Hillert M. Phase Equilibria, Phase Diagrams and Phase Transformations: Their Thermodynamic Basis
Подождите немного. Документ загружается.

7.4 Two-phase equilibria in binary systems 135
.
α
β
α is stable
α+β is stable
β is stable
AB
G
m
G
m
(b)(a)
x
B
α
x
B
β
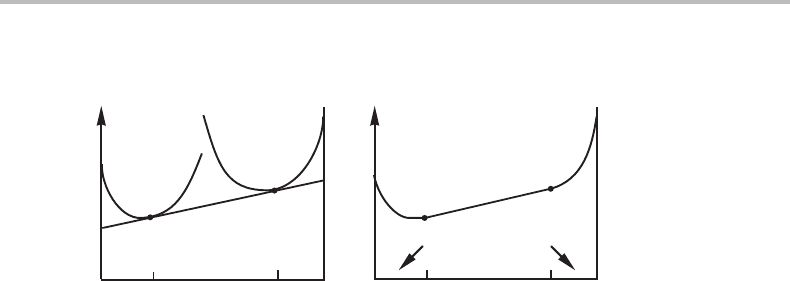
Figure 7.10 The common-tangent construction for finding the compositions of two phases in
equilibrium at given T and P.
Solution
Let the amounts of the components be N
Fe
, N
C
and N
Mn
in the old description and
N
Fe
3
C
, N
C
and N
Mn
in the new one. Denote the chemical potentials in the new description
with µ
i
. The mass balance for each element gives N
Mn
= N
Mn
, N
Fe
= 3N
Fe
3
C
and N
C
=
N
C
+ N
Fe
3
C
.
We thus get
µ
i
dN
i
=
µ
i
dN
i
= µ
Fe
dN
Fe
+ µ
Mn
dN
Mn
+ µ
C
dN
C
= µ
Fe
·
3dN
Fe
3
C
+ µ
Mn
dN
Mn
+ µ
C
dN
C
+ µ
C
dN
Fe
3
C
= (3µ
Fe
+ µ
C
)dN
Fe
3
C
+ µ
C
dN
C
+
µ
Mn
dN
Mn
.
Thus, µ
C
= µ
C
, µ
Mn
= µ
Mn
and µ
Fe
3
C
= 3µ
Fe
+ µ
C
.
7.4 Two-phase equilibria in binary systems
In a two-phase equilibrium we have the following two conditions at constant T and P
because the chemical potential for each component must be the same in the two phases.
G
α
B
= µ
B
= G
β
B
(7.27)
G
α
A
= µ
A
= G
β
A
. (7.28)
It is evidentthat these conditions can be satisfied onlybya common tangent,asillustrated
in Fig. 7.10(a). The lowest possible G
m
for each composition is shown in Fig. 7.10(b) and
it is evident that some mixture of α + β represents the stable state for an alloy between
the two tangent points.
Forastoichiometric phase with its well-defined composition, it is not possible to
define the chemical potentials since one can draw different tangents without changing
the composition of the point of tangency markedly. On the other hand, the chemical
potentials of a phase can be defined by equilibrium with a second phase. Figure 7.11(a)
illustrates this case when the second phase is a solution phase or (b) another stoichiometric
phase. When it is a solution phase, we obtain
G
β
m
= x
θ
A
· G
β
A
x
β
B
+ x
θ
B
· G
β
B
x
β
B
. (7.29)
136 Applications of molar Gibbs energy diagrams
.
β
G
m
G
m
x
B
θ
x
B
β
x
B
θ
x
B
φ
θ
µ
A
µ
B
µ
A
µ
B
θ
φ
(a) (b)
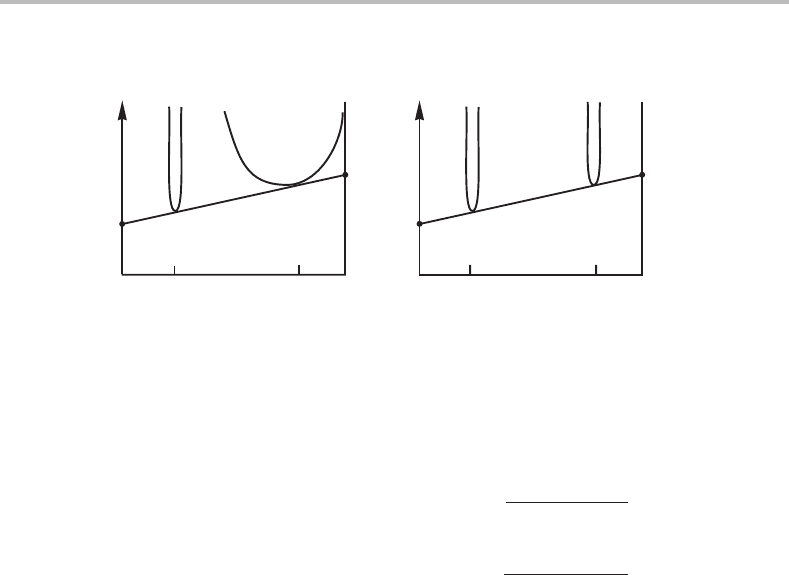
Figure 7.11 Molar Gibbs energy diagram showing the equilibrium between (a) a stoichiometric
phase and a solution phase and (b) between two stoichiometric phases.
By solving this equation, one can determine the composition of the solution phase, β,
and from the known properties of β one can then calculate µ
A
and µ
B
.InFig. 7.11(b)
both phases, θ and φ, are stoichiometric phases, and we obtain the following relations
G
θ
m
= x
θ
A
µ
A
+ x
θ
B
µ
B
; µ
A
=
x
θ
B
G
φ
m
− x
φ
B
G
θ
m
x
θ
B
− x
φ
B
(7.30)
G
φ
m
= x
φ
A
µ
A
+ x
φ
B
µ
B
; µ
B
=
x
θ
A
G
φ
m
− x
φ
A
G
θ
m
x
θ
A
− x
φ
A
. (7.31)
These equations also apply to the equilibrium between two solution phases if the G
θ
m
and G
φ
m
are evaluated for the equilibrium compositions.
Exercise 7.5
In a binary system, where the mutual solubilities are very small, there are two stable
stoichiometric phases α(A
3
B
2
) and β(AB
3
). Calculate the chemical potential of B in a
50 : 50 alloy in terms of the quantities G
α
m
and G
β
m
. Base the calculation on a construction
in the G
m
diagram.
Hint
Remember that the Gibbs energy of a two-phase state falls on the common tangent.
Start by drawing a solid line representing all stable states. It should show that both
stoichiometric phases are stable.
Solution
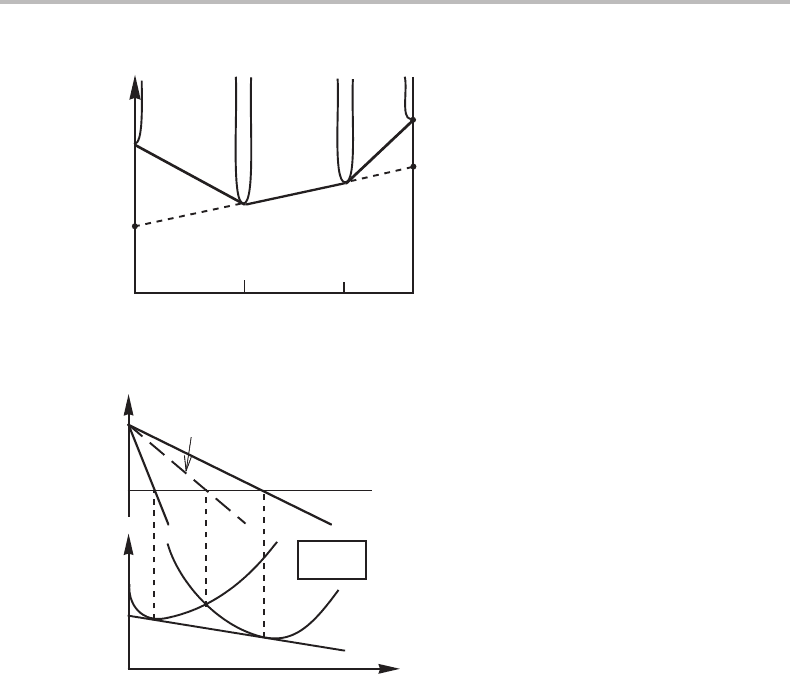
Evidently, the alloy is α + β (see Fig. 7.12). With x
α
A
= 0.6 and x
β
A
= 0.25 the con-
struction gives µ
B
= µ
α+β
B
= G
α
m
+ (G
β
m
− G
α
m
) · (x
α
A
− 0)/(x
α
A
− x
β
A
) = G
α
m
+ (G
β
m
−
G
α
m
) · 60/(60 − 25) = (60/35)G
β
m
− (25/35)G
α
m
.
7.5 Allotropic phase boundaries 137
G
m
o
G
A
o
G
B
µ
A
α+β
µ
B
α+β
α
β
A
B
Figure 7.12 Solution to Exercise 7.5.
α
β
α
β
T
T
1
T
o
G
m
x
B
T =T
1
.
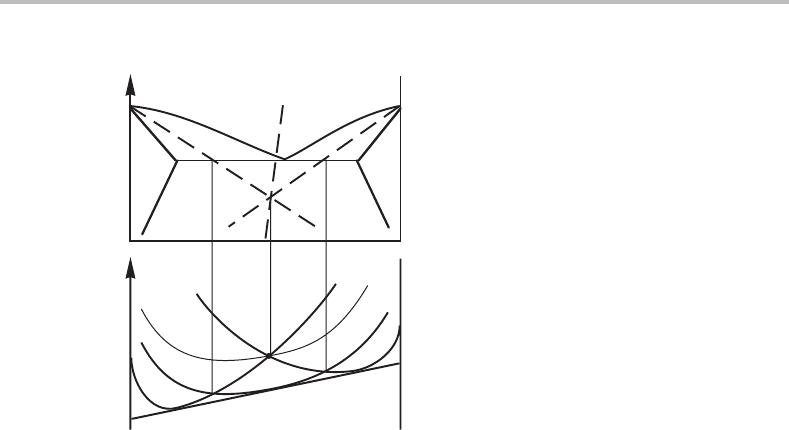
Figure 7.13 The relation between Gibbs energy curves of two phases and their two-phase fields in
the phase diagram. The dashed line is the so-called T
o
line or allotropic phase boundary.
7.5 Allotropic phase boundaries
One can sometimes draw a line inside a two-phase region to show where the two phases
would have the same Gibbs energy value if they had the same composition. It is the critical
limit for a hypothetical diffusionless phase transformation. Such a transformation is very
similar to an allotropic transformation in a pure element and the line, sometimes called
the allotropic phase boundary,isoften denoted by T
o
. This name is derived from the
word ‘allotropy’, i.e. the property of a substance of being found in two or more forms.
Figure 7.13 illustrates the relation between the allotropic phase boundary and the molar
Gibbs energy diagram.
Exercise 7.6
Consider a binary system with three phases of variable compositions and in a eutectic
equilibrium (see Section 12.5) with each other. Show reasonable positions of the three
allotropic phase boundaries. Extrapolate all of them below the eutectic temperature.
138 Applications of molar Gibbs energy diagrams
α
β
β
G
m
L
L
T
T
o
L
α
α+β
Figure 7.14 Solution to Exercise 7.6.
Hint
Consider in particular howthe three allotropic phase boundaries intersect each other when
extrapolated. Will there be one or three points of intersection? It may be instructive to
sketch a molar Gibbs energy diagram.
Solution
It is evident that T
α+L
o
and T
L+β
o
will intersect inside the α + β phase field. Consider
a G
m
diagram (Fig. 7.14)atthe eutectic temperature, showing one G
m
curve for each
phase (thick lines in the lower portion of the figure). Each one of the three intersections
is a point on a T
o
line. When the temperature is decreased, the L curve is lifted (see thin
line) relative to the other two until all three curves finally intersect in one point. There
the three T
o
lines will intersect. In the phase diagram (upper portion) we should thus
draw the T
α+β
o
line through the intersection of the other two.
7.6 Effect of a pressure difference on a two-phase equilibrium
In order to illustrate the effect of pressure, we shall consider an incompressible phase,
β. The application of a hydrostatic pressure will lift up its Gibbs energy curve by the
amount P
β
V
β
m
. This is illustrated in Fig. 7.15(a). Since V
β
m
is usually dependent on the
composition, the curve will be somewhat deformed. The construction with a tangent will
yield P
β
V
β
A
and P
β
V
β
B
where V
β
A
and V
β
B
are defined from V
β
m
in the same way as G
β
A
and G
β
B
are defined from G
β
m
(see Fig. 4.6).
The relative position of the Gibbs energy curves for different phases can change with
pressure due to differences in the molar volumes. The equilibrium conditions can thus
be modified by the application of a hydrostatic pressure. This effect is even stronger if
the pressure is applied to one of the phases only, which may happen due to the effect
7.6 Effect of a pressure difference on a two-phase equilibrium 139
G
m
(a) (b)
G
m
.
θ
∆x
B
α
α
P
β
V
A
β
G
m
β
(P
β
)
P
β
V
m
β
P
β
V
B
β
P
θ
V
B
θ
x
B
β
G
m
β
(0)
Figure 7.15 (a) Effect of a hydrostatic pressure on the molar Gibbs energy of a single phase. The
phase is assumed to be incompressible. (b) The effect of pressure in a stoichiometric phase on
the equilibrium composition of a coexisting phase not under pressure.
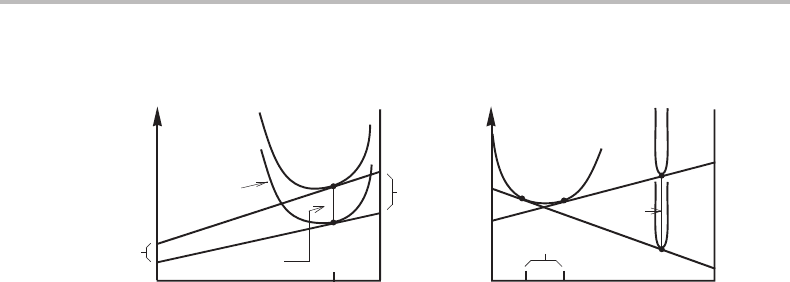
of surface energy in a curved interface. In Fig. 7.15(b) the phase under pressure is
a stoichiometric phase and its molar Gibbs energy is increased by P
θ
V
θ
m
.Wemay,for
instance, imagine that the phases are contained in a cylinder where the balance of surface
energies, σ ,atthe wall of the cylinder gives a constant radius of curvature ρ = 2σ/P
θ
.
The diagram illustrates that the solubility of θ in α is increased due to the pressure
in θ, assuming ordinary pressure in α.Itshould be emphasized that this case must be
treated with care because the two phases are under different pressures and the law of
additivity does not apply to the Gibbs energy unless special precautions are taken. This
was mentioned in Section 3.4 and will be discussed in Section 16.7.However, in applying
the common-tangent construction we have only made use of the definition of the chemical
potentials.
The effect on the solubility can be estimated if one knows the curvature of the G
α
m
curve. The difference in slope between the two tangents can be estimated as [x
α/θ
B
(P
θ
) −
x
α/θ
B
(0)] · d
2
G
α
m
/dx
2
B
if the change in composition is small. If the distance between the
two phases is reasonably constant, we obtain, by multiplying with that distance,
P
θ
V
θ
m
=
x
θ
B
− x
α
B
x
α/θ
B
(P
θ
) − x
α/θ
B
(0)
· d
2
G
α
m
dx
2
B
(7.32)
x
α/θ
B
(P
θ
) − x
α/θ
B
(0) = P
θ
V
θ
m
(x
θ
B
− x
α
B
)d
2
G
α
m
dx
2
B
. (7.33)
When α is a dilute solution we may approximate d
2
G
α
m
/dx
2
B
with RT/x
α/θ
B
according to
Eq. (7.19) and obtain
x
α/θ
B
(P
θ
) − x
α/θ
B
(0) = P
θ
V
θ
m
x
α/θ
B
RT
x
θ
B
− x
α
B
. (7.34)
This equation is often applied to a spherical interface and 2σ/r is then substituted for P
θ
.
In that form it is known as the Gibbs–Thomson equation. For large changes in solubility
one should integrate over the pressure increase and allow x
α/θ
B
on the right-hand side to
vary during the integration. For an infinitesimal increase in P
θ
we get
dx
α/θ
B
x
α/θ
B
=
V
θ
m
RT
x
θ
B
− x
α
B
· dP
θ
. (7.35)
For the case where (x
θ
B
− x
α
B
)isreasonably constant integration yields
ln
x
α/θ
B
(P
θ
)
x
α/θ
B
(0)
= P
θ
V
θ
m
RT
x
θ
B
− x
α
B
. (7.36)
140 Applications of molar Gibbs energy diagrams
G
m
(a)
G
m
(b)
G
m
β
(0) G
m
β
(0)
G
m
α
G
m
α
G
m
β
(P
β
) G
m
β
(P
β
)
P
β
V
β
m
P
β
V
β
A
P
β
V
β
B
∆x
B
β
∆x
B
∆G
m
∆x
B
α
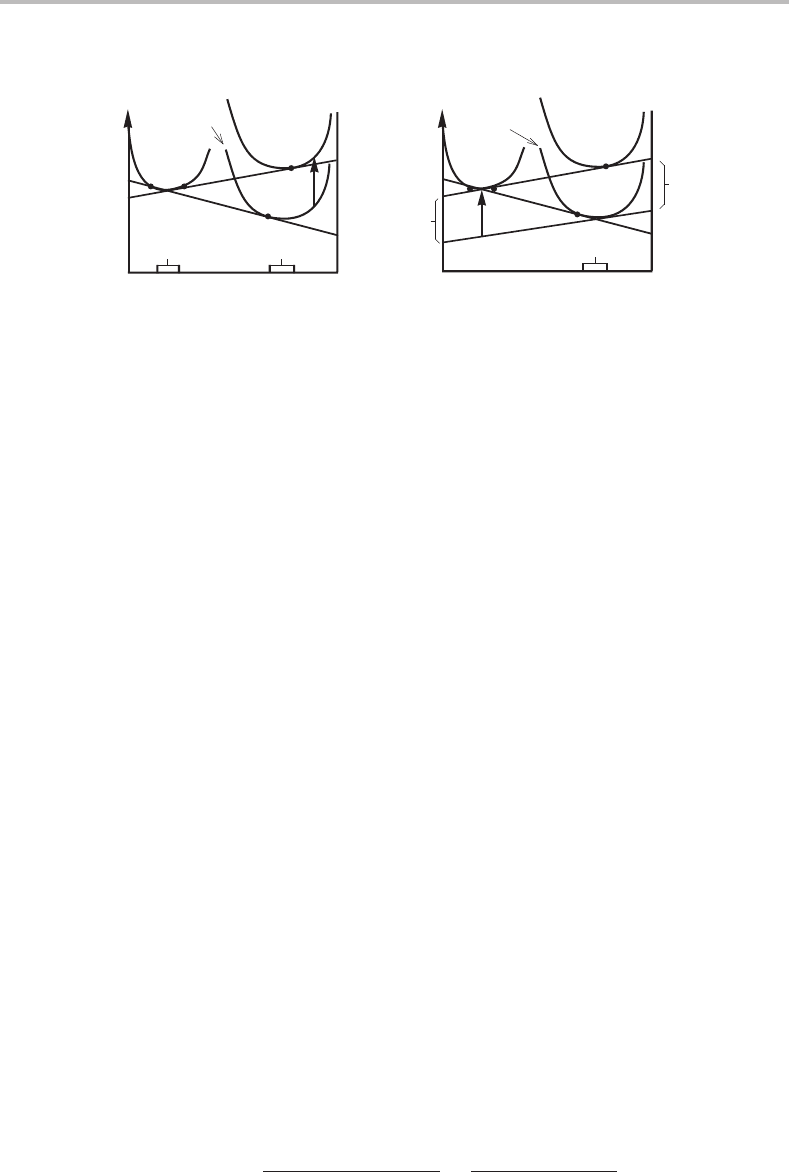
Figure 7.16 Molar Gibbs energy diagram illustrating the change in composition of a phase, β,
under pressure when in equilibrium with another phase, α.
If the phase under pressure can also vary in composition, its equilibrium composition
will also change. This case is illustrated in Fig. 7.16. The change in composition of the
phase under pressure can be evaluated from Fig. 7.16(b) where a tangent to the initial
G
β
m
curve has been drawn for the β composition of the new equilibrium. The diagram
defines a quantity G
m
which is given as
G
m
= P
β
x
α
A
V
β
A
+ x
α
B
V
β
B
, (7.37)
but also as
G
m
=
x
β
B
− x
α
B
· d
2
G
β
m
dx
2
B
·
x
β/α
B
(P
β
) − x
β/α
B
(0)
, (7.38)
if d
2
G
β
m
/dx
2
B
is reasonably constant. By equating the two expressions we obtain
x
β/α
B
(P
β
) − x
β/α
B
(0) = P
β
x
α
A
V
β
A
+ x
α
B
V
β
B
x
β
B
− x
α
B
· d
2
G
β
m
dx
2
B
. (7.39)
If the β phase is a dilute solution of B in A, then x
α
A
V
β
A
+ x
α
B
V
β
B
is approximately equal
to the molar volume for pure A in the β state, which we shall simply denote by V
β
m
, and
d
2
G
β
m
/dx
2
B
can be approximated by RT/x
β
B
according to Eq. (7.19), yielding
dx
β/α
B
/x
β/α
B
=
V
β
m
RT
x
β
B
− x
α
B
· dP
β
. (7.40)
We can take into account the effect of P
β
on both phases if they are both dilute solutions
of B in A but then we cannot treat x
β
B
− x
α
B
as a constant. However, the expressions for
dx
α/β
B
/x
α/β
B
and dx
β/α
B
/x
β/α
B
are identical for two dilute solutions,
dx
α/β
B
x
α/β
B
= dx
β/α
B
x
β/α
B
. (7.41)
Integration yields
x
α/β
B
(P
β
)
x
α/β
B
(0) = x
β/α
B
(P
β
)
x
β/α
B
(0) (7.42)
x
β/α
B
(P
β
) − x
α/β
B
(P
β
)
x
β/α
B
(P
β
)
=
x
β/α
B
(0) − x
α/β
B
(0)
x
β/α
B
(0)
. (7.43)

7.6 Effect of a pressure difference on a two-phase equilibrium 141
The quantity on the left-hand side appears in the equation for dx
β/α
B
with a slightly
different notation, (x
β
B
− x
α
B
)/x
β
B
. This ratio can thus be treated as a constant during the
integration of dx
β/α
B
, yielding
x
β/α
B
(P
β
) − x
β/α
B
(0) =
P
β
V
β
m
RT
· x
β/α
B
x
β
B
− x
α
B
(7.44)
x
α/β
B
(P
β
) − x
α/β
B
(0) =
P
β
V
β
m
RT
· x
α/β
B
x
β
B
− x
α
B
. (7.45)
It should again be emphasized that the equations in the present section were derived only
for an incompressible phase.
Exercise 7.7
The precipitation of Sn from a supersaturated solid solution of Sn in Pb sometimes
results in a lamellar aggregate of a Sn phase with very little Pb and a Pb phase with
less Sn. The aggregate, comprising alternate layers, grows into a Pb-rich matrix of the
original composition. Experimental studies have been made of the coarseness of such
a structure in terms of a quantity w, the combined width of one lamella of each phase.
When discussing theoretical predictions the measured w is compared with the critical
value w
∗
which would completely stop the growth of the Sn phase because, due to
the effect of surface energy, it would put this phase under such a high pressure that
it would be in equilibrium with the original Pb matrix in spite of its supersaturation.
This pressure can be calculated from the effect of surface energy. In one study an alloy
with x
Sn
= 0.112 was investigated at a temperature where the equilibrium value was
0.06. The investigators assumed that the new phase had the equilibrium composition,
x
Sn
= 0.06, and using the Gibbs–Thomson equation for large changes they calculated
w
∗
from w
∗
= 2σ V
m
/RT ln(0.112/0.06). They found that the observed w was about
100 times larger than w
∗
instead of twice according to a simple theory. Check their
calculation.
Hint
Make a careful analysis of what pressure the surface energy will impose on the Sn
phase under the simplifying assumption that the Pb lamellae are not under an increased
pressure.
Solution
The pressure in the Sn phase will balance the surface energy, which gives a force of 2σL
if L is the length of the lamella. The area of the edge is fw
∗
L if f is the fraction of the Sn
phase. Thus, P
∗
fw
∗
L = 2σ L. The lever rule gives f = (0.112 − 0.06)/(1 − 0.06) =
0.055. Now relate the pressure to the change in solubility, ln(0.112/0.06) = P
∗
V
m
/RT
(1 – 0.112)
∼
=
P
∗
V
m
/RT. Combining these equations yields w
∗
= 2σ /P
∗
f = 2σ V
m
/fRT
ln(0.112/0.06). The investigators missed the factor f ( = 0.055) which explains most of
the discrepancy.
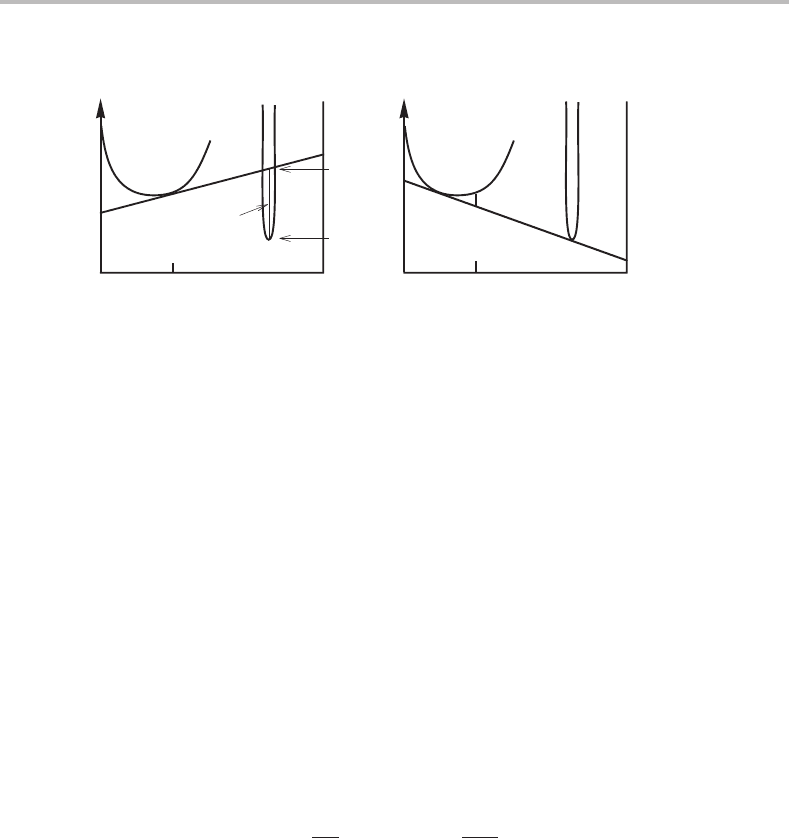
142 Applications of molar Gibbs energy diagrams
θ
.
G
m
(a) (b)
G
m
α
x
B
alloy
x
B
alloy
D =−∆G
m
θ
−∆G
m
θ
G
m
θ
Σx
i
θ
µ
i
α
α
Figure 7.17 (a) Molar Gibbs energy diagram. (b) Method for evaluation of the driving force for
the formation of a new phase θ from a supersaturated β solution.
7.7 Driving force for the formation of a new phase
When we take some A and B away from a large quantity of a solution phase, α,itislike
taking them from one reservoir each, with the chemical potentials equal to G
α
A
and G
α
B
,
respectively. As long as the amount of the α phase is large, we can take A and B in any
proportion without changing the values of G
α
A
and G
α
B
.Wecan thus form a small amount
of a new phase, θ,ofany composition without changing the Gibbs energy of the whole
system, provided that the new phase falls on the α tangent. If the new phase falls below
the tangent, the decrease counted per mole of atoms in the new phase is obtained as
− G
θ
m
= x
θ
A
· G
α
A
x
α
B
+ x
θ
B
· G
α
B
x
α
B
− G
θ
m
x
θ
B
. (7.46)
This is illustrated in the molar Gibbs energy diagram in Fig. 7.17(a).Byconvention,
the change of Gibbs energy accompanying a reaction is defined as G
m
= G
products
m
−
G
reactants
m
.Itisevident that the decrease in Gibbs energy, −G
m
,isequal to the driving
force for the precipitation of the θ phase from a supersaturated β solution, counted per
mole of θ,ifthe extent of the reaction, ξ,isexpressed as the number of moles of θ, N
θ
,
D =−
∂G
∂ξ
T,P,N
i
=−
∂G
∂ N
θ
T,P,N
i
=−G
θ
m
. (7.47)
The magnitude of the driving force for the precipitation of θ from a supersaturated
α solution, counted per mole of θ, can be estimated from the supersaturation x
α
B
in
almost the same way as the effect of pressure on solubility was evaluated. By comparing
Fig. 7.17(a) with Fig. 7.15(b) we obtain from Eq. (7.32)
D =−G
θ
m
= P
θ
V
θ
m
= x
α
B
· d
2
G
α
m
dx
2
B
·
x
θ
B
− x
α
B
. (7.48)
This is the driving force at the start of the precipitation of θ.Asthe process continues,
the supersaturation will decrease gradually and so will the driving force. It may thus be
interesting to evaluate the integrated driving force which should represent an average
value for the whole process. The method of evaluation is illustrated in Fig. 7.17(b).
One usually evaluates the integrated driving force for the transformation of the whole
system, i.e. the difference in Gibbs energy between the final α + θ mixture and the initial
supersaturated α.Itissimply given by the short vertical line in Fig. 7.17(b).
7.7 Driving force for the formation of a new phase 143
α
β
α
β
G
m
G
m
(a) (b)
G
A
β
G
A
α
G
B
α
G
A
α
G
A
β
G
B
β
G
B
α
G
B
β
x
B
β
x
A
β
−∆G
m
−∆G
m
= x
A
β
(G
A
α
−G
A
β
) + x
B
β
(G
B
α
−G
B
β
) −∆G
m
max
= G
A
α
−G
B
β
=G
B
α
−G
A
β
−∆G
m
Figure 7.18 Solution to Exercise 7.8.
Exercise 7.8
Consider the formation of a small amount of β from a large reservoir of α under condi-
tions such that the reservoir has the potentials G
α
A
and G
α
B
and the new phase has G
β
A
and
G
β
B
(accepting that such conditions can somehow be realized). (a) Construct a reasonable
molar Gibbs energy diagram and use it for deriving an expression for the driving force
per mole of β phase. Express the result in terms of the potentials and the compositions
of the two phases. (b) Suppose the composition of α has been decided. How should one
choose the composition of β in order to get the largest driving force?
Hint
(a) Using the given potentials one can draw the tangents to the two Gibbs energy curves.
Evaluate the distance between them at the proper composition. (b) In this exercise, the
tangent to the α curve is given. The question is how we can find the point on the β
curve which lies as low as possible relative to the α tangent. In principle, it can be found
without drawing the corresponding β tangent but it would be most helpful to do so, so
long as one draws that tangent correctly.
Solution
(a) See Fig. 7.18(a). (b) One should choose the composition obtained from a parallel
tangent construction (see Fig. 7.18(b)).
Exercise 7.9
Show with the construction in Fig. 7.17(b) the magnitude of the integrated driving force
counted per mole of the θ phase formed.
Hint
The magnitude is −G
m
/ f
θ
if f
θ
is the final fraction of θ in the alloy. The question is
how to find this by construction. Notice that f
θ
can be found graphically using the lever
rule.
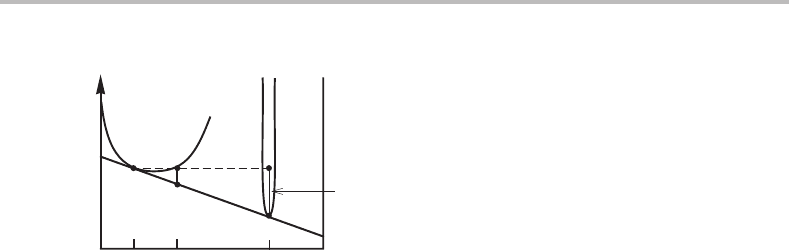
144 Applications of molar Gibbs energy diagrams
θ
G
m
α
−∆G
m
(f
θ
)(f
α
)
−∆G
m
/f
θ
Figure 7.19 Solution to Exercise 7.9.
Solution
Draw a straight line joining the final α and the initial α (Fig. 7.19). Extend the line to the
composition of θ. Also use the tangent to the final α point shown in Fig. 7.17(b). Read
the distance between intersections on the θ composition.
7.8 Partitionless transformation under local equilibrium
So far, we have mainly considered stationary states and for a state with more than
one phase we have assumed equilibrium between the phases, which is a reasonable
approximation after a long enough time at a high enough temperature. The situation is
quite different during a phase transformation but it is still common to assume that full
equilibrium is established locally at the phase interface even when it is migrating through
the material. This was introduced in Section 3.10 and is called the local-equilibrium
approximation and will now be our starting point for an examination of partitionless
transformations. The local conditions at migrating interfaces will be further discussed
in Chapter 14.
When a β →α transformation occurs in an alloy without any difference in composi-
tion between the reactant phase (also called parent phase) and the product phase (also
called daughter phase or growing phase), it is regarded as a partitionless transformation.
The two phases will fall on the same vertical line in the molar Gibbs energy diagram.
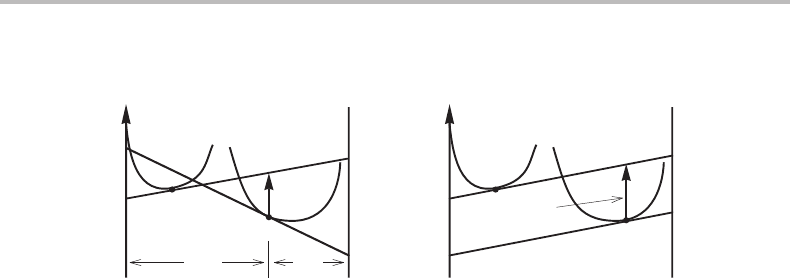
Figure 7.20(a) shows the construction for a binary system. Under constant T and P, the
driving force is given by the vertical distance between two points representing the initial
β and the growing α
D = G
β
m
− G
α
m
=−G
m
. (7.49)
It is evident that the partitionless transformation cannot possibly occur under local equi-
librium unless the composition of the phases falls on the left-hand side of the point of
intersection between the two G
m
curves.
Whether or not a transformation can actually occur under the conditions illustrated in
Fig. 7.20(a) will be discussed in Section 14.4.Anattractive possibility is illustrated in
Fig. 7.20(b).Itisbased on the assumption of local equilibrium at the interface and that
is why the common-tangent construction is used here. This illustration presumes that the
parent phase is so supersaturated that its composition falls on the equilibrium composition
