Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.


f(E
k
) of a state of energy E
k
is controlled by the “ Fermi energy ” E
F
and a smearing
parameter σ which was set to 0.2 eV in Figure 8.5 a. We have placed the term
“ Fermi energy ” in quotes because it is usually defi ned as the chemical potential
of the electronic system at some temperature. However, DFT calculations are used
to identify the ground state electron density at 0 K and the application of Equation
8.24 is simply a tool to speed up convergence. Indeed, other functions which can
smear out the occupation of the highest fi lled states can also be used. In the MgO
calculation, occupations of 2 electrons per state are obtained for the LVB and UVB
with 0 occupation numbers for all CB states. Figure 8.5 a shows that this is achieved
by placing E
F
just suffi ciently above the top of the valence band to give f(E
k
) = 1
for all UVB states. The exact position of E
F
depends on the smearing parameter
and so it is effectively a variable in the SCF procedure.
In the literature, the value of the Fermi energy is often reported as the highest
occupied state at 0 K (the “ HOMO ” state). In metals this is correct because the
boundary between fi lled and empty states corresponds to the energy of the highest
occupied state at zero Kelvin. More generally, E
F
is defi ned from the Fermi – Dirac
distribution (as Equation 8.24 with σ = k
B
T ) as the energy at which the probability
of occupancy falls to 1/2. In insulators and semiconductors, the position of the
Fermi energy is set by the detailed balance between states occupied by electrons
thermally excited to the CB and the empty states left behind in the UVB. This dif-
ference between the value of E
F
used in the smearing function and the true Fermi
energy becomes important when trying to align the energies of states of two cal-
culations, say a surface calculation and that on an isolated adsorbate. The Fermi
energy gives the chemical potential of the individual systems, which will equalize
if they are bought together. The use of E
F
from a calculation for aligning the ener-
gies of electronic states between different calculations is not a reliable way to do
this. In a calculation, the electronic energy is set by the potentials used in the
Kohn – Sham equations and should refl ect the binding energy of an electron with
respect to the vacuum. This means that the energy scales between different calcula-
tions should be directly comparable.
The band gaps between the UVB and empty CB states in Figure 8.5 are calcu-
lated as 5.1 eV for MgO (Figure 8.5 a) and 2.0 eV for TiO
2
(Figure 8.5 b). The pres-
ence of a band gap is indicative of an electrical insulator or semiconducting
material and we would expect MgO to have the larger band gap of the two materi-
als. However, it has been found that the band gap obtained in a calculation is
strongly dependent on the method used. Bredow and Gerson employed MgO as
one example in their systematic study of the effect of electron exchange and cor-
relation on the bulk properties of rock - salt structured oxides [52] . Their results are
included along with others in Table 8.4 . Using RHF, the band gap between the
UVB and CB states is substantially overestimated at 16.5 eV compared with experi-
mental values which range from 7.8 [53] to 8.7 eV [54] . In RHF calculations,
exchange is included explicitly but correlation is ignored. At the other extreme,
the results of Gillan and coworkers [55] using an LDA approach underestimated
the band gap by around 3 eV and the lattice constant is also smaller than found
experimentally. The various pure GGA - DFT results shown in Table 8.4 (BLYP,
8.2 Electronic Structure Methods 345

346 8 Theory: Periodic Electronic Structure Calculations
PBE and PW91) improve on this slightly, but still signifi cantly underestimate the
band gap and overestimate the lattice constant. This trend, for HF to overestimate
and DFT to underestimate E
g
, has been noted for a wide range of oxide materials.
In the HF case, the omission of correlation energy is the likely cause. The correla-
tion energy will most strongly infl uence the gap between bands of different occu-
pation. Therefore the energy difference between the LVB and UVB will not be in
as great an error as UVB to CB.
In the example of MgO, inclusion of GGA - DFT style correlation using the LYP
functional for the RHF method actually increases the band gap by a further 1 eV.
In addition the lattice constant optimized in this calculation is signifi cantly smaller
than the experimental reference structure [56] , so it appears that adding in correla-
tion to a HF wavefunction model is not an answer. For GGA - DFT, the self -
interaction problem is thought to be the main reason for underestimation of the
band gap, and addition of HF exchange to correct the calculated band gap has been
more successful. In Table 8.4 , the choice made in B3LYP is seen to give a band
gap in quite close agreement with experiment. The use of hybrid functionals, such
as B3LYP, to give a good compromise between the over - estimation of the band
gap in oxides by HF and its under - estimation by DFT appears to be quite general.
For example in work by Muscat and coworkers [57] , the band gaps of a wide range
of materials including MgO, Al
2
O
3
and TiO
2
are calculated using B3LYP, giving
7.3, 8.5 and 3.4 eV respectively and these compare favorably with experimental
estimates of 7.8, 9.0 and 3.0 eV [58] . Zhang and coworkers [59] have shown that
for TiO
2
the calculated band gap increases in proportion to the amount of exact
Table 8.4 Comparison of calculations on bulk MgO by various methods.
Method Basis
a)
a ( Å ) E
g
(eV) Reference
RHF O:8 - 411/1G
Mg:8 - 511/1G
4.20 16.5
RHF + LYP O:8 - 411/1G
Mg:8 - 511/1G
4.09 17.5
BLYP O:8 - 411/1G
Mg:8 - 511/1G
4.28 5.3
PBE O:8 - 411/1G
Mg:8 - 511/1G
4.25 5.3
PW91 O:8 - 411/1G
Mg:8 - 511/1G
4.25 5.2
B3LYP O:8 - 411/1G
Mg:8 - 511/1G
4.25 7.6
LDA 600 eV (pw, NC) 4.16 4.8 [55]
PBE 400 eV (pw, PAW) 4.25 5.1 this chapter
Expt 4.212 7.8 [53]
8.7 [54]
a Basis set notation such as 8 - 411/1G indicates an atomic orbital basis set using Gaussian radial
functions. This example would be referred to as a triple - ζ basis since the valence region is
represented by three basis functions, the fi rst of which is a contraction based on 4 Gaussians
(411). There is also one polarization function ( d - type function for O) indicated by /1. For
plane - wave basis sets ( pw ) the value of E
cut
is given along with an indication of the type of
pseudopotential employed: NC = Norm conserving , PAW = Projected Augmented Wave .
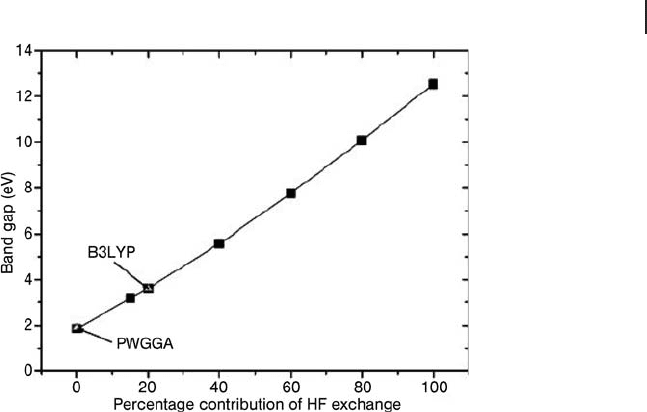
exchange included in the functional, as shown in Figure 8.6 . The optimal choice
of 13% exact exchange gives a perfect match to the band gap energy. The choice
made in B3LYP is slightly larger at 20% but the well documented performance of
this functional for molecular bond energies makes it a good compromise for giving
reliable properties of the solid and adsorbates when modeling catalytic reactions.
Further tailoring of the amount of HF exchange to use according to the solid state
property of interest has been discussed by Cora and coworkers, who also show
that the introduction of HF exchange increases electronic localization [60] .
Despite this failure to correctly represent the relative energies of the occupied
and unoccupied states correctly, HF and DFT methods have been widely applied
in the simulation of the electronic structure of oxides. Examples in the remainder
of this chapter will include comments on the reliability of these results along with
further examples of the use of hybrid functionals.
8.3
Bulk Structure: Alumina
Alumina provides a good example of a main group metal oxide that has been
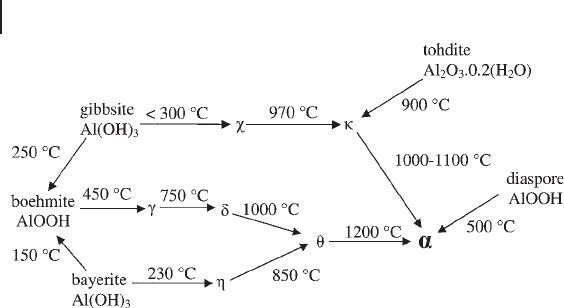
studied using a variety of simulation techniques. Alumina has several phases,
which occur during its synthesis, by heating, from aluminum hydroxides such
as gibbsite, Al(OH)
3
, or boehmite, AlO(OH). The most stable form is the dense
oxide α - Al
2
O
3
which has the corundum structure but this is obtained from the
hydroxides via a series of transition aluminas following the pathways shown in
Figure 8.7 .
In all of these structures the oxygen sub - lattice can be thought of in terms of
the stacking of hexagonal layers, familiar from close packed solids. For example,
Figure 8.6 Calculated band gap in TiO
2
as a function of
percentage HF exchange. From ref. [59] .
8.3 Bulk Structure: Alumina 347
348 8 Theory: Periodic Electronic Structure Calculations
the minerals gibbsite and bayerite are aluminum hydroxides of general formula
Al(OH)
3
. In both structures, Al is octahedrally coordinated by O atoms forming
layers of edge - sharing octahedra, one of which is shown in Figure 8.4 a. The oxygen
sub - lattice is controlled by the resulting bi - layers with the triangular faces of the
octahedra giving a roughly hexagonal arrangement. In the extended structures
these bi - layers are stacked perpendicular to the crystallographic c direction. The
two oxygen layers of the bi - layer can be assigned as A and B layers in the stacking
sequence for the oxygen sub - lattice. In bayerite, the bi - layers are stacked directly
over one another giving an ..AB.AB.AB.. sequence (Figure 8.4 b), while the struc-
ture of gibbsite has alternate bi - layers set as refl ections of one another leading to
an ..AB.BA.AB.. structure (Figure 8.4 c). The bi - layers are held together by hydro-
gen bonding via the H atoms that can be seen in the interlayer space of the atomic
representation regions of Figure 8.4 b and c. In addition, there are hydrogen bonds
between OH groups and the O ions in the same layer. The hydrogen bonding
network in these materials appears to be well ordered since the H atom positions
have been obtained in neutron (bayerite [62] ) and X - ray diffraction (gibbsite [48] )
experiments. Gale and coworkers have studied these hydroxides using a combina-
tion of plane - wave (CASTEP) and localized basis set (SIESTA) methods with the
PBE functional [47] . Despite the similarity between the two structures, gibbsite is
the more commonly observed form in nature. The plane - wave and localized orbital
calculations both gave total energies that indicate gibbsite to be more stable than
bayerite, by 7.7 and 6.3 kJ mol
− 1
, respectively. This is consistent with calorimetric
measurement of the heats of formation, which suggest that gibbsite is the more
stable by around 5 kJ mol
− 1
. In the simulation study, calculations of the vibrational
frequencies of the O
–
H stretching modes also showed that the interlayer hydrogen
bonding is stronger than the intralayer interactions.
Figure 8.7 The aluminum oxides and hydroxides and their
temperatures of interconversion. Hydroxide materials are
named along with their chemical formulae; oxides all have the
composition Al
2
O
3
and so are indicated by the Greek letter
associated with the phase. The α - phase is indicated in large
font and bold as it is the thermodynamically most stable
oxide. Adapted from reference [61] .
The trihydroxides gibbsite and bayerite were included in the work of Sautet and
coworkers along with the monohydroxides boehmite and diaspore [61] . They also
used a localized basis set within the SIESTA code and PBE functionals, but refi ned
the basis set radial functions based on reference calculations of α - Al
2
O
3
. Diaspore
has an oxygen sub - lattice with an hcp - type, ..ABAB.., stacking sequence and, as
indicated in Figure 8.7 , dehydrates directly to the low surface area oxide α - Al
2
O
3
on heating above 500 ° C. As we will see later, α - Al
2
O
3
also has a hexagonal struc-
ture, resulting in this simple relationship between diaspore and the thermody-
namically most stable alumina. Both gibbsite and bayerite dehydrate to boehmite
which is also the major component of many bauxite minerals. Its oxygen sub -
lattice has an fcc - type ..ABCABC.. packing and it is this monohydroxide that is the
precursor to the technologically important γ - , δ - and θ - aluminas.
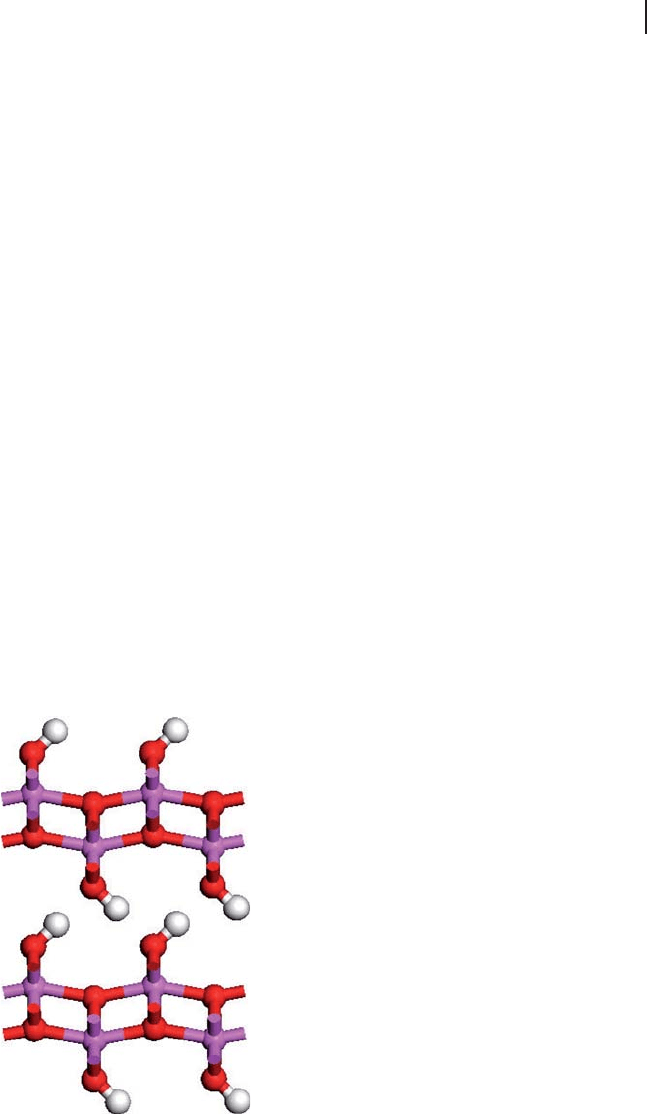
Boehmite is a layered hydroxide with the structure shown in Figure 8.8 . For the
AlO(OH) hydroxide, the intralayer hydrogen bonds of the trihydroxides have been
lost and only interlayer interactions remain. The positions of the hydrogens in this
structure are not well known experimentally and Sautet considered two possible
structures. The hydroxyls form in rows which run into the page in the view illus-
trated in Figure 8.8 and are shown here with the hydroxyls in the interlayer region
pointing to the right. There is an equivalent structure in which all hydroxyls point
left, and in real materials mixtures of these hydroxyl orientations could occur. In
a simulation we are limited by the repeating unit used, but by doubling the unit
cell in the hydroxyl row direction Sautet was able to compare structures with all
hydroxyls in a row pointing in the same direction and the case in which the
hydroxyls alternate left/right down the row direction. These structures differed by
only 0.2 kJ mol
− 1
, suggesting that the uncertainty in the experimental determina-
tion of H - positions is a result of disorder in the H - bonding network. Molecular
Figure 8.8 The structure of boehmite (AlO(OH)).
Aluminum, pink; oxygen, red; hydrogen, white.
8.3 Bulk Structure: Alumina 349
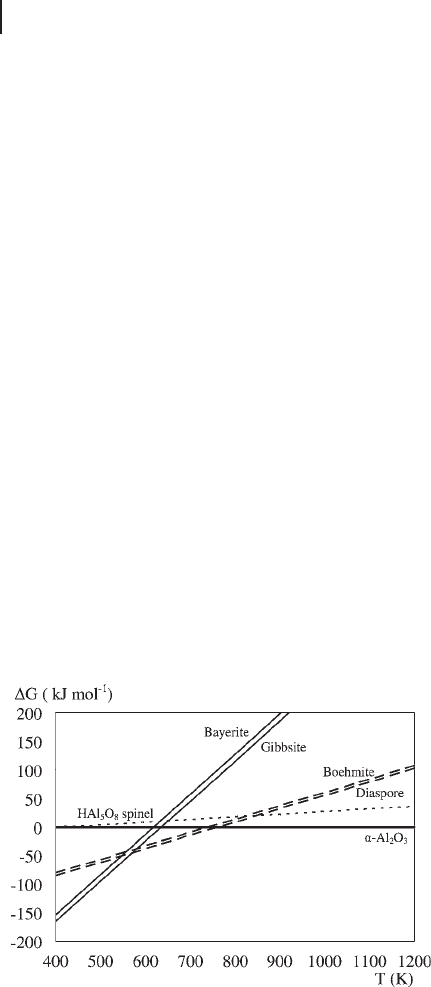
350 8 Theory: Periodic Electronic Structure Calculations
dynamics calculations, in which Newton ’ s laws are used to follow the motion of
the system based on DFT calculated forces, also show that the interchange of
hydrogens between oxygen atoms in the interlayer region is rapid, even at low
temperatures (350 – 600 K) [63] .
Sautet also used the calculated energies for the trihydroxides, monohydroxides
and α - Al
2
O
3
structures to estimate the relative stability of these materials based
on the dehydration sequence:
22 2 3
3
223
Al OH AlO OH H O Al O + H O
2
()
=
()
+=
(8.25)
Here the DFT - calculated energy differences for the oxide and hydroxide
materials were used in the place of free energies and for the internal energy of
H
2
O. Standard thermodynamic data for the enthalpy of vaporization and entropy
of water were then used to plot the relative stabilities as a function of temperature.
This plot is reproduced in Figure 8.9 . Although the temperatures of transition
do not agree closely with experiment, the sequence of stability is correct.
Trihydroxides are preferred up to around 560 K, at which point dehydration to
monohydroxide should take place. The calculated energies actually place diaspore
marginally below boehmite; however, the layered structure of boehmite is much
closer to the trihydroxide lattices and so it is kinetically preferred. The monohy-
droxides are unstable with respect to the dense oxide above 730 K. Figure 8.9 also
contains the result for HAl
5
O
8
, a spinel structure containing H atoms in the
bulk, which was proposed as a candidate new composition for γ - Al
2
O
3
based on
DFT calculations [64] . However, at all temperatures this candidate structure is
considerably thermodynamically less stable than one of the alternatives. It is more
Figure 8.9 Calculated free energies of trihydroxides (solid
lines) and monohydroxides (dashed lines) relative to α - Al
2
O
3
.
The dotted line represents the calculated free energy for a
hypothetical hydrogen spinel structure postulated for γ - Al
2
O
3
.
Adapted from reference [61] .

likely that the apparent hydrogen content of γ - Al
2
O
3
is due to surface hydroxylation
on these high surface area materials, rather than the inclusion of H in the bulk
structure.
The fi rst oxide material produced in the series from boehmite, γ - alumina, is
important in catalysis as a widely used support material for precious metal cata-
lysts. For example, Pt/Al
2
O
3
used in steam reforming catalysts takes advantage of
the high surface area of γ - alumina along with a signifi cant metal - support interac-
tion which maintains Pt dispersion [65] . The surface areas of the other transition
aluminas δ - , θ - and η - Al
2
O
3
tend to be lower and so, in this application, they are
of less direct use. However, they may appear as minor phases in γ - alumina
samples, particularly after prolonged use at high temperatures, and so their struc-
ture and properties need to be considered. In addition, aluminas have useful Lewis
acid properties that have been used in reactions such as methyl chloride synthesis
and these are known to vary according to the phase of the material [66] .
The bulk structures of the transition oxides γ - , δ - , θ - and η - alumina are all based
on an fcc ( … ABCABC..) array of oxygen ions, whereas χ - , and α - Al
2
O
3
have hcp
( … ABABAB..) oxygen sub - lattices. α - Alumina has the corundum structure, with
cations well ordered and exclusively in octahedral co - ordination sites. For this
reason, the α - polymorph has been widely studied using both atomistic potential
and periodic quantum chemical approaches and we will discuss calculations of its
surface structure in the next section. The oxygen lattice of κ - Al
2
O
3
is intermediate
with a complex … ABACABAC … stacking sequence. Its structure was solved with
the aid of periodic DFT calculations based on the PW91 functional by Yourdsha-
hyan and coworkers [67] . While κ - Al
2
O
3
can be prepared by heat treatment methods,
it is more often obtained by chemical vapor deposition ( CVD ) and so is more
widely used for wear resistant coatings, for example on cemented - carbide cutting
tools, than in catalysis [68] .
Transition aluminas are metastable with respect to the α - phase and so this is
usually used as a reference point for comparing the energies of other phases.
Wolverton and Hass have used the PW91 functional to estimate δ E ( θ – α ) and
δ E ( κ – α ) obtaining 0.04 and 0.08 eV per formula unit, respectively. They point out
that these energies are within the upper bounds set experimentally, whereas LDA
calculations by the same authors are not. These positive energy differences confi rm
that the need for high temperatures to obtain α - Al
2
O
3
in the schemes shown in
Figure 8.7 is due to a kinetic barrier between phases.
The best ordered of the fcc - type materials is θ which has the same structure as
β - Ga
2
O
3
. In turn, δ - alumina appears more ordered that the γ - phase since it is
observed in diffraction experiments by the appearance of superstructure not
present in γ - Al
2
O
3
. Analysis of the diffraction data for transition aluminas gives
cation site occupancies of less than 1, that is, it is the cation sub - lattice that is dis-
ordered. This has led to suggestions that the transition series γ → δ → θ should
be thought of as a process of ordering the cations on the interstices of the fcc
oxygen lattice [69] . From a simulation point of view, the repeat unit in a calculation
cannot have partial occupancy of cation sites and so a choice must be made to give
8.3 Bulk Structure: Alumina 351

352 8 Theory: Periodic Electronic Structure Calculations
a representative structure. The structure of γ - alumina is the most challenging from
this perspective as it contains the highest degree of disorder. It has been consid-
ered for some time to have a lattice based on a disordered cubic spinel, a sugges-
tion that was fi rst made based on X - ray diffraction data [70] , although later work
found the cell to have a tetragonal distortion [71] . However, since this is a small
effect, the spinel model has been retained by most workers. The classic spinel
MgAl
2
O
4
has an oxygen sub - lattice forming an fcc - type array with Mg
2+
in tetrahe-
dral, T
d
, and Al in octahedral, O
h
, interstitial sites. The general formula for γ - Al
2
O
3
in this system is
Al O
21
1
3
䊐
2
2
3
32
, where the symbol 䊐 is used to indicate a vacancy
in a standard spinel cation site. This structure falls into the space group
Fd m3
and a simulation cell containing of 96 oxygen atoms appears necessary to give the
required Al
2
O
3
stoichiometry with eight cation vacancy sites. There is disagree-
ment in the experimental literature on how to apportion the Al cations in γ -
alumina between the two spinel sites. In 1991, X - ray and neutron diffraction were
combined to give lattice parameters for the cubic cell [72] . The line widths of spe-
cifi c refl ections were used to suggest that the Al tetrahedral sub - lattice is extremely
disordered and that the distribution of Al between tetrahedral and octahedral sites
is roughly 50 : 50. This assignment of the occupancy ratio was diffi cult as no refl ec-
tions from the octahedral sub - lattice alone are present in the diffraction pattern.
More recently
27
Al MAS NMR has been used to show that 70 ± 2% of Al ions
occupy octahedral sites [73, 74] , which, in the spinel structure, suggests that the
octahedral sub - lattice is less disordered than the tetrahedral. Even if a simulation
is restricted by assuming that only disorder on the tetrahedral sites need be con-
sidered, the number of cation arrangements in the simplest stoichiometric cubic
cell runs into hundreds of thousands [75] . This means that a complete survey with
fi rst principles methods is impracticable. To simplify the problem, Guti é rrez and
coworkers [76] used a smaller simulation cell based on the primitive rhombohedral
unit corresponding to the
Fd m3 spinel. They began with the spinel cubic cell of
stoichiometry Mg
8
Al
16
O
32
, and replaced the Mg atoms by Al to give a cell with a
surplus of cations (Al
24
O
32
). The corresponding primitive rhombohedral cell is one
quarter the volume and so would be Al
6
O
8
. Taking three of these primitive cells
as a simulation cell and creating two vacancies gives a cell containing Al
16
O
24
. The
distribution of two vacancies, required to correct the stoichiometry, gives only 14
inequivalent arrangements and so LDA calculations on the entire set become fea-
sible. They found an energetic preference for vacancy formation on the octahedral
Al sub - lattice with the lowest energy structure after geometry relaxation having
both vacancies at octahedral sites. The lowest energy structure in which one
vacancy was at an octahedral and one at a tetrahedral site was 0.16 eV/Al
2
O
3
higher
in energy. Wolverton and Hass also identifi ed a preference for Al to occupy tetra-
hedral sites by constructing a hypothetical rock salt structured simulation cell of
formula Al
3
O
3
and then removing one Al
3+
ion. Here the oxygen sub - lattice is fcc
and all Al are initially in O
h
positions but they were able to demonstrate an energy
lowering of around 1.2 eV per formula unit on relocating one of the Al ions to a
T
d
site.

8.4 Calculation of Surface Structure 353
Another approach to obtaining the structure of γ - Al
2
O
3
is to start from the parent
boehmite hydroxide and study the mechanism of dehydration. Boehmite contains
only octahedrally coordinated Al species and so in addition to the removal of H
2
O
the migration of Al must be studied. Raybaud and coworkers [63] have used
the PW91 functional and a plane - wave basis set (VASP code) to study a possible
mechanism in which dehydration is followed by Al migration. After dehydration,
the remaining oxygen atoms in the interlayer region of boehmite (see Figure 8.8 )
bridge between the layers. It is proposed that Al ions then migrate into tetrahedral
voids in this interlayer region. Using thermodynamic free energy estimates they
discuss the relative stability of the model oxides in terms of the population of these
tetrahedral sites. As the fi rst few Al ions are moved, the free energy increases,
passing through a maximum when 6 – 9% of the Al ions have been migrated, then
a minimum is identifi ed around 30% T
d
occupation, close to the experimental
solid - state NMR estimates.
Although a spinel lattice has been used by most simulations to date, there is
some evidence that cations may also occupy interstitial sites of the fcc oxygen sub -
lattice that are not used in the spinel structure. A structure with 40% cations in
non - spinel sites has been put forward by Paglia and coworkers [77] . Their work
combined atomic potentials calculations to survey thousands of possible cation
arrangements in both spinel and non - spinel locations. Then PW91 optimizations
were carried out for selected structures with the localized basis set code SIESTA.
This sub - set of structures was used to simulate the neutron diffraction pattern for
comparison with experimental data. The tetragonal unit cell was also supported
by transmission electron microscopy and solid - state NMR [74] . The idea of cations
taking up non - spinel sites had also earlier been commented on by Wolverton and
Hass for δ - Al
2
O
3
since its cell dimensions are often quoted as non - integer multi-
ples of the γ - phase. However, the debate is still on - going, with a recent comparison
of XRD data with periodic DFT results rejecting the new structure in favor of the
conventional disordered spinel [78] .
8.4
Calculation of Surface Structure
8.4.1
Slab Models
XRD and neutron diffraction methods provide highly accurate information on the
three - dimensional structure of crystalline materials. These methods depend on
the regular array of atoms that form the bulk lattice in well ordered phases.
However, the structure of the surface of oxides is much more diffi cult to probe
experimentally, since the surface layers make up a minute fraction of the total
sample. For some catalytically important materials for which large crystalline
samples can be grown, such as α - Al
2
O
3
and TiO
2
, grazing angle XRD [79] does
show surface sensitivity. Low energy electron diffraction ( LEED ) can also give data

354 8 Theory: Periodic Electronic Structure Calculations
on surface structure both in plane and perpendicular to the surface if the intensity
of spots as a function of electron acceleration voltage, and so energy, is considered:
the LEED - IV approach [80] . However, the resolution of surface structure is still
quite low compared to bulk techniques and care must be taken with surface prepa-
ration. Here computer simulation can provide insight into the restructuring that
occurs at the interface between a solid and its environment. Since the surface
region is where most of the important processes in heterogeneous catalysis take
place, the use of theoretical methods to consider surface structure has been an
area of intense interest.
There are two main approaches to describing a surface in a periodic computer
simulation model. A truly two - dimensional model requires dedicated program-
ming to allow periodicity in the surface vector directions but not perpendicular to
the surface. Some programs do include this [31] , but most are written with periodic
boundary conditions in all three dimensions and surface calculations require cells
that isolate the surface as a repeating set of slabs. This can be done by introducing
a vacuum gap in one of the lattice vector directions. All that is required is a simula-
tion cell orientated so that the desired surface is perpendicular to, say, the
c
- vector
direction and then a repeating set of slabs is created by extending the
c
- vector
without altering the atom co - ordinates in the cell. The simulation remains three
dimensional but a gap is opened in the direction perpendicular to the Miller plane
of interest at the required cut to create two surfaces. This is best done by choosing
a unit cell in which the
a
,
b
face is the required surface, as shown in Figure 8.10 .
In this example, the MgO(110) plane is to be studied but this is not perpendicular
to any crystallographic cell vector. However if we choose alternative vectors which
are in the plane (
a
′ =
a
+
b
and
b
′ =
c
in this case) the new cell will be orientated
with the
a
′ ,
b
′ plane perpendicular to
c
′ , as shown in Figure 8.10 b. The length of
the new
c
′ lattice vector is simply the interplane spacing, d
110
. The vacuum gap is
then introduced by simply extending the
c
′ - vector.
The creation of a surface requires energy and so the energy of the slab will be
higher than that of the solid formed by closing the gap again. This means that
once the slab is generated, volume optimization can no longer be undertaken
because such a calculation should lower the energy by removing the vacuum gap.
Figure 8.10 (a) The MgO(110) Miller plane and (b) a cell
orientated to make the
c
′ - vector perpendicular to this surface.
