenation of alkanes or oxygenated compounds, Jin and coworkers [68] studied
the deposition of molybdenum oxide species on niobium oxide. The impregnation
of molybdenum oxide on Nb
2
O
5
calcined at 773 K led to a material exhibiting a
few more strong acid sites of Lewis type and hydroxyl groups of lower acidity than
the corresponding support, as confi rmed by NH
3
adsorption calorimetry and FTIR
specroscopy. In fact, the samples constituted poor catalysts for the partial oxidation
of propene.
The acid – base properties of a series of oxides of group III metals (Al
2
O
3
, Ga
2
O
3
,
In
2
O
3
) supported on niobia, prepared by incipient impregnation and with a loading
close to the theoretical monolayer, were studied by adsorption microcalorimetry
using NH
3
and SO
2
as probe molecules at 353 K. The acid site densities were found
to vary in the following order: Ga
2
O
3
/Nb
2
O
5
< I n
2
O
3
/Nb
2
O
5
< A l
2
O
3
/Nb
2
O
5
. This
means that gallium and indium oxides neutralize part of the acid sites of niobia
and present a more basic than acidic character. Since no basicity could be observed
for Nb
2
O
5
, the basicity observed for the three supported samples should be attrib-
uted to the supported oxides only. The order of basic strength as determined by
SO
2
adsorption was: Al
2
O
3
/Nb
2
O
5
>> G a
2
O
3
/Nb
2
O
5
≈ I n
2
O
3
/Nb
2
O
5
. Since it is com-
monly accepted that indium oxide is the most basic of the three amphoteric group
III oxides, the low basicity observed for this sample has been attributed to a very
poor dispersion [69] .
Cobalt, copper and nickel metal ions were deposited by two different methods,
ionic exchange and impregnation, on an amorphous silica – alumina and a ZSM - 5
zeolite. The adsorption properties towards NH
3
and NO were determined at 353
and 313 K, respectively, by coupled calorimetric – volumetric measurements. The
average acid strength of the catalysts supported on silica – alumina was stronger
than that of the parent support, while the zeolite - based catalysts had (with the
exception of the nickel sample) weaker acid sites than the parent ZSM - 5. The oxide
materials used as supports adsorbed NO in very small amounts only, and the
presence of metal cations improved the NO adsorption [70] .
Oxidation of ethene on silver catalysts to yield ethene oxide is a good example
of an industrial catalytic process with a high selectivity. In order to confi rm a pos-
sible correlation between the catalysts ’ affi nity towards oxygen and their activity
in ethene epoxidation, a heat - fl ow microcalorimeter equipped with a pulse fl ow
reactor has been used to study the reaction of oxygen at 473 K with a series of
silica - supported silver catalysts [71] . At 473 K, adsorption of oxygen at the surface
of silver is a fast process; incorporation of oxygen into deeper metal layers, though
present, is a slow process.
The differential heats of interaction were found to decrease with the amount of
oxygen consumed, but always exceeded the heat of formation of bulk silver oxides.
The average heat of formation of an oxygen monolayer varied from sample to
sample and correlated linearly with the intrinsic activity of the catalysts for ethene
9.3 Surface Properties of Oxides 413
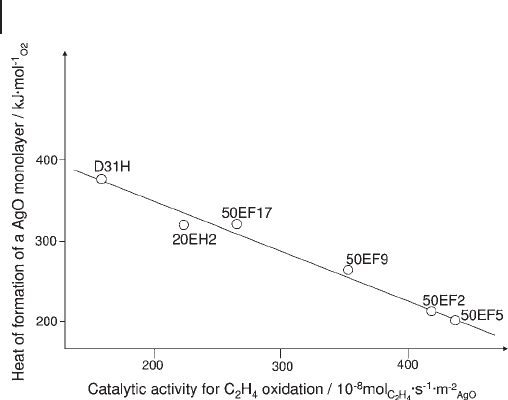
414 9 Thermal Analysis and Calorimetric Methods
oxidation (Figure 9.10 ); surface oxygen species are apparently more catalytically
active when they are less energetically bonded to the silver surface.
Ag/Al
2
O
3
catalysts with various contents have been characterized using TG,
DTA, TPD and NH
3
and CO
2
adsorption calorimetry [72] . The results were dis-
cussed in terms of silver loading and interactions with the alumina surface. An
increase of the total amount of basic sites was globally observed.
Supported Pd catalysts have been found to be the most active and promising
catalysts for methane combustion, and therefore they have been extensively studied
by adsorption calorimetry, in particular using CO as probe molecule, which can
give useful information about the surface state and active sites of the catalyst [11,
73] . Moreover, three - way catalyst s( TWC s), which are widely used for elimination
of pollutants in automobile exhausts, are also composed of precious metals (Pt
and Rh) dispersed on different supports (SiO
2
, Al
2
O
3
, etc.) to increase the exposed
metal surface. In these catalysts, other additives such as cerium oxide have impor-
tant roles as promoters, enhancing the metal dispersion, avoiding metal sintering
and enhancing the oxygen storage capacity of the catalyst.
The adsorption of CO on Pd catalysts supported on Al
2
O
3
, ZrO
2
, ZrO
2
- SiO
2
and
ZrO
2
- La
2
O
3
has been studied by Guerrero - Ruiz and coworkers [74] using both calo-
rimetry and IR spectroscopy. They found that CO was adsorbed on Pd catalysts in
three different modes, with differential adsorption heats lying in high (210 –
170 kJ mol
− 1
), medium (140 – 120 kJ mol
− 1
) and low (95 – 60 kJ mol
− 1
) value ranges,
respectively. The nature of the support, the reduction temperature and the pre -
treatment conditions affected the surface fraction of sites adsorbing CO with
specifi c heats of adsorption.
Figure 9.10 Average heat of formation of an AgO monolayer
at 473 K for a series of silica - supported silver catalysts, as a
function of their intrinsic activity for the oxidation of ethene at
the same temperature.

Supported palladium oxide catalysts present the best performance for methane
combustion in lean conditions. Consequently, the interactions between methane
and palladium oxide or metallic palladium supported on Al
2
O
3
, ZrO
2
and BN at
673 K have been studied by microcalorimetry. At this temperature, methane
reduced the palladium oxide, and the heat of reduction of palladium oxide was
shown to depend on the dispersion and the support. The lowest heats of reduction
corresponded to the highest rates of methane combustion [75] .
Oxygen, hydrogen and CO adsorption heats were measured on 2% Pt/Al
2
O
3
calcined at different temperatures, and so presenting various metal particle sizes.
It was shown that hydrogen adsorption sites have a broad site energy distribution
(attributed to the higher surface mobility of hydrogen), with an initial heat of about
120 kJ mol
− 1
. Intermediate and weak adsorption sites were not observed for carbon
monoxide and oxygen, which gave rise to uniform site energy distributions not
infl uenced by the metal particle size. The initial heats were close to 180 – 190 kJ mol
− 1
for both of them [76] .
H
2
adsorption on high surface area Rh/CeO
2
catalysts has been studied by calo-
rimetry [77] . As the reduction progresses, the mean heat of adsorption and the
amount of hydrogen adsorbed on the metal, as determined by volumetry and
calorimetry, decrease considerably. The initial heats were around 37 kJ mol
− 1
.
The oxidation of alumina - supported rhodium by oxygen in a temperature range
between 280 and 870 K has been studied using calorimetry. The heat of dioxygen
adsorption was found to vary only slightly with the dispersion of rhodium, with a
value of 294 ± 6 kJ mol
− 1
294 6
2
1
±
−
kJmol
O
[78] .
9.3.4
Binary Mixed Metal Oxides to Quaternary Metal Oxides
No general rules for predicting the basic character of mixed oxides have been
proposed up to now. On the contrary, qualitative models have been developed
concerning the generation of new acidic features upon mixing different oxides.
The models most frequently cited are those developed by Tanabe [79] and Kung
[80] .
The acid – base properties of mixed metal oxides have been found to change with
the nature of the constituents, with their relative concentrations and with the
preparation and pre - treatment procedures [81] . Accordingly, mixed oxides can be
used to obtain catalysts with the desired acid – base characteristics by appropriately
choosing the above - mentioned variables.
Regarding preparation procedures, the grafting of metal alkoxides on surface
hydroxy groups, the co - precipitation procedure and sol – gel synthesis can lead to
systems where the mixed oxides can be either close to a classical supported
impregnated oxide (for sub - monolayer coverages) or close to solid solutions or
multi - layered supported oxides. So the frontier between supported oxides and
mixed oxides cannot be well defi ned.
The acid – base properties of ceria – zirconia solid solutions [82] and ceria –
lanthana co - precipitated mixed oxides have been investigated by Cutrufello and
9.3 Surface Properties of Oxides 415
