Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

426 9 Thermal Analysis and Calorimetric Methods
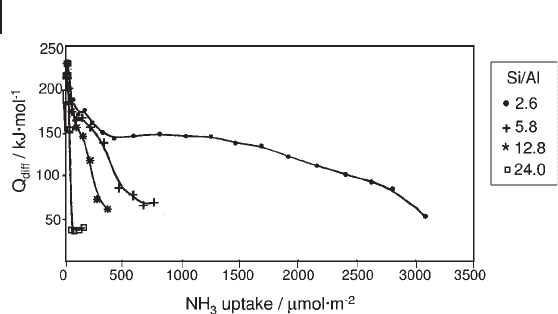
The effect of dealumination can be observed in a series of commercial
Y - faujasites with Si : Al ratios of 2.6, 5.8, 12.8 and 24. The modifi cation of the
acidity resulting from the dealumination is illustrated by the curves of differential
heats of ammonia adsorption (Figure 9.16 ).
The initial heats, in the 180 – 230 kJ mol
− 1
range, correspond to particularly strong
Lewis sites. With the exception of the most dealuminated zeolite, the curves
present a quasi plateau of heats around 140 kJ mol
− 1
(attributed to Br ø nsted sites),
then decrease to about 70 kJ mol
− 1
, a value often considered as the chemisorp-
tion – physisorption limit for ammonia. These curves indicate the existence of three
populations of sites: strong sites (Q above 150 kJ mol
− 1
), medium sites (120 < Q <
150 kJ mol
− 1
) and weak acid sites (70 < Q < 120 kJ mol
− 1
). In terms of relative site
populations, the two most dealuminated zeolites present a higher number of very
strong sites, owing to the presence of EFAL species.
Flow adsorption microcalorimetry has been used to measure the heats of adsorp-
tion of ammonia in a nitrogen carrier on the H
+
and Na
+
forms of a Y zeolite [21] .
The calorimeter was linked to a thermal conductivity detector in which the rates
of adsorption and desorption and the associated rates of heat evolution or absorp-
tion were measured simultaneously at atmospheric pressure. The authors found
that, as surface coverage increased, the sites covered fi rst were not necessarily
those with the highest molar heats of adsorption.
Calorimetric investigations of the adsorption of water, ammonia, methanol and
other small polar molecules on the Na forms of synthetic zeolites A, X and Y have
demonstrated the heterogeneous nature of the Q
diff
versus n
a
relation, which can
be explained by the successive interactions of the exchange cations at the various
crystallographic positions [14] .
The energy of interaction with ammonia and therefore the acid strength of
mordenite at low coverages are higher than the corresponding data for HY molecu-
lar sieves. The overall initial acid strengths of the H - forms of mordenite, ZSM - 5
and faujasite may be arranged in the following order: HM ( ≈ 170 kJ mol
− 1
) > H -
ZSM - 5 ( ≈ 155 kJ mol
− 1
) > H - Y ( ≈ 140 kJ mol
− 1
).
Figure 9.16 Differential heats of ammonia adsorption versus
coverage for dealuminated HY zeolites.
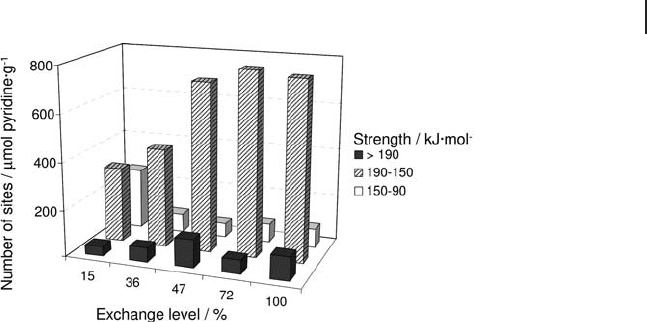
When using pyridine as a probe molecule, the heats evolved are higher than
with ammonia [36] . However, unlike ammonia, pyridine, which is a larger mole-
cule, can titrate only OH groups located in large channels. Heats of adsorption of
pyridine were measured at 573 K on a series of Na - exchanged mordenites [113] .
The curves of differential heats of adsorption versus pyridine coverage were ana-
lyzed, and Figure 9.17 shows the number of sites of a given strength as a function
of the exchange level. Three populations of sites, above 190 kJ mol
− 1
, within 190 –
150 kJ mol
− 1
and within 150 – 90 kJ mol
− 1
, are apparent (Figure 9.17 ). A moderate
increase of the population of very strong sites (heat above 190 kJ mol
− 1
) is apparent
in this fi gure, whereas the number of sites with Q
diff
values lying between 190 and
150 kJ mol
− 1
increases considerably with the exchange degree up to 47% exchange
and then remained constant above this value. These results suggest that, as long
as new hydroxyls are formed in large channels, their average acidic strength
increases. Above 47% exchange, the acidic strength of hydroxyls in the large chan-
nels remains constant, which indicates that the formation of hydroxyls taking place
in side pockets (above 50% of cation exchange) does not infl uence the properties
of hydroxyls in large channels [14] . Concomitantly, the number of sites that display
a moderate strength (between 150 and 90 kJ mol
− 1
) decreases and then remains
constant.
Adsorption enthalpies of N
2
, CO, CH
3
CN and NH
3
on H - BEA and H - MFI zeo-
lites have been measured calorimetrically at 303 K in order to assess the energetic
features of the various interactions occurring within the zeolite nanocavities,
namely: (i) specifi c adsorption on Lewis and Br ø nsted acid sites; (ii) H - bonding
interaction with hydroxyl nests; (iii) confi nement effects. Whereas CO and N
2
single out contributions from Lewis and Br ø nsted acid sites, CH
3
CN and NH
3
are
not preferentially adsorbed on Lewis sites, suggesting that adsorption on Br ø nsted
sites is competitive with that on Lewis sites. For CO adsorbed on a Lewis - rich
H - β zeolite, the initial Q
diff
value is ≈ 70 kJ mol
− 1
, compared with 60 kJ mol
− 1
for
H - ZSM - 5 [114] .
9.3 Surface Properties of Oxides 427
Figure 9.17 Acid site strength distribution of Na,H -
mordenites as a function of the exchange level. Pyridine
adsorption at 573 K, activation at 773 K.

428 9 Thermal Analysis and Calorimetric Methods
Alkali - exchanged zeolites are basic solids and are recognized as effective cata-
lysts for a wide range of organic transformations, such as aldol condensation,
alkylation and etherifi cation. The basic strength and catalytic activity of alkali -
exchanged zeolites can be increased by the occlusion of alkali metal oxide clusters
via impregnation and decomposition of alkali metal compounds such as acetates
or hydroxides. Oxides of cesium and potassium have been synthesized in the
supercages of zeolite X and the alkali - loaded zeolites have been characterized by
both CO
2
adsorption microcalorimetry and stepwise TPD ( STPD ) of CO
2
[115] . For
a loading of about two alkali metal atoms per zeolite supercage, the majority of
CO
2
adsorption sites at 373 K were characterized by a ∆ H
ads
of about 80 – 100 kJ mol
− 1
.
The CO
2
adsorption capacity of CsO
x
/CsX increased linearly with the number of
excess cesium atoms, but the strength of most adsorption sites seemed to be unaf-
fected by the amount of excess cesium species. The basic strengths were found to
vary in the order CsO
x
/CsX > CsO
x
/KX > K O
x
/KX. However the differential heats
at low CO
2
coverage were about the same for all samples. The values of CO
2
adsorp-
tion capacities from STPD were slightly higher than those from adsorption micro-
calorimetry [115] .
Silicalite can be regarded as a new polymorph of silica, presenting adsorption
characteristics close to those of a ZSM - 5 zeolite with an extremely high Si : Al ratio.
The heats of adsorption of both quadrupole (N
2
and CO
2
) and non - polar (Ar, O
2
,
CH
4
, C
2
H
6
and SF
6
) gases were determined on a silicalite in order to study the
effect of adsorbate size and polarity on the energetics of adsorption in zeolites
[116] . Silicalite was classifi ed as a relatively homogeneous adsorbent compared to
X - type zeolite. The accuracy of the calculated isosteric heats of adsorption was
estimated to be ± 2% for heats larger than 20 kJ mol
− 1
and ± 5% for heats smaller
than 20 kJ mol
− 1
. Examples of calorimetrically measured heats for adsorption of
pure SF
6
and CO
2
on a silicalite sample bonded with alumina have also been
reported and compared with calculated isosteric heats [117] .
ETS - 10 is a titanosilicate with a three - dimensional 12 - ring pore system and a
very high ion - exchange capacity. The integral heats of adsorption of monoalkyl-
amines on ETS - 10 have been measured by isothermal calorimetry as a function
of the n - alkylamine concentration [118] . The heats vary in the order methyl < ethyl
< propyl < butyl amine.
Copper - exchanged ETS - 10 catalysts with low and high degrees of exchange have
been prepared and studied using calorimetry of adsorption of NO, CO, C
2
H
4
and
NH
3
probe molecules [119] . Moreover, DSC curves of reduction by hydrogen as a
function of temperature yielded useful information concerning the identifi cation
of supported species and the determination of the intermediate oxidation states of
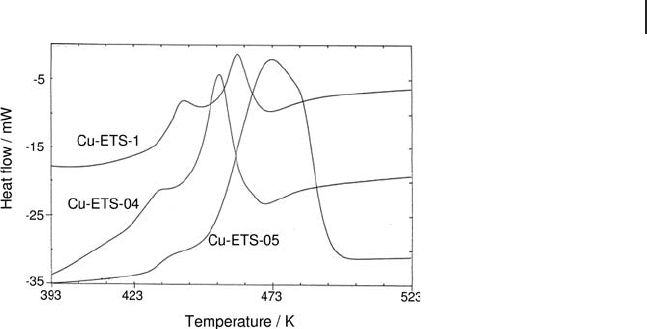
copper during reduction (Figure 9.18 ).
The low - temperature shoulder for the under - exchanged Cu - ETS - 04 and Cu - ETS -
1 samples can be assigned to the reduction of Cu
2+
to Cu
+
and the main peak to
the reduction of Cu(I) to Cu(0), which confi rms that Cu(I) species are predominant
in these samples, while in the over - exchanged sample Cu - ETS - 5, most of the Cu
2+
species are in close contact and not inserted in the support.
A fairly large number of papers have been published on the use of calorimetry
to study the acidity of mesoporous materials [14] .
Mesoporous molecular sieves are close to microporous zeolites in their methods
of preparation and principal regions of application. These materials are usually
synthesized by using supermolecular templates and, in particular, the micelles of
cationic surfactants [17] .
According to the data from calorimetric investigations of the adsorption of
ammonia, unlike silica gel, the pure - silica mesoporous sieve MCM - 41 possesses
specifi c although weak acidity. The introduction of titanium or zirconium, and
particularly aluminum, ions into the structure of this sieve substantially increases
the acid strength of the sorbent [120, 121] .
Using ammonia as probe, the strength of the acid centers of the investigated
samples can be arranged in the following order: silica gel < Si - MCM - 41 < Si,Zr -
MCM - 41 < Si,Ti - MCM - 41 < Si,Al - MCM - 41. The heats of NH
3
adsorption on the
latter sample with molar ratio SiO
2
: Al
2
O
3
= 32 : 8 are 130 – 150 kJ mol
− 1
, which is
close to the heats of interaction of ammonia with a ZSM - 5 zeolite.
Spectroscopic and calorimetric investigations of the adsorption of acetonitrile
[122] have shown that the sorbent Si,Al - MCM - 41 has strong Lewis (aprotic) acidity
and poorly defi ned Br ø nsted (protic) acidity [17] . The presence of strong Lewis
centers on Si,Al - MCM - 41 was confi rmed by fl ow microcalorimetry for the adsorp-
tion of 1 - butanol from a solution of n - hexane [123, 124] . Depending on the alumi-
num content of the structure, the number of strong Lewis centers on the surface
of this sample was found to vary in the range of 43 to 70% of the total number of
active centers.
9.4
A Case Study: Vanadia Catalysts
It is known that catalytic activity in selective oxidation of hydrocarbons can
be related to the co - operative action of an oxidizing function and an acidic
function. From this perspective, the determination of both the redox and the acidic
Figure 9.18 Heat fl ow profi les for reduction under H
2
fl ow as
a function of temperature for various Cu - ETS samples.
9.4 A Case Study: Vanadia Catalysts 429

430 9 Thermal Analysis and Calorimetric Methods
properties of the catalytic centers, as carried out in the present study using pulsed
adsorption and fl ow calorimetry, becomes a topic of renewed importance.
Vanadium oxide is known to catalyze oxidation of hydrocarbons, and the selec-
tive ODH of ethane over V
2
O
5
catalysts has been extensively studied. The acid – base
and redox characters of vanadium pentoxide, V
2
O
5
/SiO
2
, V
2
O
5
/ γ - Al
2
O
3
, V
2
O
5
/TiO
2
,
V
2
O
5
/TiO
2
/SiO
2
, V
2
O
5
/CeO
2
, V
2
O
5
/Nb
2
O
5
and V
2
O
5
/MgO catalysts have been
investigated by adsorption microcalorimetry of probe molecules and/or TPR/TPO
calorimetric experiments respectively.
NH
3
adsorption calorimetry experiments performed on bulk V
2
O
5
indicated a
weak acidity, with an initial heat of adsorption of 70 kJ mol
− 1
and a large heteroge-
neity [125] .
TPR/TPO experiments were performed in a TG - DSC apparatus on a bulk V
2
O
5
[125] using a gas fl ow of C
2
H
6
, C
2
H
4
or H
2
(diluted in helium) as the reducing
agents, at a heating rate of 5 K min
− 1
. The reduction temperatures and heat fl ows
depended greatly on the reducing agent, with reduction heats increasing in the
order C
2
H
6
< C
2
H
4
< H
2
. According to the weight loss, a similar reduction extent
occurred whether hydrogen, ethane or ethene was used as reducing agent, corre-
sponding to the formation of V
2
O
3
in all cases. In the case of ethene, various
intermediate suboxides could be deduced by examining the derivative of the
thermogravimetric curve. Following each reduction, re - oxidation studies were
performed under oxygen fl ow in the same equipment, clearly showing different
steps in the re - xidation process.
Regular pulses of pure ethane on bulk V
2
O
5
maintained at 823 K in a microcalo-
rimeter linked to a gas chromatograph provided kinetic data of theoretical signifi -
cance, as well as an insight into the mechanism of the reduction process. The
results of this work carried out using mainly calorimetric techniques led to the
conclusion that diffusion of oxygen from the bulk is predominant in the selective
oxidation of ethane and that the redox process plays a more important role than
the acidic sites in the case of unsupported vanadium pentoxide.
V
2
O
5
/SiO
2
catalysts prepared by grafting or wet impregnation methods have also
been studied in the ethane ODH reaction. The surface characteristics and the
reactivity of these catalysts have been investigated using microcalorimetry linked
to other techniques such as volumetric studies and thermogravimetry. The vanadia
amounts ranged from 1 to 20 wt%. The numbers of acid sites of the supported
catalysts were found to increase with vanadium loading, leading to materials with
a moderately acidic character [126, 127] .
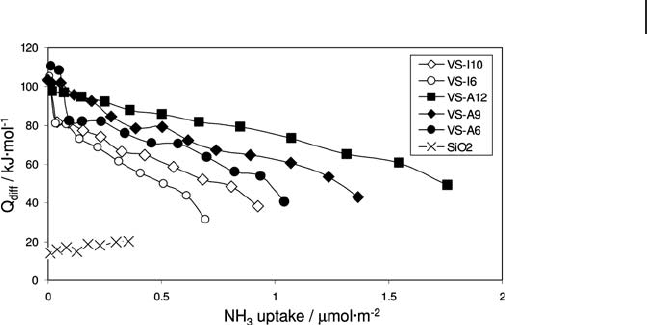
Compared to bulk vanadia, higher values of the initial heats of NH
3
adsorption
have been measured for the V
2
O
5
/SiO
2
catalysts (78 to 124 kJ mol
− 1
). Considering
the overall value of chemisorbed ammonia versus the vanadium content, the
population of titrated acid sites was slightly higher for the grafted V
2
O
5
/SiO
2
cata-
lysts than for the samples prepared by wet aqueous impregnation for similar V
2
O
5
loadings. A linear relation was also observed between the integral enthalpy of
ammonia adsorption and the vanadium content of the catalysts.
Interaction with the support was enhanced after catalytic reaction, as indicated
by the detection of new strong Lewis acid sites by DRIFT spectroscopy measure-
ments and higher heats of ammonia adsorption [128] . The catalytic activity
increased with the vanadium content and the ammonia uptake of the V
2
O
5
/SiO
2
catalysts, that is with their acidity. However, selectivity to ethane appeared to be
more closely related to the coordination of the vanadium atoms than to the acidic
character of the surface of the V
2
O
5
/SiO
2
catalysts. The activity and selectivity to
ethane were enhanced on V
2
O
5
/SiO
2
especially for very low vanadium contents,
presumably corresponding to isolated vanadium cations, which are more easily
reduced in ethene as shown by DSC. As stated above, both the number and the
strength of the acid sites of the silica - supported catalysts increased as the vanadia
loading increased, independently of the preparation method. Comparing catalysts
at similar V
2
O
5
loadings prepared by impregnation and by atomic layer deposition
( ALD ) it appears that the ALD preparation yields a V
2
O
5
phase characterized by a
higher number of acid sites and a stronger surface acidity than the preparation by
impregnation (Figure 9.19 ) [129] . ALD is a gas - phase layer - by - layer processing
method based on surface - saturating precursor adsorption on the support, thus
allowing a high dispersion of the deposited species.
The acid – base properties of V
2
O
5
/ γ - Al
2
O
3
catalysts prepared by the impregnation
method have been characterized by ammonia, pyridine and sulfur dioxide adsorp-
tion microcalorimetry. Sulfur dioxide adsorption made it possible to differentiate
a vanadate layer from free alumina.
Ammonia and sulfur dioxide adsorption experiments also showed that,
at low vanadium coverage, a large part of the vanadate layer is bound to acid – base
pairs of alumina. At low vanadium coverage ( < 3 wt% V
2
O
5
), the acidic character
of the V
2
O
5
/ γ - Al
2
O
3
catalyst can be ascribed to vanadium - free alumina, whereas
at higher vanadium coverage it is largely attributed to vanadate compounds
[130] .
9.4 A Case Study: Vanadia Catalysts 431
Figure 9.19 Differential heats of ammonia adsorption over a
silica support and silica - supported vanadia catalysts prepared
by ALD (fi lled symbols) and impregnation (open symbols).
VS - A6 and VS - I6 on one hand, and VS - A12 and VS - I10 on the
other hand, have comparable vanadia contents.
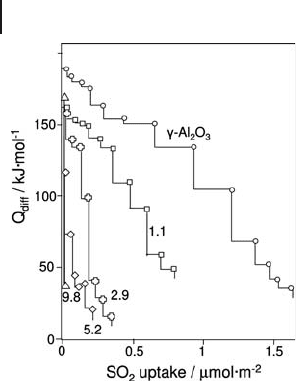
432 9 Thermal Analysis and Calorimetric Methods
Figure 9.20 displays the differential enthalpy of SO
2
adsorption at 353 K as a
function of the probe uptake on samples with various vanadium contents and on
pure γ - Al
2
O
3
.
The adsorption of sulfur dioxide of the catalysts rapidly decreased with the
vanadium content of the samples. As sulfur dioxide is not chemisorbed on bulk
vanadium pentoxide and also appears not to chemisorb on vanadate species, on
V
2
O
5
/ γ - Al
2
O
3
catalysts this probe can be considered to be selective for the titration
of the basic sites of vanadium - free alumina. Thus, by selectively probing uncovered
alumina, SO
2
further allows the distinction between the acid – base features of the
vanadium oxide layer and those of uncovered alumina.
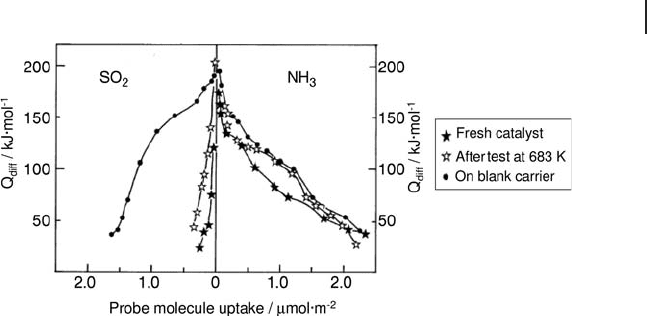
The acid – base properties of a 5.2 wt% V
2
O
5
/ γ - Al
2
O
3
catalyst have also been
studied after performing measurements of catalytic activity for ethane conversion
to ethene by microcalorimetry of adsorption of NH
3
and SO
2
at 353 K. Figure 9.21
displays the acidic and basic features of this catalyst before and after the catalytic
run. The acid – base features of γ - Al
2
O
3
are also reported for reference. This fi gure
confi rms that V
2
O
5
is preferentially anchored on the basic sites of the alumina,
resulting in a large decrease in basicity. It was also observed that some additional
acid sites (0.1 µ mol m
− 2
) have appeared after the catalytic run. This amount corre-
sponds to 5% of the overall acidic sites of the catalyst. It is interesting to note the
same increase in basic sites. These features are consistent with the restoration of
some acid – base pairs of γ - Al
2
O
3
and show the overall stability of the acid – base
character of the vanadium oxide layer [131] .
A 20 wt% V
2
O
5
/ γ - Al
2
O
3
catalyst has been subjected to a TPR in ethane and re -
oxidation in air using TG - DSC equipment, and compared to bulk vanadium pent-
oxide. Reduction in ethane occurs at a lower temperature on the V
2
O
5
/ γ - Al
2
O
3
catalyst (717 compared with 794 K for bulk vanadium pentoxide). The exothermic
Figure 9.20 Differential heat of sulfur dioxide adsorption at
353 K as a function of the probe uptake on V
2
O
5
/ γ - Al
2
O
3
catalysts with different wt% of V
2
O
5
as indicated in the fi gure.
enthalpy evolved in the reduction is much higher on the alumina - supported cata-
lyst. A reduction step by itself is an endothermic process. The observed exothermic
enthalpies involve two terms: an enthalpy of reaction (exothermic) and an enthalpy
due to lattice oxygen migration (endothermic). It was deduced from the calorimet-
ric data that lattice oxygen migration to react with ethane requires less energy on
the V
2
O
5
/ γ - Al
2
O
3
catalyst, that is lattice oxygen mobility is improved on that
catalyst.
Nano - sized V
2
O
5
/SiO
2
, V
2
O
5
/Al
2
O
3
, V
2
O
5
/TiO
2
and V
2
O
5
/TiO
2
/SiO
2
can be pre-
pared by ALD. Compared to liquid - phase preparations, the technique gives greater
benefi ts in terms of species dispersion and uniformity.
Alumina - , silica - and titania - supported vanadium oxide systems with V
2
O
5
load-
ings ranging from 3 to 12 wt%, corresponding to 0.02 to 0.09 V/(Al, Si, Ti) atomic
ratios, were prepared by ALD and compared with the corresponding impregnated
catalysts [129] . The surface acidic properties of the supports and catalysts were
investigated using ammonia adsorption microcalorimetry to determine the number
and strength of the surface acid sites. Deposition of V
2
O
5
on alumina and titania
supports gave rise to catalysts with lower numbers of acid sites than the respective
supports, while for the samples prepared on silica, an increase of the number of
acid sites was observed after V
2
O
5
deposition, thus confi rming the study performed
by Le Bars and coworkers [131] . As a common trend, the surface acid strength was
greater for the ALD catalysts than for the impregnated ones, suggesting a stronger
interaction of the VO species with the support centers, which act as electron attrac-
tor centers creating Lewis - like vanadium species.
Synergistic interactions of highly dispersed vanadia and titania species on silica
have been shown to control the acidity, reducibility and thus the overall activity of
the V
2
O
5
/TiO
2
/SiO
2
catalysts in the selective oxidation reaction of o - xylene to
phthalic anhydride [132] . Using ammonia adsorption calorimetry and pyridine
adsorption IR spectroscopy it was shown in this study that the strong Lewis acidity
of a pure titania support was masked by the deposition of vanadia, the decrease
in the number of acid sites being especially pronounced in the case of the highly
9.4 A Case Study: Vanadia Catalysts 433
Figure 9.21 Differential enthalpies of adsorption of ammonia
and sulfur dioxide at 353 K on 5.2 wt% V
2
O
5
/ γ - Al
2
O
3
.

434 9 Thermal Analysis and Calorimetric Methods
dispersed ALD samples. On V
2
O
5
/TiO
2
/SiO
2
samples, the better dispersion of
active species in the ALD samples was verifi ed by the acidity measurements. The
number of strong, mainly Lewis, acid sites increased in the ALD catalysts with
sub - monolayer titania coverage. In the catalysts with high titania content, the ALD
vanadia species masked the acidity better than the species prepared by impregna-
tion. It was also shown using ammonia and o - xylene adsorption microcalorimetry
that the presence of strong surface acidic and o - xylene adsorption sites decreased
the activity of the catalysts in the test reaction of o - xylene oxidation. Medium -
strength and strong o - xylene adsorption sites, with evolved heats between 120 and
150 kJ mol
− 1
, seemed to be the most detrimental for the catalytic activity.
In another study [133] , microcalorimetric adsorption of reactants and products
on supported vanadia catalysts for the selective oxidation of propene to acetone
was used to provide information about the surface bonding strengths that may be
used for the microkinetic analysis of the corresponding surface reactions. The
initial heat for water adsorption on the studied V
2
O
5
/TiO
2
catalyst is lower than
90 kJ mol
− 1
, indicating that water is reversibly adsorbed at temperatures higher
than 360 K. The heat of adsorption of acetone on the reduced V
2
O
5
/TiO
2
catalyst
is about 100 kJ mol
− 1
, which can be used to estimate the activation energy for
desorption of acetone from the catalyst [133] .
The acid – base properties of V
2
O
5
/CeO
2
catalysts prepared by the wetness impreg-
nation technique have been determined by NH
3
and CO
2
adsorption calorimetry
at 423 K [134] . Variations in V
2
O
5
loading and calcination temperature brought
about changes in the surface structure of the dispersed vanadium species, and
hence in the surface acidic and redox properties. CeO
2
possessed fairly strong
surface acidity and basicity. Addition of V
2
O
5
enhanced the surface acidity as well
as the redox properties, but decreased the surface basicity. Calcination of the
10 wt% V
2
O
5
/CeO
2
catalyst at 873 K resulted mainly in the formation of CeVO
4
on
the surface, which showed low surface acidity and redox ability.
Microcalorimetry was also used to measure the heats of adsorption of ethanol
and 2,2,2 - trifl uoroethanol as a function of coverage at 300 K for a 6 wt% V
2
O
5
/CeO
2
sample. The calorimetric data showed a constant heat of adsorption of 65 kJ mol
− 1
[135] .
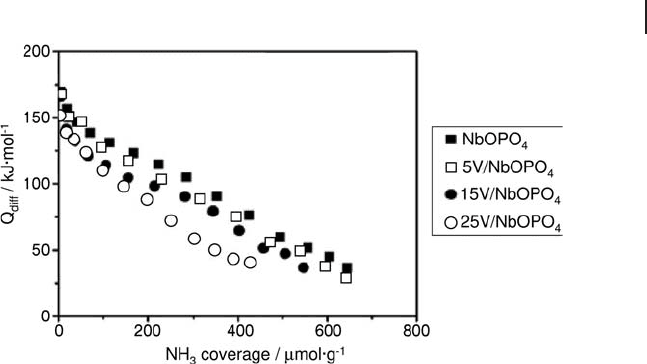
V
2
O
5
catalysts supported on niobyl phosphate (NbOPO
4
) with V
2
O
5
loadings
from 5 to 25 wt% were prepared by incipient wetness impregnation, and the
surface acidity was determined by microcalorimetry using NH
3
as a probe mole-
cule. The results suggested that the loaded V
2
O
5
weakened the surface acidity of
NbOPO
4
while increasing the proportion of weak acid sites (Figure 9.22 ). The
surface of NbOPO
4
presented a purely acidic character and weakened the redox
properties of supported V
2
O
5
[136] .
Magnesium vanadates, also known as VMgO catalysts, are known to be effi cient
catalysts for the ODH of light alkanes. Corma and coworkers [137] suggested that
the incorporation of small amounts of V on a MgO surface notably modifi ed not
only the redox properties of the system but also the acid – base character of the
oxygen species present on the catalyst surface. These acid – base properties were
related to the nucleophilic character of the lattice oxygen anions.
A series of VMgO catalysts with various compositions and surface areas were
characterized at 353 K, using adsorption calorimetry of NH
3
and SO
2
to probe their
acid – base properties. The acid strength of the VMgO samples increased up to
14 wt% of V loading. For higher loadings, the strong acidity decreased, while
weaker acid sites developed above 25 wt%. The basicity of the VMgO samples
remained nearly unchanged for loadings up to 25 wt% V. At higher loadings, the
basicity decreased, and vanished for the 45 wt% V loading sample [138] .
All these studies have demonstrated the usefulness of calorimetry for studying
the surface characteristics of vanadia - based catalysts. Among the surface proper-
ties of vanadia catalysts of relevance to their catalytic activity in selective oxidation
reactions, acidity is one of the most signifi cant.
Protonic acidity of the support is not desirable as it leads to side - reactions such
as polymerization or cracking of organic molecules at high temperatures and
coking. Complete coverage of the support by a V
2
O
5
phase might give rise to Lewis -
like vanadium centers, which are characterized by a lower acid strength than the
acid centers of the oxide supports.
For vanadia catalysts supported on alumina, titania or niobyl phosphate, the
main trend evidenced by ammonia adsorption microcalorimetry is a remarkable
decrease of the number of acid sites, together with a decrease of the total acid
strength compared with the acidic properties of the bare support. By contrast,
owing to the neutral behavior of the silica surface, the acidity of V
2
O
5
phases
deposited on silica becomes much more apparent. A similar trend was observed
on the basic magnesia and amphoteric ceria. A decrease in basicity was observed
on all amphoteric supports ( γ - Al
2
O
3
,TiO
2
,TiO
2
/SiO
2
, CeO
2
), allowing the distinc-
tion between acid – base features of the vanadium oxide layer and those of the
uncovered support.
9.4 A Case Study: Vanadia Catalysts 435
Figure 9.22 Differential heats of ammonia adsorption versus
coverage for various niobyl phosphate supported vanadia
samples.
