Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

170 Karlsson and Björnsson
5. Add 1.25 mL of 8 M Gu-HCl, and 1.25 mL of H
2
O, and 1.25 mL of SAT reagent. Mix for
15 min. Centrifuge at 3300g for 15min. Transfer the supernatant to another tube. Deter-
mine absorbance of a suitable volume at 605 nm.
6. Add 22.5 mL of AB reagent. Leave for 2 h. Centrifuge at 3300g for 1h. Remove the
supernatant and discard. Let the tube drain upside down.
7. Add 10 mL of DMSO washing solution and mix until the pellet is suspended. Mix for 15 min.
8. Centrifuge at 3300g for 20 min. Remove the supernatant and determine absorbance of a
suitable volume at 605 nm. Let the tube drain upside down.
9. Dissolve the pellet in 1000 µL of Gu-prop-H
2
O solution (prepared without Ficoll). Mix
for 30 min or until the pellet is completely dissolved. Divide into four 2 mL propylene
vials (approximately 250 µL/vial).
10. Add 1750 µL of Prop-gu solution. Leave for 1h to precipitate the GAG/PG.
11. Centrifuge at 12,000g for 15 min. Remove the supernatants carefully, using a syringe, to
new tubes. Determine absorbance of a suitable volume at 605 nm. Loss of GAG/PG may
occur during the propanol precipitation/centrifugation in steps 10–11. Such losses are
revealed by comparison of the total absorbances calculated in step 11 and 13 (see Table 2).
12. Add 750 µL of Prop-Tris solution to the pellet. Mix. Centrifuge at 12,000g for 15 min.
Remove the supernatant and discard. Let the tube drain upside down. Enzymatic degrada-
tion of GAG/PG is inhibited if propanol is present in the pellet. Propanol may be evapo-
rated by drying at 37°C. Too intensive drying should be avoided, since the GAG/PG may
be difficult to redissolve.
13. Dissolve the pellet in a suitable buffer (see Note 1). Take a suitable volume for quantitation
(see Subheading 3.1.). Calculate total absorbance and the amount of GAG/PG.
Calculation. The recovery of GAG/PG in each step may be monitored by taking
the absorbance of a suitable portion and calculating the total absorbance. Care should
be taken not to exceed the actual absorbance range of the instrument since most ELISA
photometers are only reliable below an absorbance of 2.0. Calculate the total absor-
bance as follows: total volume of supernatant /volume in ELISA well × A
605
. The
approximate amount of GAG/PG is obtained by multiplying by 5.
The following results in Table 2 were obtained when 250 µg of shark CsC were
dissolved in 25 mL of human blood plasma. The absorbance was measured in each of
the steps indicated above. The purified GAG/PG was dissolved in 500 µL 4 M guani-
dine-HCl and 100 µL were taken for final quantitation (see Subheading 3.1.).
Comments: The figure obtained for step 5—supernatant overestimates the amount
of GAG/PG since it also contains some co-precipitating dye. This dye is removed by
washing in DMSO. The figure obtained for step 8—supernatant overestimates the
amount of dye washed away since the molar absorbance coefficient of Alcian blue is
higher in DMSO than in guanidine-HCL solution. The figure obtained for step 11—
supernatant is always higher than that obtained by final quantitation of the redissolved
purified GAG/PG sample. This is presumably because of GAG/PG losses during the
propanol precipitation/centrifugation in step 11 or by incomplete solubilization in step 13.
3.6. Dot-Blot Reflectance Assay (see Table 3)
The Alcian blue–GAG complexes are collected on a PVDF membrane, by filtration
in a dot-blot apparatus, and the stain quantitated as reflectance by scanning and densi-
tometry. The assay requires 10 µL of sample and has a measuring range of 10–800 ng

Alcian Blue Quantitation 171
of GAG. No interference from plasma proteins is evident when chondroitin sulfate,
keratan sulfate or heparan sulfate is dissolved in a blood plasma with low endogenous
GAG content (see Fig. 8). The dot-blot assay is sensitive enough to measure the
amount of GAG released upon coagulation; that is serum values (see Fig. 10) are
much higher than plasma values (see Fig. 9).
1. Put 10 µL of either blank (water), sample, calibrator, or control in duplicate in a 96-well
polystyrene microplate. All six calibrator levels, plus blank, should be used, as the cali-
bration curve is nonlinear.
2. Add 20 µL of a 1/1 mixture of 8 M GuHCl and SAT reagent.
3. Mix on a microplate shaker at 200 rpm for 15 min.
4. Add 200 µL of cold AB reagent. Mix as above for 60 min.
5. Assemble a 96-well MilliBlotD apparatus according to the instructions of the manufac-
turer, using a PVDF membrane wetted and blocked for 1 h in 1% (v/v) Triton X-100.
6. Add 200 µL of 0.4 M Gu-HCl/0.1% (v/v) sulfuric acid/0.25% (v/v) Triton X-100 to each
well immediately upon assembly (see Note 2). Evacuate approximately 100 µL by suc-
tion. Close the outlet tubing with a rubber stopper.
7. Transfer 200 µL of the samples from the microplate using an eight-channel pipet and the
reverse pipetting technique. Precipitates of GAG/PG–Alcian blue may form during incu-
bation in step 4. Any precipitates present at this point can be dispersed by repetitive
pipetting in the microplate well before transfer.
8. Evacuate the wells by suction.
9. Add 200 µL of 50% (v/v) ethanol in 0.05 M MgCl
2
and evacuate the wells by suction.
Repeat once
10. Remove the membrane from the apparatus and wash briefly in distilled water before air
drying. High blank values are caused by degraded Alcian blue stain. Degradation may
occur both in dry form and in liquid preparations (stock or AB reagent).
11. Scanning. Scanning is performed in the reflectance color mode with the gamma curve set
at 1.0 and the dynamic range adjusted by positioning the 256 gray scales over the actual
range displayed in the histogram window. The membrane is placed on top of one trans-
parent plastic sheet in order to increase the sensitivity. The green and blue channels are
discarded. The red channel is saved in gray-scale mode, Fig. 8.
12. Densitometry. The Scan Analysis software for Macintosh from Biosoft
®
(Cambridge, UK)
is used for densitometry (see Note 3). The manual mode is used without background
subtraction, and the densitometry is performed on a rectangle encompassing two dots of a
Table 3
Dot-Blot Reflectance Assay
Calibrator Dilution H2O GAG in 10µl
80 16 µL CsC stock 1984 800
40 1000 µL 80 mg/L 1000 400
20 1000 µL 40 mg/L 1000 200
10 1000 µL 20 mg/L 1000 100
5 1000 µL 10 mg/L 1000 50
2.5 1000 µL5 mg/L 1000 25
1.25 1000 µL 2.5 mg/L 1000 12.5
172 Karlsson and Björnsson
Fig. 9. Dot-blot assay of different GAGs in serum. Commercial preparations of glycosami-
noglycans were dissolved in water or in serum (0-80 mg/L) and quantitated with Alcian blue
using the dot-blot assay-❏- Chondroitin-6-sulfate; 䉬 Keratan sulfate; -❍- Heparan sulfate;
open symbols, GAG dissolved in water. Closed symbols, GAG dissolved in serum.
Fig. 10. Dot-blot assay of different GAGs in blood plasma. Commercial preparations of gly-
cosaminoglycans were dissolved in water or in blood plasma (0-80 mg/L) and quantitated with
Alcian blue using the dot blot assay. -❏- Chondroitin-6-sulphate; 䉬 Keratan sulphate; -❍-
Heparan sulphate; open symbols, GAG dissolved in water; closed symbols, GAG dissolved in
blood plasma.

Alcian Blue Quantitation 173
duplicate sample. The non linear relationship between reflectance and concentration is
evident (see Figs. 9 and 10).
13. Curve fitting. The arbitrary units obtained in Scan Analysis are pasted into the Ultra Fit
software from Biosoft
®
and multiplied by 0.01 in order to get manageably sized fac-
tors (see Note 3). The densitometry units (x axis) are plotted against the calibrator
values (y axis). The Cubic equation (a third-degree polynomial) is used for curve fitting.
In order to prevent minima appearing within the fitted curve, parameter c should be re-
stricted to values
> 0. The fitted equation is used to calculate the concentration of un-
known samples.
4. Notes
1. The pellet will always contain some degraded Alcian blue, which is not water soluble and
can be sedimented by centrifugation.
2. Uneven spots caused by air bubbles present underneath the membrane are prevented by
always having excess fluid beneath and above the membrane when assembling the appa-
ratus and applying the samples. Therefore, buffer should be added to each well immedi-
ately upon assembly.
3. Densitometry and curve fitting may be performed with any suitable software. A third-
degree polynomial or any other suitable equation may be used for curve fitting.
References
1. Scott. J. E. (1970) Histochemistry of Alcian blue I. Metachromasia of Alcian Blue,
Astrablau and other cationics and other phthalocyanin dyes. Histochemie 21, 277–285
2. Björnsson, S. (1993) Simultaneous preparation and quantitation of proteoglycans by pre-
cipitation with Alcian Blue. Anal. Biochem. 210, 282–291.
3. Björnsson, S. (1993) Size dependent separation of proteoglycans by electrophoresis in
gels of pure agarose. Anal. Biochem. 210, 292–298.
4. Björnsson, S. (1998) Quantitation of proteoglycans as glycosaminoglycans in biological
fluids using an Alcian Blue dot blot analysis. Anal. Biochem. 256, 229–237.
5. Farndale, R. W., Sayers, C. A., and Barrett A. J. (1982) A direct spectrophotometric
microassay for sulfated glycosaminoglycans in cartilage cultures. Connect. Tissue Res. 9,
247–248.
6. Farndale, R. W., Buttle, D. J., and Barrett A. J. (1986) Improved quantitaion and discrimi-
nation of sulphated glycosaminoglycans by use of dimethylmetylene blue. Biochim.
Biophys. Acta. 883, 173–177.

Cellulose Acetate Electrophoresis of GAGs 175
175
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
17
Cellulose Acetate Electrophoresis of Glycosaminoglycans
Yanusz Wegrowski and Francois-Xavier Maquart
1. Introduction
Electrophoresis on cellulose acetate membrane (zone electrophoresis) is a common
method for qualitative and semiquantitative analysis of glycosaminoglycan (GAG)
mixtures. The advantage of this method is its simplicity, rapidity, the possibility of
processing several samples at the same time, and the low cost of analysis. Apart from
cellulose acetate strips and electrophoresis apparatus, usually applied in diagnostic
laboratories for serum protein electrophoresis, no special equipment is needed (see
Note 1). Several original papers and book chapters describe this technique in different
running conditions; the most common is a monodimensional electrophoresis in biva-
lent cation buffer or pyridine/formiate buffer (1–3). However, no simple system can
separate all known GAGs in one run. The separation of different GAGs in one dimen-
sion can be done by a several steps method described by Hopwood and Harrison (4),
requiring careful temperature control and selective ethanol precipitation after each
run. Alternatively, the GAGs can be separated by a two-dimensional method (5), but
only one sample at once may be applied on the cellulose acetate sheet. The scope of
this chapter is to describe a simple method for rapid separation and visualization of the
most common GAGs from tissue or cell culture. Comparison of electrophoretic pro-
files before and after selective enzymatic treatment (see Chapters 32-36) or HNO
2
depolymerization of heparin/heparan sulfate (6) allows characterization in a single run
of the major fractions of GAGs in a given sample.
Glycosaminoglycans should be displaced from the protein core of proteoglycans
before analysis. This can be done by exhaustive digestion of proteins with nonspecific
proteases such as pronase or papain (7). Apart from corneal keratan sulfate, which is
bound to protein via an N-glycosyl linkage between N-acetylglucosamine and the amide
group of asparagine, O-glycosyl covalent bonding can be alternatively disrupted by a
beta-elimination reaction, which liberates all linked GAGs from the protein core (8).
The GAG samples have to be desalted and sufficiently concentrated (see Note 2). Tissue
176 Wegrowski and Maquart
or body fluids usually contain sufficient amounts of GAGs to be detected by cationic
dye staining without radioactive labeling (see Chapter 16). The limit of Alcian blue
staining in the case of electrophoresis is about 100 ng of individual GAG. The tech-
niques that use Safranin O (9) or ruthenium red (10) staining have a detection limit
even 100-fold lover, but they are complicated to use. When working with cell culture,
one can easily radiolabel the GAGs with
35
S-sulfate and/or
3
H-glucosamine. Tritium
labeling also permits the study of nonsulfated GAGs, such as hyaluronan or chon-
droitin. The methods for
35
S-sulfate labeling as well as beta-elimination are described
elsewhere (8).
We present here two simple variants of cellulose acetate electrophoresis. The first
one, published originally by Wessler (11), permits the separation of hyaluronan,
heparan sulfate, galactosaminoglycans (dermatan and chondroitin sulfates), and hep-
arin in 0.1 M HCl. The second one was used to separate galactosaminoglycans in 0.1
M zinc acetate, pH 5.1. If the electrophoresis is performed first in zinc acetate and then
in HCl, the separation of five glycosaminoglycans can be done. The simple method of
selective detection of
35
S-sulfate vs
3
H-glucosamine is also described.
2. Materials
2.1. Electrophoresis of Glycosaminoglycans
1. Cellulose acetate strips and electrophoresis apparatus (e.g., Titan III cellulose acetate plates
and Titan Zip Zone Chamber from Helena Research Laboratories, Beaumont, TX, USA, or
Sebiagel
®
and Electrophoresis Apparatus from Sebia, Issy-les-Moulineaux, France).
2. Glycosaminoglycan standards (Sigma, St. Louis, MO, USA). Dissolve each glycosami-
noglycan in deionized water at concentration 1 mg/mL. This is stable indefinitely if frozen.
3. 0.1 M zinc acetate buffer, acidified to pH 5.1 with acetic acid.
4. 0.1 M HC1.
5. Absolute ethanol.
6. Phenol red (Sigma). Saturated aqueous solution.
7. A microsyringue for sample application (e.g., Hamilton).
2.2. Staining with Alcian Blue
1. Stock solution of Alcian blue 8GX (Sigma): Prepare 0.4% (m/v) solution in absolute
ethanol. Filter through cotton or Kleenex or Büchner funnel. Stable indefinitely in the dark.
2. Staining buffer: 0.05 M Natrium acetate containing 0.1 M MgCl
2
, pH 5.8. Sodium azide
0.05 % (m/v), may be added to prevent bacterial development. Store at 4°C.
3. Staining solution: Mix 1-to-1 stock Alcian solution with staining buffer. May be reused
several fold until the formation of precipitate.
4. Destaining solution: Mix 1-to-1 staining buffer with ethanol.
2.3. Autoradiography of Glycosaminoglycans
1. Glycerol 2% (v/v) in absolute ethanol.
2. PPO solution: Dissolve PPO (2,5-diphenyloxasol, Merck, Darmstadt, Germany), 2%
(m/v) in glycerol-containing ethanol.
3. Autoradiography cassette with intensifying screens and autoradiography film.
4. Saran Wrap foil.
5. Developing facilities.
Cellulose Acetate Electrophoresis of GAGs 177
3. Method
3.1. Electrophoresis
1. Soak cellulose acetate membrane for at least 10 min in electrophoresis buffer with gentle
agitation (see Note 3).
2. Fill the electrode chambers with the same buffer.
3. Blot the membrane in filter paper to remove the excess of liquid. Do not dry. Immediately
install in electrophoresis apparatus (see Note 4).
4. Starting 8 mm from one border and about 1 cm from cathode (–) end, mark with a phenol red
a series of 5-mm traits indicating the places for sample loading. Leave a space of 5 mm
between the traits. Let the liquid dry.
5. Apply samples or standards by portions not exceeding 3 µL. Let the liquid dry every time.
Optimal loading quantity for staining is the equivalent of 1 µg of standard GAG and, for
autoradiography, 40,000 cpm as
35
S-sulfate.
6. Run the electrophoresis at room temperature for 2 h at constant voltage. The current at the
beginning should not exceed 1 mA/cm of width for zinc acetate or 1.5 mA/cm for HCl
(see Note 5).
7. If the electrophoresis is performed in 0.1 M HCl, fix the membrane (see step 9). If it is
done in zinc acetate buffer, remove the membrane and soak it for about 1 min in 0.1 M
HCl. At the same time, fill the buffer tanks of apparatus with 0.1 M HCl.
8. Blot and reinstall the membrane. Continue the electrophoresis for 1 h at the same
current.
9. After electrophoresis, the GAGs are fixed by soaking the membrane for about 1 min in
absolute ethanol.
3.2. Staining
1. Soak the membrane in Alcian blue staining solution for 30 min with gentle agitation.
2. Wash the membranes in three baths of destaining solution (see Note 6).
3. Wash the membranes in water if scanning or photography have to be performed. The color
is stable for several days. Figure 1a shows an example of electrophoresis of tissue GAGs
performed in zinc acetate/HCl solutions, followed by Alcian blue staining.
4. To dry the membranes, place them in anhydrous methanol for 1 min with gentle agitation,
then transfer for exactly 1 min to freshly prepared 18% (v/v) acetic acid in methanol. The
membranes are removed, deposited on glass plates, and dried at 80°C for 10 min. After
cooling at room temperature, the membranes are unstuck from the glass plate with a spatula
or scalpel. They may be stored indefinitely.
3.3. Autoradiography
1. Wash the membrane containing the radiolabeled GAGs in ethanolic glycerol. If the
labeling was performed with only one isotope, wash the membrane directly in PPO
solution (see step 2). Dry between Whatman filter papers. Install in cassette. Cover
with Saran foil and autoradiography film in a dark room. Keep at –80°C for 24 h for
40,000 cpm as
35
S-sulfate deposited. Less isotope needs longer exposition time.
Develop the film. Figure 1b shows an example of electrophoresis in 0.1 M HCl and
direct autoradiography.
2. For tritium autoradiography, wash the membrane in PPO solution. Dry and reexpose as
above. (Fig. 1c).
3. Optionally, after autoradiography, the bands can be excised, dissolved in xylene, and
counted by liquid scintillation.
178 Wegrowski and Maquart
4. Notes
1. Any horizontal electrophoresis apparatus may be easily adapted to support cellulose acetate
strips. The strips are lying in the gel platform and are connected with the electrode cham-
bers (buffer tanks) by Whatman no. 1 filter paper. Do not submerge the membrane.
2. The salts provoke lateral diffusion of GAGs. It can be easily eliminated by dialysis or
gel-filtration. Precipitated GAGs can be desalted by successive washing with 90%
ethanol and absolute ethanol, drying, and dissolving in water. For practical reasons,
maximal volume for electrophoresis should not excess 10 µL.
3. Use zinc acetate or HCl as electrophoresis buffer. Keep the liquid at 4°C before electrophoresis.
4. The membranes do not dry in the electrophoresis tank when the extremities sink in
the buffer.
5. The electrophoresis in zinc acetate buffer is performed at 80 V for a membrane of 10 cm
length and that performed in 0.1 M HCl at 40 V for the same membrane dimension.
6. If necessary, the membrane can be destained overnight at room temperature in a covered
tank, to avoid ethanol evaporation.
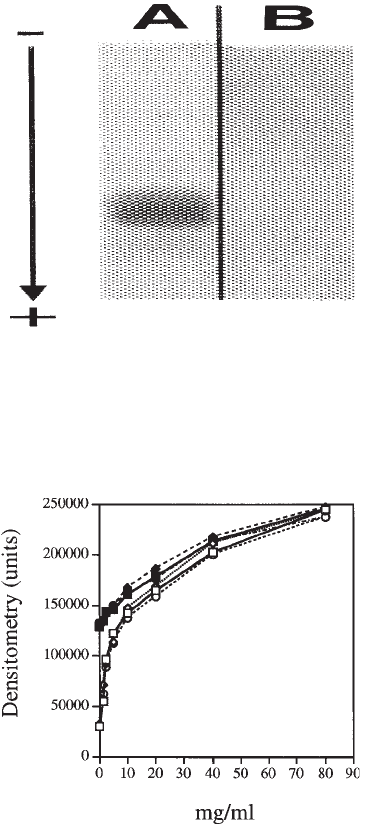
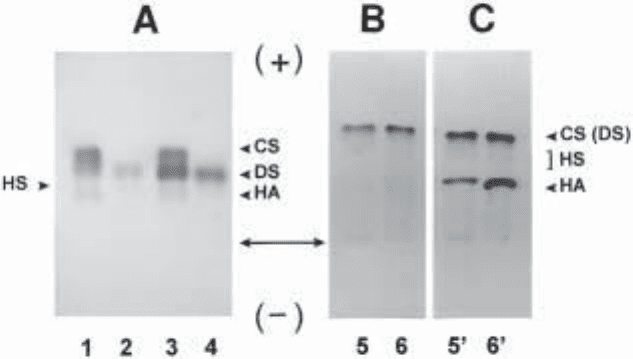
Fig. 1. Examples of electrophoresis in zinc acetate and HCl of tissue GAGs (A) and electro-
phoresis in HCl of
35
S-sulfate and
3
H-glucosamine labeled GAGs extracted from MRC5
fibroblast’s culture medium (B,C). Panel (A) shows the separation of 1 µg (as uronic acid) of
GAGs from fibrous tissue obtained from patient with type IV Ehlers-Danlos syndrome (1,2)
and control fibrous tissue (3,4) before (1,3) and after (2,4) chondroitinase AC treatment (12).
The GAGs were stained with Alcian blue. Note the higher proportion of dermatan sulfate in
control tissue. The panels at right (B,C) show the electrophoresis of the secreted, radiolabeled
GAGs from control cells (5,5') and the cells stimulated with 5 ng/mL each of TGFβ and EGF
(6,6'). Ten thousand cpm of
35
S-sulfate were deposited on each lane. The electrophoresis was
autoradiographied before (B) and after (C) impregnation with 2% (m/v) PPO. The migration
position of chondroitin sulfate (CS), dermatan sulfate (DS), heparan sulfate (HS), and
hyaluronan (HA) standards are indicated on the margins. The bi-directional arrow indicates the
application points. Anode and cathode extremities are indicated by (+) and (–), respectively.
Cellulose Acetate Electrophoresis of GAGs 179
References
1. Morris, J. E., Canoy, D. W., and Rynd, L. S. (1981) Electrophoresis with two buffers in
one dimension in the analysis of glycosaminoglycans on cellulose acetate strips. J.
Chromatogr. 224, 407–413.
2. Beeley, J. C. (1985) Characterization of charge, in Glycoprotein and Proteoglycan Tech-
niques (Burdon, R. H. and van Knippenberg, P. H. eds.), Elsevier, Amsterdam, The Neth-
erlands, pp. 88–94.
3. Kodama, C., Kodama, T., and Yosizawa, Z. (1988) Methods for analysis of urinary gly-
cosaminoglycans. J. Chromatogr. 429, 293–313.
4. Hopwood, J. J. and Harrison, J. R. (1982) High-resolution electrophoresis of urinary gly-
cosaminoglycans: an improved screening test for the mucopolysaccharidoses. Anal.
Biochem. 119, 120–127.
5. Hata, R. and Nagai, Y. (1973) A micro-colorimetric determination of acidic glycosami-
noglycans by two dimensional electrophoresis on a cellulose acetate strip. Anal. Biochem.
52, 652–656.
6. Cifonelli, J. A. (1976) Nitrous acid depolymerisation of glycosaminoglycans. Meth.
Carbohydr. Chem. 7, 139–142.
7. Breen, M., Weinstein, H. G., Blacik, L. J., Borcherding, M. S., and Sittig, R. A. (1976)
Microanalysis and characterization of glycosaminoglycans from human tissue via zone elec-
trophoresis. Meth. Carbohydr. Chem. 7, 101–115.
8. Rider, C. C. (1998) Analysis of glycosaminoglycans and proteoglycans, in Glycoanalysis
Protocols (Hounsell, E. F., ed.), Humana, Totowa, NJ, pp. 131–143.
9. Lammi, M. and Tammi, M. (1988) Densitometric assay of nanogram quantities of
proteoglycans precipitated on nitrocellulose membrane with Safranin O. Anal. Biochem.
168, 352–357.
10. Gaffen, J. D., Price, F. M., Bayliss, M. T., and Mason, R. M. (1994) A ruthenium-103 red
dot blot assay specific for nanogram quantities of sulfated glycosaminoglycans. Anal.
Biochem. 218, 124–130.
11. Wessler, E. (1971) Electrophoresis of acidic glycosaminoglycans in hydrochloric acid: a
micro-method for sulfate determination. Anal. Biochem. 41, 67–69.
12. Wegrowski, Y., Bellon, G., Quereux, C., and Maquart, F. X. (1999) Biochemical alterations
of uterine leiomyoma extracellular matrix in type IV Ehlers-Danlos syndrome. Am. J.
Obstet. Gynecol. 180, 1032–1034.

GAG Disaccharide Composition by CZE 181
181
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
18
Disaccharide Composition
in Glycosaminoglycans/Proteoglycans Analyzed
by Capillary Zone Electrophoresis
Nikos K. Karamanos and Anders Hjerpe
1. Introduction
During the past decade, the use of fully automated equipment for capillary electro-
phoresis (CE) has made this a routine method for the study of soluble analytes. The
separation of such compounds in capillaries (20–200 µm id) and in strong electric
fields (around 50 kV/m) seems to be exceptionally efficient for separating both large
and small molecules. The most common type of CE is capillary zone electrophoresis
(CZE), which is done with the separation buffer free in an otherwise empty capillary.
These separations, in principle, share some features not only with gel electrophoresis
but also with high-performance liquid chromatography (HPLC), and they provide a
unique combination of both analytical techniques. CZE is therefore an alternative to
HPLC and it is sometimes advantageous because there are no problems related to
laminar flow and other wall effects.
Other advantages of CZE, compared to HPLC and gel electrophoresis, are that it is
more friendly to the user and the environment. Only minute amounts of solvents are
needed, and the use of aqueous separation buffers in open capillaries eliminates the
need for toxic organic solvents and acrylamide.
The migration of analytes toward the detector depends on two main factors: electro-
phoretic mobility (EM), due to the net charge of the analyte; and electroosomotic flow
(EOF) of the free solution, caused mainly by dissociation of silanol groups in the capil-
lary glass wall and migration of resultant H
3
O
+
toward the anode. Both these effects can
be modulated to change the separation. The net charge of the analyte is readily modified
by ion pairing or ion suppression. The EOF of the solution depends on the pH and can be
completely blocked by the inclusion of detergents in the separation buffer or by coating
the inner capillary surface with hydrophobic agents or surfactants.
