Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

182 Karamanos and Hjerpe
Modern detectors yield an electropherogram that is similar to a chromatogram. The
ideal flow characteristics and resultant high resolution provide a considerably higher
sensitivity than HPLC in terms of analyte amounts, while the short light paths through
the detection window of the capillary often necessitate concentrations similar to those
of HPLC. Stacking of injected material, however, sometimes creates deviations from
this general rule to give exceptional sensitivity (1). Another way to improve the sensi-
tivity is to derivatize the analytes with fluorescent tags, allowing the use of laser-
induced fluorescence (LIF) detection (2,3).
The proteoglycans (PGs) with their glycosaminoglycan (GAG) side chains are
involved in a wide range of biological processes. Although the GAGs are synthesized
to yield only a few main types, the possible variations in structure are enormous (4).
Our knowledge of the structural background of specific GAG interactions and their
biological importance increases with improved methods for elucidation of the fine
structure. In such studies, CE can be powerful as an alternative tool or complement to
other analytical techniques. The various methods developed in this context have
recently been reviewed (5,6).
In this chapter we present protocols for the analysis of ∆-disaccharides obtained
from digestion of GAGs with specific lyases. These separations can be used to obtain
information on the total amounts of hyaluronan (HA) and galactosaminoglycans
(GalAGs)—i.e., chondroitin sulfate (CS) and dermatan sulfate (DS) (7)—and the disac-
charide composition of these GAGs, the latter including the type of uronic acid in DS,
sulfation patterns in CS/DS (1) or heparan sulfate (HS)/heparin, and the extent of N-acetyla-
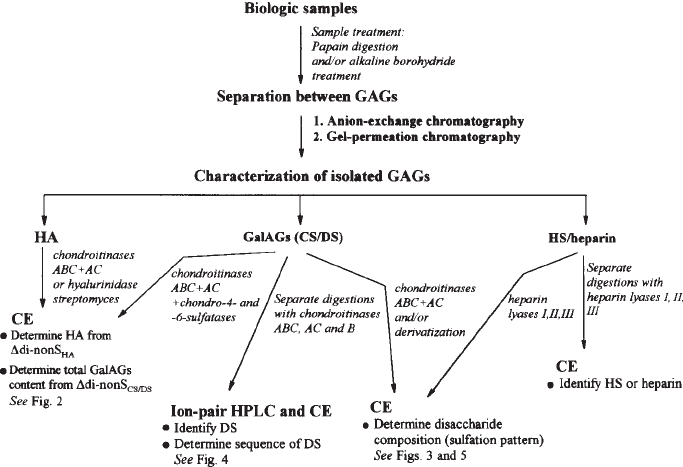
tion in heparin/HS (8,9). A strategy for analyzing all these GAGs is shown in Fig. 1.
Fig. 1. A possible strategy to identify and analyze GAGs.
GAG Disaccharide Composition by CZE 183
To our knowledge, there are no CZE-based methods for analyzing keratan sulfate dis-
accharide composition, although the availability of specific keratanases would make
such separations possible.
2. Materials
2.1. Total Content of HA and GalAGs
1. Digestion buffer: 25 mM Tris-HCl, pH 7.5. The buffer is passed through a 0.2-µm mem-
brane filter and kept at –20°C pending use.
2. Standard HA/CS solution: Prepare stock solutions with HA and CS (preferably use CSA of
mammal origin), each containing 1.0 mg/mL. Determine the exact GAG content in each
solution colorimetrically (for example, by the carbazole reaction). Make a standard solution
with 1 mL of each stock solution and 8 mL of the digestion buffer. Divide in portions and
store at –20°C until use.
3. HA/GalAGs degradation buffer: Dissolve chondroitinases ABC and AC as well as
chondrosulfatases-4 and -6 in the digestion buffer, so as to give 1 unit/mL. An equi-unit
mixture of all lyases is then prepared by mixing 10 µL of each enzyme to produce 40 µL
of the buffer containing 0.01 unit of each enzyme (see Note 1).
4. Capillary: Uncoated fused-silica (75-µm id, effective length 50 cm) (see Note 2).
5. Operating buffer: 15 mM sodium orthophosphate buffer, pH 3.0. The buffer is passed
through a 0.2-µm membrane filter, divided into portions of 1 mL, and kept at –20°C
pending use (see Note 3).
6. 0.1 M NaOH, prepared in 2 × distilled water and passed through a 0.2-µm membrane filter.
7. Centricon 3 membrane (cutoff 3000 daltons) microfuge tubes.
2.2. Structural Characterization of GalAGs
2.2.1. Analysis of
∆
-Disaccharides Using Ultraviolet Detection
1. Digestion buffer I: 50 mM Tris-HCl, pH 7.5. The buffer is passed through a 0.2-µm mem-
brane filter and kept at –20°C pending use.
2. Standard ∆-disaccharides: Prepare a stock solution (1.0 mg/mL) of nonsulfated (∆di-nonS
CS/DS
),
monosulfated (∆di-mono2S, ∆di-mono4S, and ∆di-mono6S), disulfated [∆di-di(4,6)S,
∆di-di(2,4)S, ∆di-di(2,6)S, previously referred to as E, B, and D types] and trisulfated
[∆di-tri(2,4,6)S, also referred to as ∆di-triS] chondro-/dermato-derived ∆-disaccharides by
dissolving the various disaccharides in digestion buffer I. Make serial dilutions (1/10,000,
1/5,000, and 1/1,000) in digestion buffer I so as to prepare standard ∆-disaccharide solu-
tions of 0.1, 0.2, and 1.0 µg/mL.
3. GalAGs degradation buffer: Dissolve chondroitinases ABC and AC in digestion buffer I to
give 1 unit/mL. An equi-unit mixture of both lyases is then prepared by mixing 10 µL of
each enzyme solution with 20 µL of digestion buffer I to produce 40 µL of the GalAG
degradation buffer containing 0.01 unit of each chondroitinase (see Note 1).
4. Operating buffer: 15 mM sodium orthophosphate buffer, pH 3.0. The buffer is passed
through a 0.2-µm membrane filter, divided in portions of 1 mL, and kept at –20°C, pending
use (see Notes 3 and 4).
2.2.2. Analysis of
∆
-Disaccharides Using Laser-Induced Fluorescence
1. Derivatizing agent: 0.1 M 2-aminoacridone (AMAC) is dissolved in glacial acetic acid/
DMSO (3/17 v/v).
2. Reductive agent: 1 M NaCNBH
3
dissolved in 2 × distilled water.
184 Karamanos and Hjerpe
2.2.3. Structural Characterization of DS
1. DS degradation buffer I: Chondroitinase AC is prepared by dissolving the enzyme in
digestion buffer I to give 1 unit/mL. Then 10 µL of the enzyme solution is mixed with
30 µL of digestion buffer I to produce 40 µL of DS degradation buffer I containing 0.01
unit of the enzyme.
2. Digestion buffer II: 50 mM Tris-HCl, pH 8.0. The buffer is passed through a 0.2-µm
membrane filter and kept at –20°C, pending use.
3. DS degradation buffer II: Chondroitinase B is prepared by dissolving the enzyme in diges-
tion buffer II to give 1 unit/mL. Then 10 µL of the enzyme solution is mixed with 30 µL of
digestion buffer II to produce 40 µL of DS degradation buffer II, containing 0.01 unit of the
enzyme (see Note 1).
2.3. Disaccharide Composition of heparin/HS
1. Digestion buffer III: 20 mM sodium acetate/acetic acid buffer, pH 7.0. The buffer is passed
through a 0.2-µm membrane filter and kept at –20°C pending use.
2. Standard ∆-disaccharides: Prepare a stock solution (1.0 mg/mL) of nonsulfated (acetylated
∆di-nonS
HS
, also referred to as a∆di-nonS
HS
or nonacetylated ∆di-nonS
HS
), monosulfated
(a∆di-mono6S
HS
, ∆di-mono6S
HS
, a∆di-mono2S
HS
, ∆di-mono2S
HS,
and ∆di-monoNS
HS
),
disulfated [a∆di-di(2,6)S
HS
, ∆di-di(2,6)S
HS
, ∆di-di(2,N)S
HS,
and ∆di-di(6,N)S
HS
], as
well as trisulfated [∆di-tri(2,6,N)S
HS
, also referred to as ∆di-triS
HS
] heparin-/HS-
derived ∆-disaccharides, by dissolving the various disaccharides in digestion buffer III.
Make serial dilutions (1/10,000, 1/5,000 and 1/1,000) in digestion buffer III to pre-
pare standard ∆-disaccharide solutions of 0.1, 0.2, and 1.0 µg/mL.
3. Heparin/HS degradation buffer: Dissolve heparin lyases I (EC 4.2.2.7), II (no EC number),
and III (EC 4.2.2.8) in digestion buffer III to give 1 unit/mL. An equi-unit mixture of all
three lyases is then prepared by mixing 10 µL of each enzyme solution with 10 µL of
digestion buffer III to produce 40 µL of the heparin/HS degradation buffer containing 0.01
unit of each heparin lyase (see Notes 1 and 5).
4. Operating buffer: 15 mM sodium orthophosphate buffer, pH 3.5. The buffer is passed
through a 0.2-µm membrane filter, divided in portions of 1 mL, and kept at –20°C pending
use (see Notes 3 and 6).
3. Methods
3.1. Total Content of HA and GalAGs
Biological samples or isolated GAGs can be easily analyzed for their content of
HA and total GalAGs, that is, CS and DS, by complete depolymerization of these
GAGs with a combination of specific lyases and chondrosulfatases. Combining
chondroitinases ABC and AC, both HA and GalAGs are converted to ∆-disaccha-
rides. Those derived from HA are nonsulfated and those from GalAGs contain vari-
ous numbers of sulfates. Most sulfate groups in mammals are esterified at C-4 and C-6
of the GalNAc and are eliminated by using digestion with both chondrosulfatases-4
and -6. Therefore, all HA is recovered in the ∆di-nonS
HA
peak and both CS and DS in
the ∆di-nonS
CS/DS
peak (see Fig. 1). These two peaks are completely resolved and the
contents of HA and total GalAGs are estimated using the following protocol. (See Note 7.)
1. Dissolve the samples containing approximately 0.1–10 µg of HA and/or GalAGs in 10
µL of digestion buffer. Place 10 µL of the HA/CS standard solution in separate vials.
GAG Disaccharide Composition by CZE 185
2. Add 40 µL of HA/GalAGs degradation buffer to the samples and standards. Digest for 90
min at 37°C (see Notes 8 and 9).
3. To remove nondegraded HS and protein/proteoglycans, centrifuge the digestion mixture on
a Centricon 3 membrane at 11,000g for 5 min (see Note 10).
4. Place a 5 - 10-µL portion of the filtrate in the electrophoresis vials and keep at 4°C pend-
ing use.
5. Start the CE instrument (see Note 11) and set the detector wavelength at 232 nm.
6. Prepare the software of CE instrument as follows:
a. Rinse the capillary with 0.1 M NaOH for 1 min.
b. Condition the capillary with operating buffer for 4 min (see Note 12).
c. Inject the sample at the cathode (reversed polarity), using the pressure mode
(500 mbar × s) (see Note 13).
d. Run the samples for 15 min at 40 kV/m and 25°C (see Notes 2 and 14).
e. Before each run, rinse and condition the capillary as in steps 6a, b.
7. After every four injections of the samples, inject the standard solution to check the perfor-
mance of the electrophoresis.
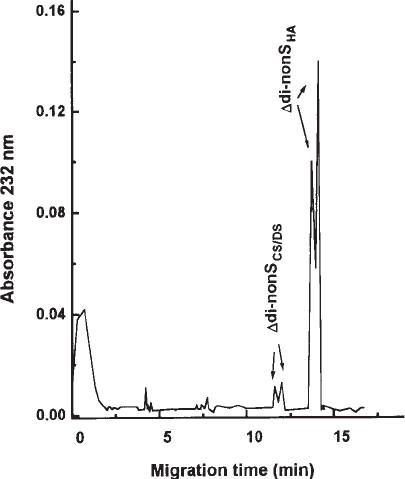
8. Calculate the amount of HA and total GalAGs, using the peak areas estimated and the
standard curve (see Fig. 2) (see Notes 8, 15 and 16).
3.2. Disaccharide Composition of GalAGs
3.2.1. Analysis of
∆
-Disaccharides Using Ultraviolet Detection
Following digestion of samples containing CS and/or DS with both chondroitinases
ABC and AC, all constituent nonsulfated and variously sulfated disaccharides of these
Fig. 2. Typical CZE profile obtained by combined digestion of HA and CS and/or DS with
chondroitinases ABC and AC and chondrosulfatases-4 and -6. (Reprinted with permission
from ref. 7.)
186 Karamanos and Hjerpe
GAGs are recovered as ∆-disaccharides. All CS/DS-derived ∆-disaccharides are
completely resolved and determined using direct UV detection with the following
protocol.
1. Dissolve the samples containing approximately 0.1–10 µg of CS and/or DS in 10 µL of
digestion buffer I.
2. Add 40 µL of GalAGs degradation buffer to the samples and digest for 90 min at 37°C
(see Note 7).
3. Centrifuge the digestion mixture in a microfuge tube at 11,000g for 5 min (see Note 8).
4. Place aliquots of the supernatant in the electrophoresis vials and keep at 4°C pending use.
5. Start the CE instrument, adjust the detector wavelength at 232 nm, prepare to use the method
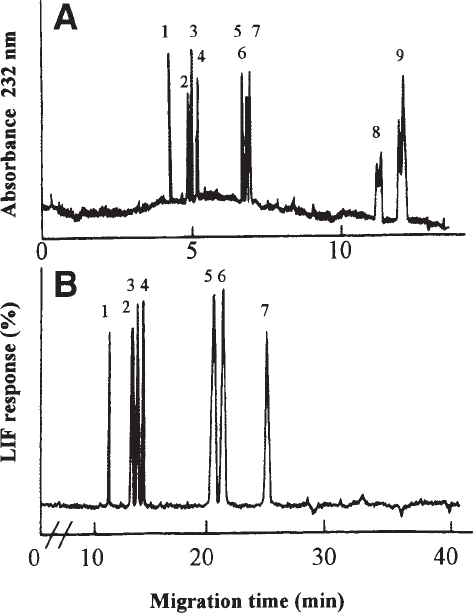
and analyze the samples, as described under Subheading 3.1, steps 6–8. (see Fig. 3A).
Fig. 3. Electropherograms showing the resolution of GalAG-derived ∆-disaccharides follow-
ing degradation of CS/DS with chondroitinases ABC and AC. (A) Direct UV detection at 232 nm
and (B) derivatization of the ∆-disaccharides obtained with fluorescent tag 2-aminoacridone and
detection using LIF (λ
exc
488 nm). In both cases, analysis is performed using 15 mM phosphate
buffer, pH 3.0. Peaks: 1 = ∆di-tri(2,4,6)S; 2 = ∆di-di(2,6)S; 3 = ∆di-di(2,4)S; 4 = ∆di-di(4,6)S; 5
= ∆di-mono2S; 6 = ∆di-mono4S; 7 = ∆di-mono6S; 8 = ∆di-nonS
CS/DS
; 9 = ∆di-nonS
HA
. Reprinted
from refs. 1 and 2, copyright 1995, 1999 Elsevier Science, with permission.
GAG Disaccharide Composition by CZE 187
3.2.2. Analysis of
∆
-Disaccharides Using Laser-Induced
Fluorescence (LIF)
To obtain higher sensitivities, LIF detection can be used to determine CS- and/or
DS-derived ∆-disaccharides. Therefore, following the same digestion scheme as in
Subheading 3.2.1., the variously sulfated CS- and/or DS-derived ∆-disaccharides
can be easily converted to fluorescent derivatives by reductive amination with
AMAC. CS/DS-derived sulfated ∆-disaccharides are completely separated by
reversed-polarity CZE and determined with Ar-laser source LIF detection, using the
following protocol.
1. Evaporate standard ∆-disaccharides and digestion mixtures in a microfuge tube at low
temperature.
2. Add 5 µL of each of the derivatizing and reductive agents to the dry residues.
3. Incubate the mixture at 45°C for 2 h (see Note 7).
4. Evaporate the mixtures to dryness, reconstitute in 50% DMSO, transfer to the electrophore-
sis vials and keep at 4°C pending use.
5. Start the CE instrument (see Note 11) and adjust the LIF detector at an excitation wave-
length of 488 nm.
6. Prepare the software of the CE instrument, as follows:
a. Rinse the capillary with 0.1 M NaOH for 1 min and 2 × distilled water for 0.5 min.
b. Condition the capillary with operating buffer for 4 min.
c. Introduce the samples at the cathode (reversed polarity), using the pressure mode
(500 mbar × s) (see Note 13).
7. Run the samples for 30 min at 40 kV/m and 25°C (see Notes 2 and 14). Estimate the
amount, as described under Subheading 3.1., steps 7 and 8. (see Fig. 3B; see Note 16).
3.2.3. Structural Characterization of DS
DS is a copolymer GAG constructed by both GlcA- and IdoA-containing repeating
disaccharide units. From a biological point of view, it is often important to know
whether DS is present in a GAG preparation. Differential digestion with chondroitinase
ABC and chondroitinase AC or B may provide useful information on this aspect. When
the total amount of ∆-disaccharides recovered following digestion by chondroitinase
AC is less than that obtained by ABC, this is evidence of the presence of DS. Further-
more, comparing the amount of ∆-disaccharides recovered, using separate digestions
with chondroitinase AC and B, we may well determine whether DS is rich in IdoA or
GlcA and, at the same time, the sulfation pattern of IdoA- and GlcA-containing disac-
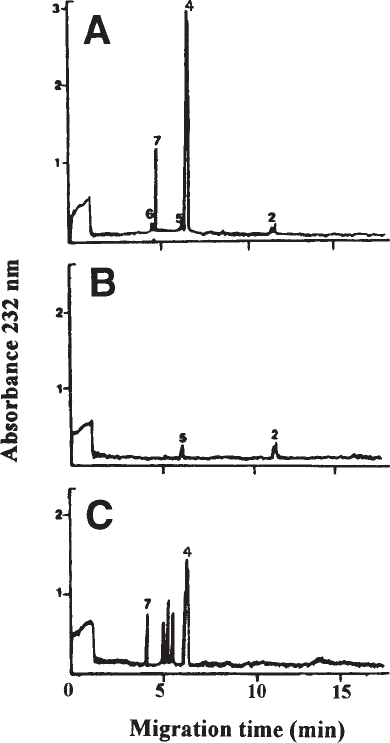
charides (see Fig. 4). The protocols are presented below.
1. Dissolve separately the samples containing DS in 10 µL of digestion buffers I and II (see Note 18).
2. To each one of them, add 40 µL of DS degradation buffers I and II, respectively, and
incubate for 90 min at 37°C.
3. Centrifuge the digestion mixture in a microfuge tube at 11,000g for 5 min.
4. Place aliquots of the supernatant in the electrophoresis vials and keep at 4°C pending use.
5. Start the CE instrument (see Note 11), adjust the detector wavelength to 232 nm, prepare
the method, and analyze the samples as described under Subheading 3.1, steps 6–8.
188 Karamanos and Hjerpe
3.3. Disaccharide Composition of heparin/HS
Heparin and HS can be almost quantitatively (>90%) degraded to ∆-disaccharides
by using all three heparin lyases (I, II, and III) in combination (See Note 5). All vari-
ously sulfated ∆-disaccharides and those containing N-acetylated and unsubstituted
GlcN at the amino group are completely resolved, using reversed-polarity CZE,
according to the following protocol.
Fig. 4. Analysis of DS isolated from porcine skin following separate digestion with
chondroitinase ABC (A), AC (B) and B (C). The electropherograms obtained show that DS is
rich in IdoA-containing disaccharides (very low susceptibility to chondroitinase AC in contrast to
that obtained with chondroitinase B) and that most of the GlcA-containing disaccharides of the
DS chain occur in short sequences (extra peaks representing ∆-oligosaccharides are obtained
when DS is treated with chondroitinase B). For identity of peaks (see Figure 3). Reprinted from
ref. 1, copyright 1995, Elsevier Science, with permission.
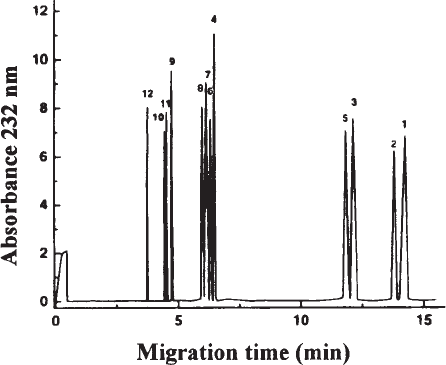
GAG Disaccharide Composition by CZE 189
1. Dissolve the samples containing approximately 0.1–10 µg of heparin and/or HS in 10 µL
of digestion buffer III.
2. Add 40 µL of heparin/HS degradation buffer to the samples and add 1 µmol calcium acetate
from a 2 M stock solution. Digest at 37°C overnight (see Note 8).
3. Centrifuge the digestion mixture in a microfuge tube at 11,000g for 5 min (see Note 9).
4. Place aliquots of the supernatant in the electrophoresis vials and keep at 4°C pending use.
5. Start the CE instrument (see Note 11), adjust the detector wavelength to 232 nm, prepare
the method, and analyze the samples as described under Subheading 3.1., steps 6–8
(see Fig. 5).
4. Notes
1. Digestion buffers containing the various lyases and chondrosulfatases should be divided
into small portions of 50–200 µL and kept in sealed plastic tubes at –20°C. Enzyme-
containing solutions should not be frozen and used again after thawing.
2. In each protocol, a certain capillary diameter and length are given. When capillaries with
different characteristics are available, the resolution may be tested using external standards.
Voltage can also be modified (±5–10 kV) to affect the rate of migration and resolution.
However, if no complete resolution is obtained, use the proposed capillary and the recom-
mended conditions. Large amounts of injected material may cause peak broadening. Opti-
mal peak shape can be obtained with £100 ng disaccharide injected.
3. Buffers and samples used in capillary electrophoresis should be handled carefully so as to
protect the capillary from particles and air bubbles. Therefore, all solutions should be passed
through a 0.2-µm membrane filter and degassed in an ultrasonic bath for 5–10 min.
Samples dissolved in low volumes are preferably centrifuged at 11,000g for 5 min.
4. A pH of 3.0 in the operating buffer is important for the resolution of the variously sulfated
∆-disaccharides and should be carefully adjusted. The separation is partly based on ion
Fig. 5. CZE profile showing the resolution of all 12 heparin-/HS-derived ∆-disaccharides. Analy-
sis is performed with 15 mM phosphate buffer, pH 3.50. Peaks: 1 = a∆di-nonS
HS
;
2 = ∆di-nonS
HS
; 3 = a∆di-mono6S
HS
; 4 = ∆di-mono6S
HS
; 5 = a∆di-mono2S
HS
;
6 = ∆di-mono2S
HS
; 7 = ∆di-monoNS
HS
; 8 = a∆di-di(2,6)S
HS
; 9 = ∆di-di(2,6)S
HS
;
10 = ∆di-di(2,N)S
HS
; 11 = ∆di-di(6,N)S
HS
; 12 = ∆di-tri(2,6,N)S
HS
. Reprinted from ref. 8, with
permission of Wiley - VCH, STM Copyright of Licenses.
190 Karamanos and Hjerpe
suppression (pK
a
of the carboxyl groups is just above 3 and slightly different in the vari-
ous disaccharides). Slightly modified pH values (±0.1 pH unit) therefore affect the net
charge and the separation of ∆di-mono4S and ∆di-mono6S and decreased pH may cause
close to infinite retardation of nonsulfated ∆-disaccharides.
5. Due to the different specificities of the three heparin lyases (type of uronic acid and posi-
tion of GlcN sulfate), it is possible to obtain separate information on HS- and heparin like
sequences in the analyte (9). The amounts of enzyme recommended represents an excess
to warrant maximal yield of disaccharides, but can be expensive when analyzing large
series of samples. Less than 10% of these amounts is sufficient, but one should then be
sure that the amounts of GAG is 1 mg or less.
6. The pH of 3.50 in the operating buffer and reversed polarity are critical for the resolution
of the heparin- and HS-derived ∆-disaccharides carrying N-acetylated, N-sulfated, or
unsubstituted GlcN. The pH should be checked carefully, since slightly modified pH values
during electrophoresis (±0.1 pH) affect the separation (see Note 4). Nonsulfated,
nonacetylated ∆-disaccharide migrates very slowly and can sometimes be difficult to recover
at all. In this case, it can be recommended to run one separation in reversed polarity as above,
followed by a second separation with normal polarity, moving this ∆-disaccharide by EOF.
7. Biological samples or tissue extracts taken for analysis of HA and total GalAGs content can be
concentrated by precipitation with 4 volumes of 90% (v/v) ethanol, also containing 2.5% (w/
v) sodium acetate. Small amounts of dextran can be used as carrier. Digestion buffer contain-
ing the lyases and chondrosulfatases is then added directly to the precipitate. Following
heating to stop the enzymic effects, the digests should be centrifuged at 11,000g for 5 min.
8. Incubation times and the units of the enzymes indicated should not be exceeded, since some
enzyme preparations may contain contaminating lyase activities. Enzymic digestions should
be terminated by boiling the incubation mixtures in a water bath for 1 min.
9. Evaporation during incubation necessitates the use of sealed, capped tubes. Drops on the
tube walls are recovered at the same time as particles are removed by centrifugation at
11,000g for 5 min.
10. Removal of chondroitinase-resistant macromolecules is obtained by ultrafiltration in
Centricon 3 membrane tubes. However, overly long centrifugation times may cause the
membrane to dry and break and therefore should be avoided. In the absence of experience
with the handling of these membrane tubes, use them only once.
11. When the capillary is mounted into the capillary cassette, care should be taken to keep the
detection window clean. Preferably, use gloves when handling the capillary!
12. Due to some electrolysis during the electrophoretic separation, the operating buffer char-
acteristics (ionic strength and pH) may be affected. Therefore, replace the operating buff-
ers frequently (at least every 5 runs) and run frequent standards (once for each new buffer
vial) to ensure uniform performance.
13. When using pressure injection, these protocols give suitable parameters for pressure
(mbar) × time (s). When working with low concentrations, it is always possible to increase
the injection times without significantly affecting the resolution. However, use the same
injection conditions for samples and standards.
14. Electrical current and temperature should be constant during electrophoresis, since changes
may give extra peaks or baseline drift. The current should therefore be monitored through-
out the run. Temperature also influences the viscosity of the solution and may therefore
affect resolution and quantification. Performance can be considerably improved, by creating
a “concentration zone” during injection. If the analyte is dissolved in water with as little
electrolyte as possible, the conductivity in the zone of injected material will be low, hence
GAG Disaccharide Composition by CZE 191
increasing the electric field through the injected solvent. This causes the analyte to move
rapidly through this zone and it becomes concentrated when entering the running buffer
(similar to what happens in TLC when using a concentration zone). To obtain such condi-
tions one can use volatile buffers for digestion (for example, ammonium formate instead
of those conventionally recommended), followed by evaporation of the digested material
and redissolving it in pure water. The EOF will push the injected low-electrolyte solvent
backward out of the capillary, not interfering at the detection window.
15. The amounts of HA and GalAGs are preferably given in terms of uronic acid per milliliter,
since the presence of crystal water and the poorly defined weights of the counterions make
it difficult to determine the weight of a GAG preparation correctly. Therefore, GAG stan-
dards with a well-defined amount of uronic acid (carbazole reaction) can be used. This also
monitors the efficiency of the enzymic digestion. Alternatively, the quantity of HA and total
GalAGs in samples can be determined, based on standard curves and using commercially
available ∆-disaccharides, as under Subheading 3.2.
16. Disaccharides sulfated in uronic acid migrate earlier than the main nonsulfated peak (mainly
in the region of monosulfated disaccharides). However, in most CS preparations from
mammalian tissues this peak will be minute and the calculation more readily made when
relating to a mammalian CS standard. When analyzing CS/DS preparations with significant
amounts of uronic acid sulfation, also include a ∆di-di(2,4)S standard (see Subheading
2.2.1) when digesting the standard mixture.
16. LIF detection of AMAC-conjugated ∆-disaccharides increases the sensitivity at least 100
times more than that obtained by UV detection at 232 nm of underivatized ∆-disaccharides.
However, the AMAC derivatives of the nonsulfated ∆-disaccharides do not appear on the
electropherogram, due to the effect of the fluorochrome. The advantage of this
derivatization is its sensitivity, which permits the study of the sulfation pattern with
less sample present. The AMAC labeling also allows the non reducing end of the GAG
chain to be studied. This fragment does not carry the UV-absorbing ∆
4,5
-structure.
Recent studies indicate that this part of the chain may be of particular biological impor-
tance (10,11).
18. When DS is digested with only one of the chondroitinases AC or B, these digests will also
contain ∆-oligosaccharide fragments. They can be identified in the electropherogram, thereby
providing information also regarding longer DS sequences.
References
1. Karamanos, N. K., Axelsson, S., Vanky, P., Tzanakakis, G. N., and Hjerpe, A. (1995)
Determination of hyaluronan- and galactosaminoglycan-derived disaccharides by high-per-
formance capillary electrophoresis at the attomole level. Applications to analyses of tissue
and cell culture proteoglycans. J. Chromatogr. A 696(2), 295–305.
2. Lamari, F., Theocharis, A., Hjerpe, A., and Karamanos, N. K. (1999) Ultrasensitive capil-
lary electrophoresis of sulfated disaccharides in chondroitin/dermatan sulfates by laser-
induced fluorescence after derivatization with 2-aminoacridone. J. Chromatogr. B. 730,
129–133.
3. Lamari, F. and Karamanos, N. K. (1999) High-performance capillary electrophoresis as
a powerful analytical tool of glycoconjugates. J. Liq. Chromatogr. Rel. Technol. 22,
1295–1317.
4. Karamanos, N. K. (1999) Proteoglycans: biological roles and strategies for isolation and
determination of their glycan constituents, in Proteome and Protein Analysis (Kamp, M.,
Kyriakides, D., and Choli-Papadopoulou, T. eds.), Springer-Verlag, Heidelberg, Germany,
pp. 341–363.
