Hillert M. Phase Equilibria, Phase Diagrams and Phase Transformations: Their Thermodynamic Basis
Подождите немного. Документ загружается.


6.9 Le Chatelier’s principle 125
Exercise 6.9
Test Le Chatelier’s principle on the change of temperature and pressure when an amount
of heat is added to a two-phase system of water vapour and liquid water under constant
volume. Suppose that evaporation is initially very slow due to a thin film of oil.
Hint
Remember that dU = dQ–PdV.Atconstant V we thus have dU = dQ and could choose
U as the variable that is changed by an action from the outside. Its conjugate variable
is–1/T. The internal variable ξ may be identified with the amount of vapour.
Solution
Identify U with X
b
and − 1/T with Y
b
.For a stable system we get, at constant ξ,
i.e. before any change of the amount of vapour, 0 < (∂(−1/T )/∂U)
ξ
. This means that
−1/T, and thus also T, has increased due to the increase of U.
At the higher temperature the equilibrium vapour pressure will be higher. In a second
stage of the process there will thus be evaporation and the temperature will decrease in
agreement with Le Chatelier’s principle, 0 < (∂(−1/ T )/∂U )
D=0
< (∂(−1/T )/∂U)
ξ
.
On the other hand, the pressure has increased during the first stage due to the heating
of the vapour present from the beginning. During the second stage, the pressure will
increase further, in apparent contradiction to Le Chatelier’s principle. However, pressure
is not conjugate to U,which was the variable that was changed to a new value in the
experiment.
The result is far from trivial because there would be further evaporation during the
second stage only if the increase in pressure of the initial vapour due to its heating is
smaller than the increase of the equilibrium vapour pressure due to the heating of the
water. By relying upon Le Chatelier’s principle we may thus conclude that the heating of
the vapour gives a smaller increase of the pressure than the heating of the water would
increase the equilibrium vapour pressure.

7
Applications of molar Gibbs
energy diagrams
7.1 Molar Gibbs energy diagrams for binary systems
In this chapter we shall derive some useful thermodynamic relations relating to phase
equilibria under constant temperature and pressure, sometimes in exact form but some-
times using approximations in order to bring the final expressions into a suitable form.
We shall see how property diagrams for the molar Gibbs energy can be used in such
derivations. Most of the applications will make use of the tie-line rule (see Section 4.5).
As an introduction, some basic properties of solutions must be discussed and, in the
present section, a simple solution model will be described. A more thorough discussion
will be given in Chapter 20.
Let us first consider a case where a solution phase α can vary in composition over a
whole binary system from pure A to pure B. It is then convenient to compare the G
m
value at any composition with the value one can read on the straight line between the two
end-points, sometimes called the end-members of the solution. The difference is often
called the Gibbs energy of mixing and is denoted with a superscript M. It is illustrated
in Fig. 7.1.Itisdefined by the following equation
G
α
m
= x
A
o
G
α
A
+ x
B
o
G
α
B
+
M
G
α
m
. (7.1)
Awarning should be issued regarding the interpretation of
M
G
m
. Usually it is defined
with reference to the straight line between points representing the pure components in
the same state as the phase under consideration, i.e. the end-members of the solution.
However, sometimes it is defined with reference to a different state for one of the compo-
nents, for instance the state which is most stable at the temperature under consideration.
This is illustrated in Fig. 7.1(b) where pure B is more stable as β than as α.
The Gibbs energy diagram gives information on the partial molar Gibbs energies
for the two components, i.e. the chemical potentials. For a single phase one can use
the construction explained for V
m
in Fig. 4.6 and illustrated for G
m
in Fig. 4.7.Itis
now demonstrated again in Fig.7.2(a) and is in agreement with the following relations,
which are examples of the more general expression for all partial quantities, derived in
Section 4.1.
G
α
B
= G
α
m
+ (1 − x
B
)dG
α
m
/dx
B
= G
α
m
+ x
A
dG
α
m
/dx
B
(7.2)
G
α
B
− G
α
A
= dG
α
m
/dx
B
. (7.3)
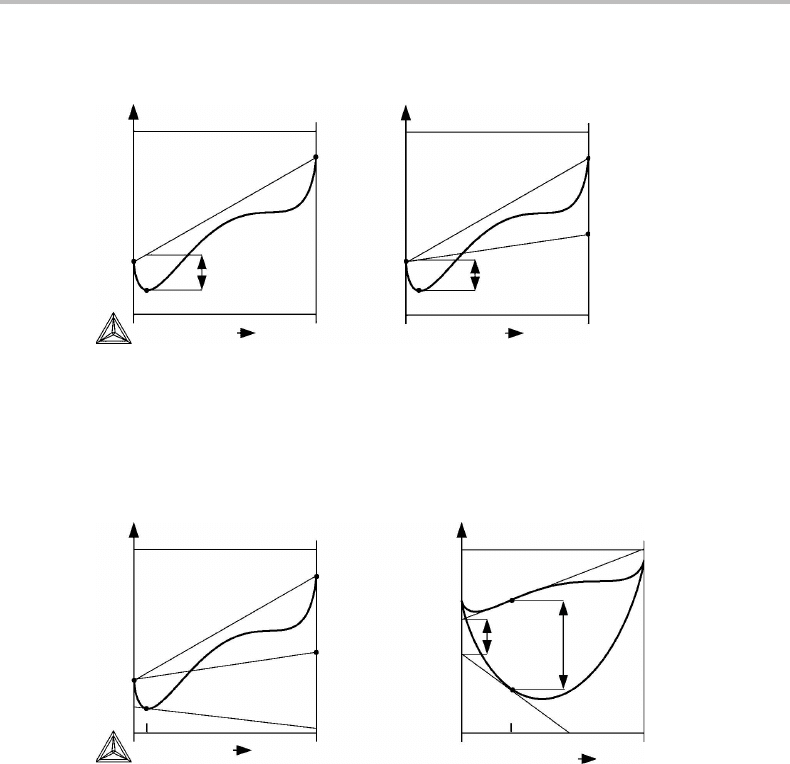
7.1 Molar Gibbs energy diagrams for binary systems 127
G
m
(a) (b)
G
m
o
G
A
o
G
A
α
o
G
B
o
G
B
α
o
G
B
β
M
−G
m
M
−G′
m
x
B
x
B
Figure 7.1 Molar Gibbs energy diagram for a binary system illustrating the definition of the
Gibbs energy of mixing. The end-members of the solution are used as references in (a) but a
more stable state of B is used in (b).
G
m
(a) (b)
G
m
o
G
A
o
G
B
o
G
B
β
G
B
(x ′
B
)
x ′
B
0 1.0
G
A
(x ′
B
)
G
A
ideal
(x ′
B
)
E
G
A
(x ′
B
)
E
G
m
x ′
B
x
B
x
B
Figure 7.2 (a) Tangent construction to obtain a chemical potential. (b) Definitions of excess
quantities for an alloy of composition x
B
.
An important contribution to the Gibbs energy of mixing comes from the entropy of
mixing of the two kinds of atoms. In Section 19.8 we shall consider the case where they
are distributed at random and shall find that the entropy of mixing of one mole of atoms
will then be −R(x
A
ln x
A
+ x
B
ln x
B
). A solution with only this contribution to the Gibbs
energy of mixing is regarded as an ideal solution
G
idealα
m
= x
A
o
G
α
A
+ x
B
o
G
α
B
+ RT(x
A
ln x
A
+ x
B
ln x
B
). (7.4)
Using Eq. (4.5) relating partial quantities to molar quantities we obtain
G
idealα
B
=
o
G
α
B
+ RT ln x
B
. (7.5)
A comparison with the ideal solution is given in Fig. 7.2(b). The curve for G
ideal
m
shows
that the term RT(x
A
ln x
A
+ x
B
ln x
B
)isnegativeand makes the G
m
curve look like a
hanging rope. It is the main cause of the stability of solutions.

128 Applications of molar Gibbs energy diagrams
It is common to summarize all other contributions to the Gibbs energy with a term
called the excess Gibbs energy and denoted by
E
G
α
m
.
G
α
m
= G
ideal
m
+
E
G
α
m
= x
A
o
G
α
A
+ x
B
o
G
α
B
+ RT(x
A
ln x
A
+ x
B
ln x
B
) +
E
G
α
m
. (7.6)
This is also illustrated in Fig. 7.2(b).Inthe same way we may define the mixing and
excess quantities for the partial Gibbs energies,
G
α
B
= G
ideal
B
+
E
G
α
B
=
o
G
α
B
+ RT ln x
B
+
E
G
α
B
. (7.7)
M
G
α
B
= RT ln x
B
+
E
G
α
B
. (7.8)
It is evident that the mixing and excess quantities can be calculated directly in the same
way as G
α
B
.
M
G
α
B
=
M
G
α
m
+ (1 − x
B
)d
M
G
α
m
dx
B
=
M
G
α
m
− x
A
d
M
G
α
m
dx
A
(7.9)
E
G
α
B
=
E
G
α
m
+ (1 − x
B
)d
E
G
α
m
dx
B
=
E
G
α
m
− x
A
d
E
G
α
m
dx
A
. (7.10)
It should be emphasized that one cannot give an absolute numerical value to the partial
Gibbs energies,
o
G
α
B
or
o
G
α
A
, because there is no natural zero point for Gibbs energy.
Numerical values can be given only to differences in Gibbs energy between two states.
Thus we can give a value to G
α
B
−
o
G
α
B
, and another value to G
α
B
−
o
G
β
B
,where pure
α–B and pure β–B are regarded as two different choices of reference states for B. Such
avalue gives the vertical distance between two points (see the B-axis in Fig. 7.2(a)).
For alloys, a numerical value can be given only to differences between two states of the
same composition. The two lines representing
M
G
m
and
M
G
m
in Fig. 7.1(a) and (b),
respectively, are thus vertical. This stems from the fact that one cannot compare the
Gibbs energies for A and B. When starting to construct such a diagram one can give the
o
G
α
A
−
o
G
β
B
line any convenient slope.
If one has chosen the end-members of an A–B solution to define the reference states,
o
G
A
and
o
G
B
, then it is evident that the excess Gibbs energy is zero at the two sides of
the system where G
m
is equal to
o
G
A
or
o
G
B
.For a dilute solution of B in A we may
thus try to approximate
E
G
m
as Lx
A
x
B
,anexpression that goes to zero on both sides.
This is the regular solution approximation, and using the equation relating G
B
to G
m
we
find
G
α
B
=
o
G
α
B
+ RT ln x
B
+
E
G
α
B
=
o
G
α
B
+ RT ln x
B
+ L
α
x
2
A
. (7.11)
It is common to introduce the chemical activity, a
B
, through the expression
G
α
B
=
o
G
α
B
+ RT ln a
B
=
o
G
α
B
+ RT ln x
B
+ RT ln f
B
, (7.12)
where
a
B
= f
B
x
B
, (7.13)
and f
B
is called the activity coefficient of B. The activity is thus defined as
a
B
= exp
G
α
B
−
o
G
α
B
RT
. (7.14)
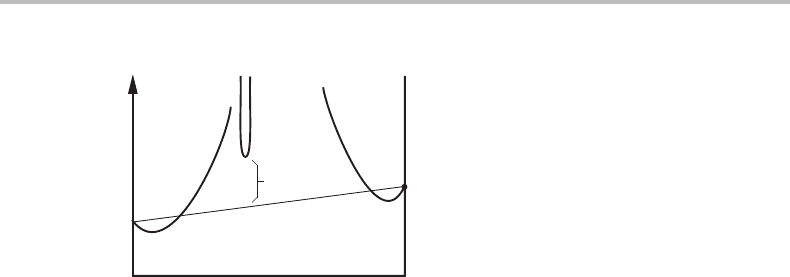
7.1 Molar Gibbs energy diagrams for binary systems 129
β
α
θ
AB
G
m
o
G
A
α
∆
o
G
m
θ
o
G
B
β
Figure 7.3 Molar Gibbs energy diagram illustrating the definition of the standard Gibbs energy
of formation,
o
G
θ
m
,ofacompound θ.
The activity coefficient is obtained through
RT ln f
B
=
E
G
α
B
. (7.15)
Foradilute solution, i.e. low x
B
,wemay thus write
RT ln f
B
=
E
G
α
B
= L
α
x
2
A
∼
=
L
α
or f
B
∼
=
exp(L
α
/RT). (7.16)
With this approximation, the activity is proportional to the content. This is called Henry’s
law. We also find for low x
B
,
RT ln f
A
=
E
G
α
A
+ (1 − x
A
)d
E
G
α
m
dx
A
= L
α
x
2
B
∼
=
0. (7.17)
The value of f
A
is thus unity close to the A side. This is called Raoult’s law. When
Henry’s law holds for B and Raoult’s law holds for A, then we obtain
dG
α
m
dx
B
= G
α
B
− G
α
A
=
o
G
α
B
+ RT ln x
B
+ L
α
−
o
G
α
A
− RT ln x
A
(7.18)
d
2
G
α
m
dx
2
B
= RT/x
B
+ RT/x
A
= RT(x
A
+ x
B
)/x
A
x
B
= RT/x
A
x
B
∼
=
RT/x
B
.
(7.19)
Foranintermediary phase, which does not extend to the pure components, one must
always refer the Gibbs energy to the values of the components in selected states, usu-
ally their stable states. For a phase with a well-defined composition one often talks
about the standard Gibbs energy of formation (see Fig. 7.3). From that diagram we
obtain
o
G
θ
m
=
o
G
θ
m
− x
A
o
G
α
A
− x
B
o
G
β
B
. (7.20)
This quantity is often denoted by
f
G
θ
m
or
f
o
G
θ
m
.Itisimportant to mention the reference
states to which it refers and also the amount of material being considered, for instance
1 mole of atoms or 1 mole of formula units (like, e.g., Cr
23
C
6
).
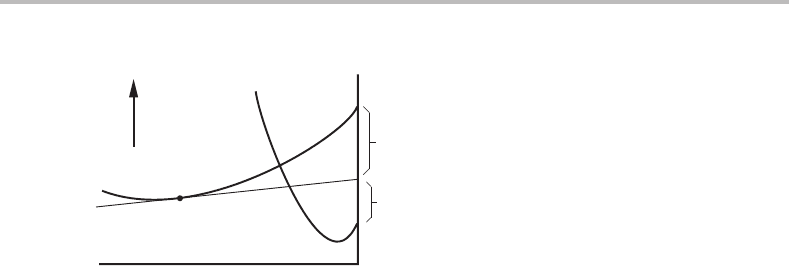
130 Applications of molar Gibbs energy diagrams
β
B
G
m
−RT ln0.9
−RT ln1.04
α
α
β
Figure 7.4 Solution to Exercise 7.1.
Exercise 7.1
An α solution in the A–B system has a
B
= 0.9at1000 K when pure α–B is used as
reference state. Calculate a
B
referred to another state of B, called β–B, which is more
stable than α–B by 1200 J/mol. Illustrate with a G
m
diagram.
Hint
The position of the point on the B-axis representing G
α
B
does not depend upon the choice
of reference state. We can thus equate any two expressions for G
α
B
.
Solution
a
refα
B
= 0.9;
o
G
α
B
+ RT ln(a
refα
B
) = G
α
B
=
o
G
α
B
+ RT ln(a
refβ
B
);
a
refβ
B
= a
refα
B
exp[(
o
G
α
B
−
o
G
β
B
)/RT] = 0.9 × exp(1200/8.3145 × 1000) = 1.04. Since
this is > 1, the α solution is supersaturated with B in comparison with the stable β state
of B (see Fig. 7.4).
Exercise 7.2
Fe
3
Cismetastable at all temperatures and could thus decompose into an Fe-rich phase
and graphite. At 1169 K the stable Fe phase (γ ) dissolves about 1.24 mass% C. Mea-
surements have shown that the Gibbs energy of formation of Fe
3
Cat1169 K is negative
(–1620 J/mole of formula units). Explain how this can be reconciled with the fact that
Fe
3
Cisnot stable by sketching a G
m
, x
C
diagram.
Solution
Fe
3
Cfalls 1620 J/mol below the line of reference between pure Fe and pure graphite
(dashed line in Fig. 7.5)but it falls above the common tangent representing the γ +
graphite equilibrium. Thus, Fe
3
Cismore stable than a mixture of the pure elements but
less stable than a mixture of an Fe–C solution and pure C.
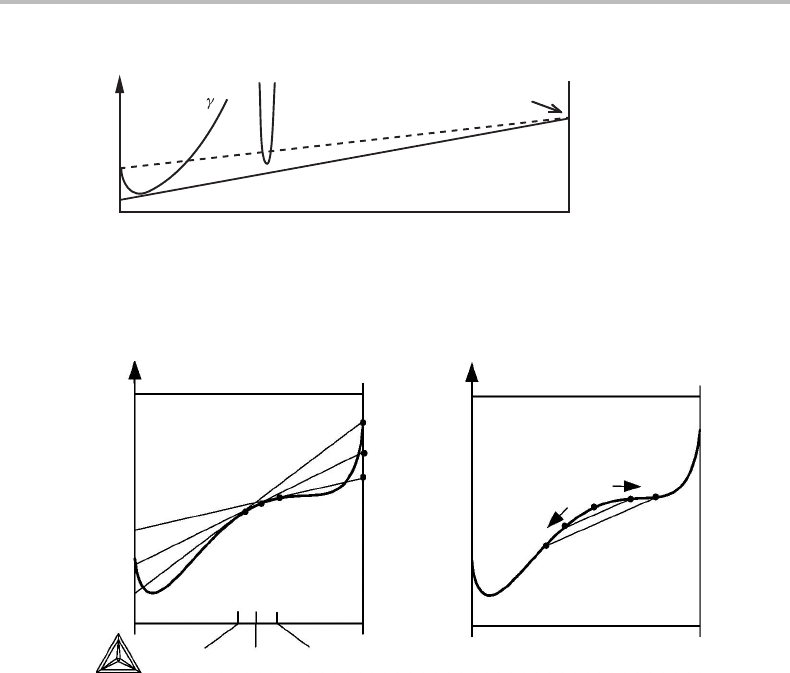
7.2 Instability of binary solutions 131
G
m
Fe
graphite
C
Fe
3
C
Figure 7.5 Solution to Exercise 7.2.
G
m
(a) (b)
G
m
x
1
x
2
x
3
G
B
(x
1
)
G
B
(x
2
)
G
B
(x
3
)
Figure 7.6 (a) Construction showing that a negative curvature results in a decrease of G
B
when
x
B
is increased. (b) Demonstration that a system between the points of inflexion is unstable. A
small difference in composition will increase spontaneously.
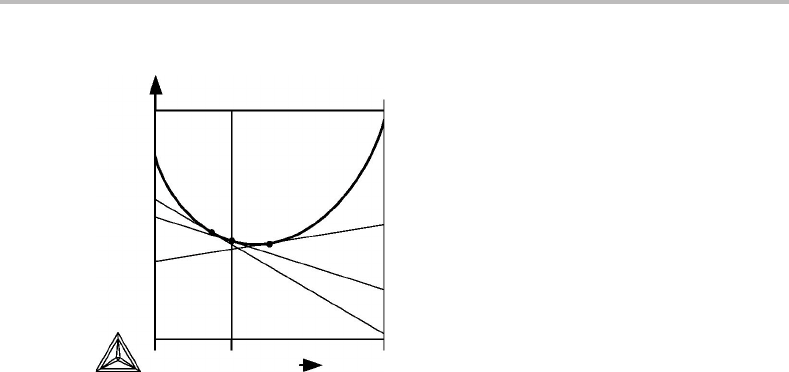
7.2 Instability of binary solutions
In Figs 7.1 and 7.2 we have sketched molar Gibbs energy curves, each with two minima
and a central region where ∂
2
G
m
/∂x
2
B
is negative. This region falls between two points
of inflexion and according to Section 6.6 they should define the limit of stability. In
Fig.7.6(a) tangents havebeen drawnat some compositions betweenthe points of inflexion
and it can be seen that G
B
decreases when x
B
increases. According to Eq. (6.27) this is
also a violation of the condition of stability.
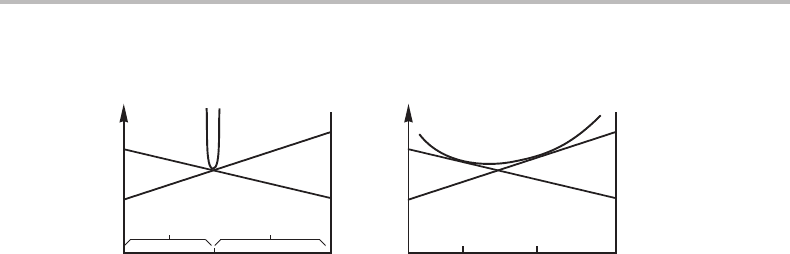
The change in the total Gibbs energy of the system, when one half of the system grows
richer in A and the other one in B, is illustrated in Fig. 7.6(b). The tie-line rule requires
that the total Gibbs energy of a mixture is represented by a point on the line connecting
the points representing the two parts. Since the overall composition is not changed, the
total Gibbs energy moves down along a vertical line at the alloy composition (not shown
here). Such a system is thus unstable against fluctuations in composition.
132 Applications of molar Gibbs energy diagrams
G
m
G
A
1
G
B
3
G
B
2
G
B
1
G
A
2
G
A
3
0 1.01/3
x
B
Figure 7.7 Solution to Exercise 7.3.
Exercise 7.3
In a binary solution one usually discusses two activities, a
A
and a
B
, and for a stable
system each of them increases monotonously with the content of the same component.
However, one may define the activity of an intermediary species, e.g. A
2
B. Prove that
a
A
2
B
also increases as the content of A
2
B increases in a stable system and thus has a
maximum at the very composition of A
2
B.
Hint
Instead of the chemical activity, a
A
2
B
, let us consider the chemical potential µ
A
2
B
,which
is equal to 2µ
A
+ µ
B
, i.e. 2G
A
+ G
B
,or, even better, µ
A
2/3
B
1/3
, i.e. (2G
A
+ G
B
)/3). It
may be studied in a G
m
, x
B
diagram.
Solution
Forany alloy the tangent in the G
m
, x
B
diagram gives G
A
and G
B
on the two sides
and the intersection of the tangent with a vertical line at x
B
= 1/3 thus gives (2G
A
+
G
B
)/3. By inspection it is evident that the intersection has its highest position for the
alloy x
B
= 1/3 (see Fig. 7.7). Otherwise, the G
m
curve must have a negative curvature
somewhere.
7.3 Illustration of the Gibbs–Duhem relation
The molar Gibbs energy diagram in Fig. 7.8(a) shows a stoichiometric compound, θ,
with a well-defined composition A
a
B
b
, possibly because it is a crystalline phase with
two sublattices. Often, the composition of such a phase cannot vary appreciably without
avery steep increase of the Gibbs energy. It is thus practically impossible to vary N
A
and keep N
B
constant and the definition of partial Gibbs energy, given in Section 4.1,
7.3 Illustration of the Gibbs–Duhem relation 133
AB
θ
µ′′
B
µ′′
B
µ′
B
µ′
B
µ′
A
µ′′
A
µ′
A
µ
′′
A
x ′′
B
x ′
B
G
m
(a) (b)
G
m
AB
b = x
B
θ
x
B
θ
a = x
A
θ
.
α
Figure 7.8 Molar Gibbs energy diagram for (a) a binary stoichiometric phase θ and (b) a binary
solution phase α.Ifµ
A
is controlled by some method, then the value of µ
B
is given in both cases.
cannot be used. However, the tangent construction in Fig. 7.2 can still be used. For this
case we shall prefer to talk about chemical potentials and use the notations µ
A
and
µ
B
. The situation is not drastically different for a phase with variable composition (see
Fig. 7.8(b)). For both types of phase one may select the value of µ
A
and the value of µ
B
will then be fixed. One could also talk about the chemical potential of the compound,
µ
A
a
B
b
.From Section 4.8 we get
G
A
a
B
b
= µ
A
a
B
b
=
a
s
i
µ
i
= aµ
A
+ bµ
B
; µ
B
= (µ
A
a
B
b
− aµ
A
)/b. (7.21)
By comparing the values defined by two different tangents we find for a stoichiometric
compound, because µ
A
a
B
b
is fixed,
x
A
(µ
A
− µ
A
) + x
B
(µ
B
− µ
B
) = 0, (7.22)
where a = x
A
and b = x
B
if a + b is chosen as 1. This may be regarded as the Gibbs–
Duhem relation integrated for a phase with constant composition.
Foraphase with variable composition one may also select the value for µ
A
, and the
value for µ
B
will then be fixed by the expression for the molar Gibbs energy,
G
m
= x
A
µ
A
+ x
B
µ
B
; µ
B
= (G
m
− x
A
µ
A
)/x
B
, (7.23)
but here G
m
varies with the composition and the composition varies with the choice of
µ
A
,asdemonstrated for a solution phase α in Fig. 7.8(b). The Gibbs–Duhem relation
holds
x
A
dµ
A
+ x
B
dµ
B
= 0, (7.24)
but not in the integrated form given for the stoichiometric compound, because the tangents
do not intersect in a point on the G
m
curve.
In a ternary system one may have a solution between two binary stoichiometric phases
if they are isomorphic (have the same structure). Figure7.9 shows a Gibbs energy diagram
for such a case. The composition can now be varied with one degree of freedom and we
may consider two components or end-members A
a
C
c
and B
a
C
c
.
This kind of solution may be represented by the formula (A, B)
a
C
c
and the composition
may be represented by molar contents defined as
x
B
a
C
c
= N
B
a
C
c
/(N
A
a
C
c
+ N
B
a
C
c
), (7.25)
134 Applications of molar Gibbs energy diagrams
A
B
C
G
m
θ
.
θ
µ′
B
µ′
A
µ′′
A
µ
θ
A
a
C
c
µ
θ
A
a
C
c
µ
θ
B
a
C
c
µ
θ
B
a
C
c
A
a
C
c
B
a
C
c
µ′′
C
µ′
C
(a) (b)
Figure 7.9 Molar Gibbs energy diagram for a ternary solution between two binary
stoichiometric phases.
where the two Ns represent the moles of formula units. Another method of representation
is based on the molar contents evaluated for each sublattice, the so-called site fractions
which were discussed in Section 4.7.
From Fig. 7.9 it is evident that the molar Gibbs energy for this kind of solution phase
can be expressed in the following ways if a + c = 1.
G
m
= x
A
µ
A
+ x
B
µ
B
+ x
C
µ
C
= y
A
µ
A
a
C
c
+ y
B
µ
B
a
C
c
. (7.26)
The diagram in Fig. 7.9(a) with two possible tangent planes shows that the values of
µ
A
a
C
c
and µ
B
a
C
c
are well defined by the composition but µ
A
,µ
B
and µ
C
are not.
Exercise 7.4
Consider the chemical potential of Fe
3
Cinasolution phase of Fe, C and Mn, using basic
principles. Show that it is actually equal to 3µ
Fe
+ µ
C
by making a calculation using
Section 4.6.
Hint
In the Fe–C–Mn system we usually use Fe, C and Mn as the components but now we
should change to a new set of components. Fe
3
Cisone component and the others could
be C and Mn. Notice that dG =
µ
i
dN
i
cannot change its value just because we change
the set of components to be considered.
