Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

1010 28. Bioconjugation in the Study of Protein Interactions
Protocol
1. Dissolve the protein(s) to be studied in 20 mM HEPES buffer, pH 7.5, at a concentration
of 5–10 M.
2. Dissolve together in one solution the desired heavy and light crosslinker analogs in dry
DMSO at an equal concentration of up to 100 mM. Use either the pair BS
2
G-d
4
/BS
2
G-d
0
or BS
3
G-d
4
/BS
3
G-d
0
, but do not mix the different sized crosslinkers together. The heavy
and light analogs of the same type should always be dissolved at equivalent concentra-
tions in DMSO to prepare the stock solution.
3. Add a quantity of the crosslinker solution to the protein solution to obtain at least a
10-fold molar excess of the crosslinkers over the concentration of the protein. Studies
should be done at several levels of crosslinker addition to determine the optimal conjuga-
tion conditions (i.e., 10-, 50-, 100-, and 200-fold excess).
4. Quench the reaction by the addition of NH
4
HCO
3
to a fi nal concentration of 20 mM.
The optimal time course for the reaction should be determined by removing portions
of the solution at different points, starting at about 5–10 minutes and extending out to
2 hours in length.
5. Analyze the quenched reaction by SDS electrophoresis, Western blotting, and mass spec
analysis.
1.3. Formaldehyde
By Far the simplest bifunctional crosslinking agent is formaldehyde. Although structurally a
mono-functional aldehyde compound, formaldehyde reacts with proteins via a two-step reaction
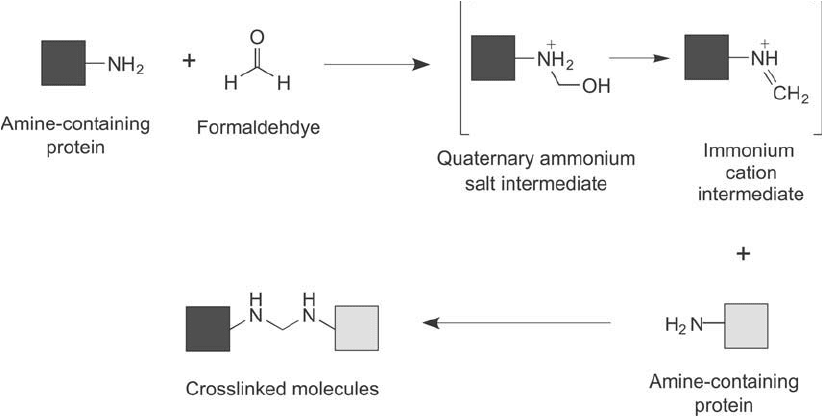
Figure 28.4 Formaldehyde can be used to capture protein interactions if it is used at low concentrations. The
reaction proceeds through modifi cation of a protein to create an intermediate immonium cation, which then
goes on to react with a neighboring protein to form the crosslinked product via secondary amine bonds.
to yield a methylene bridge crosslink between two amines on proteins and other molecules, thus
it behaves as though it is bifunctional in nature ( Figure 28.4 ). The reaction of formaldehyde
with proteins is rapid and effi cient, and it has long been used to fi x cells and tissue samples
for preservation, staining, and probing. Formaldehyde quickly penetrates cells and at the right
concentration, results in locking biomolecules in place, preventing diffusion, morphological
changes, or loss of proteins through solubilization .
Formaldehyde also can be used at limiting concentrations to crosslink interacting proteins,
while avoiding the extensive global crosslinking typically obtained when fi xing cells. At rela-
tively low concentrations, formaldehyde will link together only those proteins and other amine-
containing molecules within proximity to one another and presumably, therefore, undergoing
specifi c biological interactions (Prossnitz et al., 1988; Skare et al., 1993; Derouiche et al., 1995;
Orlando et al ., 1997; Orlando, 2000; Hall and Struhl, 2002; Vasilescu et al ., 2004).
Guerrero et al. (2006) used this technique along with the quantitative mass spec strategy
called SILAC (stable isotope labeling of amino acids in cell culture; Ong et al., 2002) to iden-
tify the yeast proteins that interact with the 26 S proteasome.
The following protocol is a generalized method that summarizes the publications on the use
of formaldehyde for capturing interaction proteins. The ranges indicated for concentrations of
reactants and time of the reaction need to be optimized for each protein interaction studied.
Protocol
1. Grow cells and wash into ice-cold 10 mM PBS buffer, pH 6.8.
2. Add formaldehyde to a fi nal concentration of 0.125–1 percent (w/w) (optimize to fi nd
the best concentration level for the particular protein being studied).
3. React at room temperature to 37°C for 5–60 minutes (optimize to fi nd the best reaction
time for the protein being studied).
4. Wash cells and solubilize pellet in SDS-PAGE sample buffer.
5. Heat at 37°C for 10 minutes to fully solubilize and maintain crosslinked proteins, and
then enrich specifi c complexes by immunoprecipitation using an immobilized antibody
specifi c for the bait protein that was used. Alternatively, heat at 96°C for 20 minutes to
solubilize and break all crosslinks (this may be used as a control).
6. Analyze interacting proteins by electrophoresis, Western blotting, and mass spectrometry.
1.4. Protein Interaction Reporters
Standard homobifunctional crosslinkers, such as those described in the previous sections, can
capture protein interactions effectively through covalent linkages, but they create severe chal-
lenges for analyzing exactly what proteins have been conjugated. This is not just the result of
the large number of protein interacting partners that get crosslinked; it is also a result of the
wide variety of products that can form from the process, including side reactions, nonspecifi c
conjugations, and crosslinkers that only reacted with one protein. Attempting to deconvolute
the identity of true protein interacting complexes created by large-scale crosslinking of complex
samples is the major problem of all conjugation techniques used to study protein interactions.
A new type of crosslinking strategy may overcome this problem, as it takes advantage of
defi nitive mass spec identifi cation after the chemical crosslinking of interacting proteins. The
1. Homobifunctional Crosslinking Agents 1011
1012 28. Bioconjugation in the Study of Protein Interactions
protein interaction reporter (PIR) reagent as described by Tang et al. (2005) is based on a
bifunctional crosslinker concept, but having the additional feature of containing two mass spec
cleavable bonds within a specially designed spacer arm. The cleavable parts of the molecule
consist of two RINK groups, which are amide-releasing, acid-cleavable components based
on the solid phase peptide synthesis resin fi rst described by Rink (1987), and containing the
4-(2 ,4 -dimethoxyphenylaminomethyl)phenoxy linker on each side of a central mass reporter
tag ( Figure 28.5 ). On both ends of the PIR compound, NHS esters provide amine reactivity to
capture any interacting proteins in a complex sample, such as within a cell or lysate. In use,
the PIR crosslinker fi rst is reacted with a sample to form conjugates and then the proteins are
subjected to proteolysis to create peptides. Some of the peptides that are formed will be conju-
gated together through the PIR crosslinks. This complex mixture of peptides then is subjected
to mass spec analysis using a mass spectrometer capable of MS
2
or MS
3
separations.
Second-generation PIR reagents have been designed to include an affi nity handle branch-
ing off from the central reporter group ’s free carboxylate. In one such design, a biotin group
is built at the end of a hydrophilic PEG spacer ( Figure 28.6 ). The PEG arm provides increased
water-solubility to the overall reagent, which is otherwise rather hydrophobic. The biotin group
can be used to detect or capture crosslinked interacting proteins out of complex solutions. For
instance, immobilized streptavidin can be used to pull down any proteins modifi ed with the
biotin–PIR reagent. Alternatively, a complex solution may be separated by electrophoresis and
the modifi ed proteins identifi ed after Western blotting through the use of a streptavidin conju-
gate detection complex.
The use of a crosslinker containing MS labile bonds along with a mass reporter group can sig-
nifi cantly reduce the complexity of fi nding and identifying coupled peptides in a huge amount of
mass spec data. In the fi rst dimension of a MS separation, the overall mass of the PIR-crosslinked
peptides can be accurately determined as a single peak in the MS spectrum. The resultant mass
correlates to the sum of the two crosslinked peptides plus the intervening PIR spacer which links
them together.
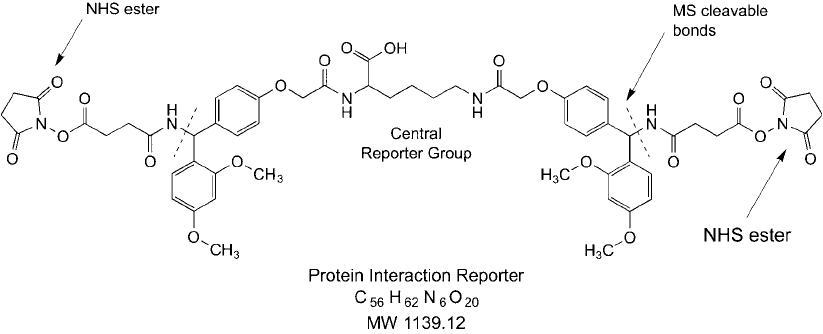
Figure 28.5 This PIR compound contains NHS esters at both ends to capture interacting proteins through
amide bond formation. It also contains MS cleavable bonds that release a central reporter group, which can be
used to identify crosslinked peptides by mass spec.
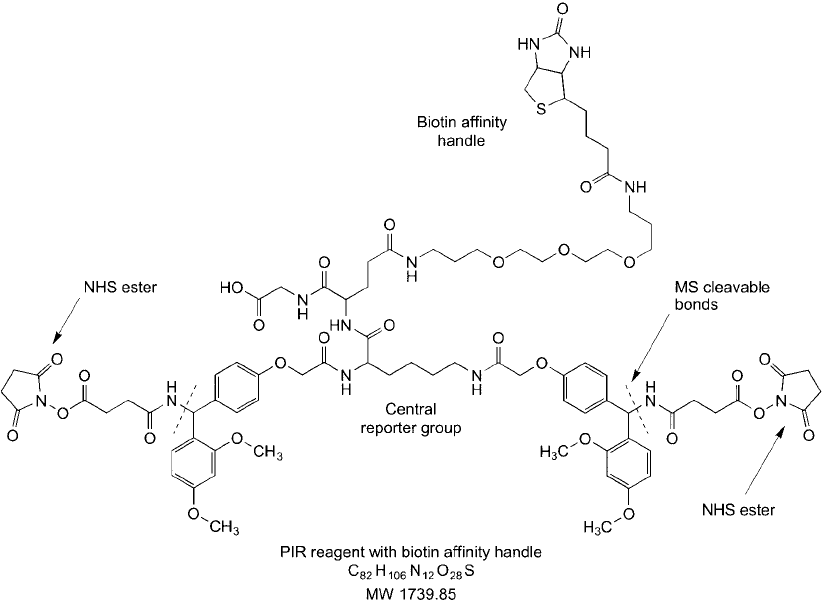
Figure 28.6 A trifunctional PIR compound that contains two NHS esters to capture interacting pro-
teins through amide bond formation and a PEG–biotin arm to permit isolation of crosslinked proteins on
(strept)avidin supports.
With bifunctional NHS ester reagents, one of three modifi cation products can occur with
proteins: (1) a dead-end linkage wherein one end of the crosslinker has attached to an amine
group within a protein and the other end has hydrolyzed and not formed an attachment, (2) an
intra-protein crosslink wherein the PIR reagent has been coupled at both ends to amines within
the same protein, or (3) an inter-protein crosslink wherein both ends of the PIR reagent have
been coupled with amines on two different protein molecules ( Figure 28.7 ).
The second stage of an MS
2
analysis can be done to bombard with more energy the mass
products of the fi rst stage separation in order to fragment the proteolytically digested complexes.
At this point, the mass reporter group within the PIR crosslink is released due to breakdown of
the two labile RINK bonds within the reagent structure (see Figure 28.5 ). Mass spec cleavage of
these groups and release of the reporter results in one of two potential mass signatures depend-
ing on if both ends of the PIR reagent have reacted (which gives a reporter mass m/z 711) or
if only one end has reacted (a dead-end; reporter mass m/z 828). The resulting mass spectrum
then shows either two or three peaks, depending on the type of modifi cation formed from the
initial PIR reaction. This process also releases the crosslinked peptides without disrupting the
1. Homobifunctional Crosslinking Agents 1013
1014 28. Bioconjugation in the Study of Protein Interactions
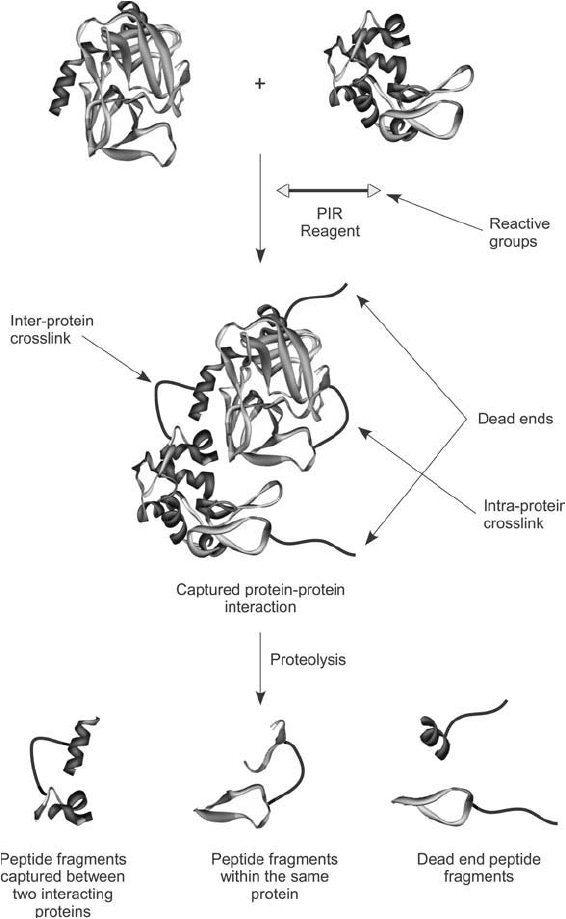
Figure 28.7 Reaction of a PIR compound with a protein sample can result in several products, all of which
can be identifi ed by mass spec analysis. A true inter-protein crosslink can occur that links two interacting pro-
teins together, which is the desired product. However, the crosslinking process also may result in intra-protein
crosslinks or dead ends wherein only one end of the PIR reagent has coupled to a protein.
peptide backbone and thus leaves a small part of the PIR compound attached to each peptide
fragment, minus the reporter group.
The specifi c fragmentation pattern observed at this stage distinguishes the kind of crosslink
or modifi cation that was initially formed. In this regard, a single modifi ed peptide peak plus a
reporter peak of mass m/z 828 indicates a dead-end modifi cation with no value in determining pro-
tein interactions. Alternatively, a single peptide fragment peak plus a reporter group peak of mass
m/z 711 indicates an intra-molecular crosslink made between regions of the same protein, which
also is not of interest. However, a fragmentation pattern containing two labeled peptide peaks plus
a reporter peak of mass m/z 711 indicates a successful conjugation between two protein molecules,
which may be indicative of a true protein–protein interaction. Note that alternative designs of a
PIR-type reagent containing other reporter structures, including those with a biotin handle and a
PEG spacer, will result in different reporter fragmentation mass values than those stated here.
The use of PIR compounds to study protein interactions is a signifi cant advance over the use of
standard homobifunctional crosslinkers. The unique design of the PIR reagent facilitates decon-
volution of putative protein interaction complexes through a simplifi ed mass spec analysis. The
software can ignore all irrelevant peak data and just focus analysis on the two labeled peptide
peaks, which accompany the reporter signal of appropriate mass. This greatly simplifi es the bio-
informatics of data analysis and provides defi nitive conformation of protein–protein crosslinks.
Finally, knowledge of the peptide masses that resulted from the PIR conjugation provides
information to identify the parent proteins from which they originated. Peptide mass and
sequence databases now are suffi ciently developed to provide rapid confi rmation of protein–
protein interaction partners.
The following protocol is designed for treating cells with the PIR reagent to study protein
interactions in vivo. It is based on the method of Tang et al. (2005). The use of the PIR com-
pound to treat intact cells results in the crosslinking of proteins both on the cell surface and
within the cell, which indicates that the reagent is able to cross the cell membrane.
Protocol
1. Dissolve the PIR compound in dry DMSO to make a 100 mM stock solution.
2. Grow cells in media to a density of about OD
600
nm
1.2 and harvest in mid-log phase.
Centrifuge cells at 3,200 rpm to pellet them and wash 3 times with ice-cold PBS (150 mM
sodium phosphate, 100 mM NaCl, pH 7.5).
3. Suspend the cells in 1 ml of PBS and add an aliquot of the dissolved PIR compound to
bring the fi nal concentration to 1 mM.
4. React at room temperature with gentle shaking for 5 minutes.
5. Quench the reaction by the addition of 50 l of 1 M Tris, pH 7.5.
6. Wash the cells 5 times by centrifugation with cold PBS to remove excess PIR reagent and
any secreted proteins.
7. Lyse the cells using a detergent lysis buffer suitable for the cell type being treated.
Centrifuge the lysate at 15,000 rpm for 40 minutes at 4°C to remove insoluble material.
Collect the supernatant and discard the pellet. At this point, the soluble protein fraction
may be analyzed by electrophoresis, if desired.
8. Remove unreacted PIR reagent and reaction by-products by gel fi ltration or dialysis.
9. Precipitate the protein with TCA to further remove any remaining salts and detergent.
Centrifuge to pellet the precipitated protein, wash the pellet with TCA, and centrifuge again.
Redissolve the washed pellet in 100 l of 100 mM NH
4
HCO
3
, pH 7.8, containing 8 M urea.
1. Homobifunctional Crosslinking Agents 1015
1016 28. Bioconjugation in the Study of Protein Interactions
10. Reduce protein disulfi des by adding dithiothreitol (DTT) to a fi nal concentration of
5 mM and incubate for 30 minutes at 60°C. Add iodoacetamide to a fi nal concentration
of 25 mM to alkylate the thiols. React for 1 hour in the dark.
11. Dilute the solution 4-fold with 100 mM 25 mM NH
4
HCO
3
, and then add 20 g trypsin
to digest the proteins. Incubate at 37°C for 4 hours or overnight at 30°C with shaking.
12. Purify the tryptic peptides by chromatography on a C18 column to remove salts (follow
the manufacturer ’s directions for peptide purifi cation). Dry the eluent and redissolve the
peptides in 20 l 0.1 percent formic acid.
13. Analyze the purifi ed peptides using two-dimensional LC–MS/MS.
2. Use of Photoreactive Crosslinkers to Study Protein Interactions
The use of crosslinking agents containing at least one photoreactive group provides reagents
that can be activated at a desired point after bait proteins have been allowed to interact and
bind to prey proteins. The availability of the fi rst photoreactive homobifunctional or hetero-
bifunctional compounds permitted protein interactions to be studied at a new level of specifi -
city. Unlike the use of spontaneously reactive homobifunctional reagents, the incorporation of
a photoreactive group helps to prevent nonselective crosslinking and uncontrolled polymeriza-
tion of proteins that may or may not be specifi cally interacting.
In use, a bait protein fi rst is modifi ed with the spontaneously reactive (thermoreactive) end
of a photoreactive heterobifunctional compound, while protecting the solution from light expo-
sure. The modifi ed bait protein then is allowed to interact with a sample containing other pro-
teins and biomolecules, which may contain prey proteins able to interact with it. Finally, the
sample mixture is exposed to UV light to activate the photoreactive end of the crosslinker, caus-
ing conjugation of this group with any nearby interacting molecules. The rapid reaction rate of
the activated photoreactive intermediates assures that they don ’t survive long enough to cause
much conjugation to proteins just due to random collisions. Interacting proteins, however, that
are in proximity to the modifi ed bait protein are more likely to be captured through reaction
with the activated photoreactive group.
The following sections discuss the application of several photoreactive heterobifunctional
crosslinkers to the study of protein interactions.
2.1. Sulfo-SAND, SANPAH, and Sulfo-SANPAH
These three photoreactive crosslinkers are described in terms of their properties and reactivi-
ties in the section on Amine-Reactive and Photoreactive Crosslinkers in Chapter 5, Section 3.
They represent early examples of the use of standard phenyl azide photoreactive compounds
for the study of protein interactions. All of them either contain an amine-reactive NHS ester or
the charged and water-soluble analog, a sulfo-NHS ester ( Figure 28.8 ). SANPAH is uncharged
and hydrophobic and thus provides membrane permeability for studying intracellular protein
interactions in whole cells (Mikhailov et al., 2001; Kota and Ljungdahl, 2005). Sulfo-SAND
and Sulfo-SANPAH, however, possess a negatively charged sulfonate group, which prevents
them from passing through cell membranes, and thus are membrane impermeable (Uckun et al.,
1995; Gaudet, et al., 2003). The sulfonated compounds may be used with whole cells to study
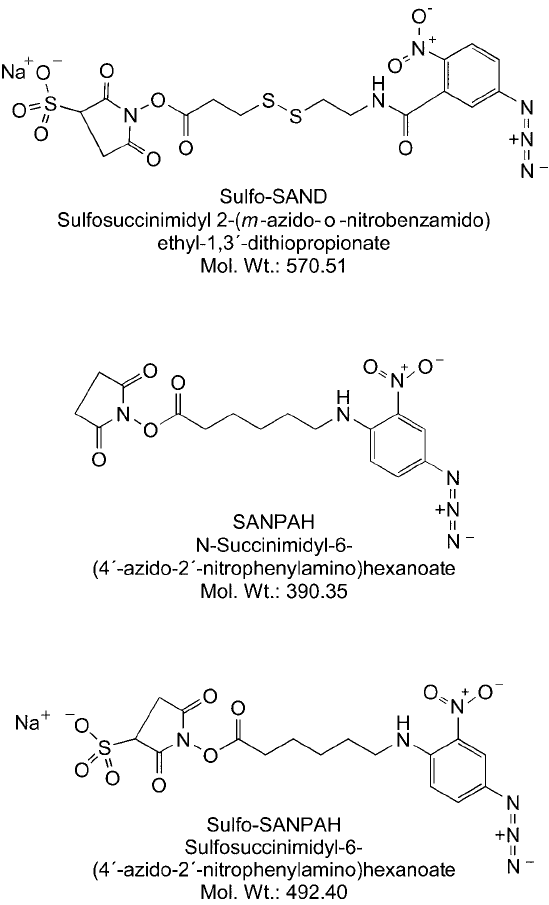
Figure 28.8 The heterobifunctional crosslinkers sulfo-SAND, SANPAH, and sulfo-SANPAH contain an amine-
reactive (sulfo)NHS ester on one end and a photoreactive phenyl azide group on the other end. Sulfo-SAND
allows release of conjugates by reduction of its internal disulfi de bridge.
cell-surface protein interactions, although in use, their concentration should be limited to pre-
vent internalization by active transport into cells.
Sulfo-SAND, SANPAH, and Sulfo-SANPAH all contain a nitrated phenyl azide photoreac-
tive group. The presence of the nitro group shifts the optimal wavelength for photoactivation
2. Use of Photoreactive Crosslinkers to Study Protein Interactions 1017
1018 28. Bioconjugation in the Study of Protein Interactions
to higher wavelengths, thus avoiding the potential for biomolecule damage due to UV
irradiation (photoactivation occurs at 320 –350 nm). Sulfo-SAND provides one further option
for studying interacting proteins. It has a cleavable disulfi de containing cross-bridge that per-
mits recovery of any prey proteins that have been captured by the photo-coupling process
(McMahan and Burgess, 1994; Uchiyama et al., 2002). Finally, each of these photoreactive
crosslinkers has phenyl azide rings that undergo ring expansion to the 7-membered ring, dehy-
droazepine intermediate, which reacts primarily with amines on target molecules. Thus, the
proteins that are captured by these crosslinkers typically are coupled through secondary amine
linkages to the photoreactive end of the reagents. A general protocol for the use of these com-
pounds to study protein interactions is given at the end of the next section.
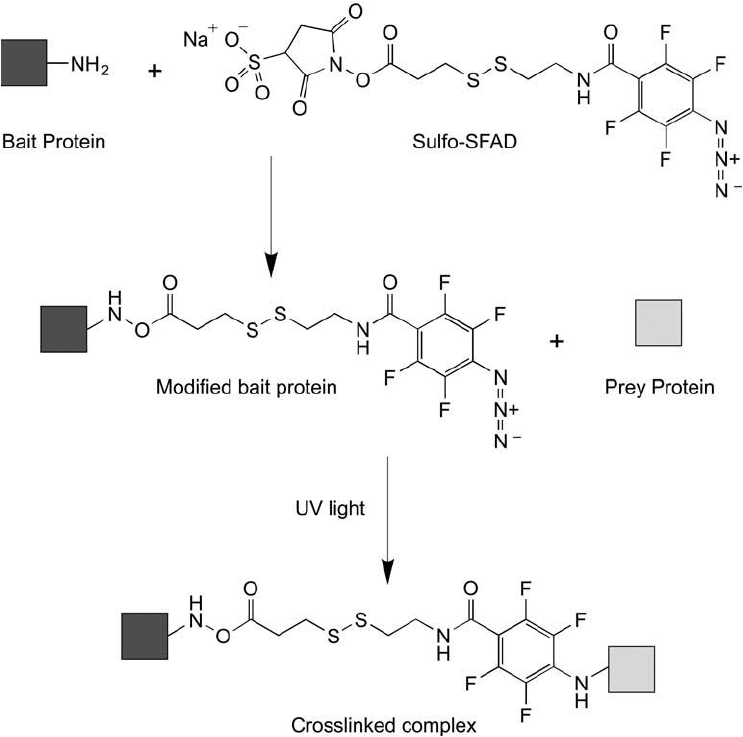
2.2. Sulfo-SFAD
Sulfo-SFAD is sulfosuccinimidyl-[perfl uoroazidobenzamido]-ethyl- 1,3 -dithiopropionate, an
amine reactive and photoreactive crosslinker with more advanced properties than the photore-
active reagents discussed above. This reagent contains a sulfo-NHS ester to provide increased
water-solubility prior to conjugation, and this group will react with an amine on proteins and
other biomolecules to form an amide linkage. Sulfo-SFAD also contains a perfl uorophenyl
azide group that has better photo-insertion capability than the original unsubstituted phenyl
azide group. The reason for this enhanced yield is that after photoactivation, the nitrene inter-
mediate can ’t react with the phenyl ring itself and undergo ring expansion to a dehydroazepine
as happens with typical unsubstituted phenyl azides. The result is that the nitrene survives
long enough to react with neighboring molecules in the immediate molecular vicinity, such as
proteins that are interacting with the modifi ed bait protein. Perfl uorophenyl azides thus have
higher yields of conjugation, and they can react by insertion into C H bonds, N H, unsatu-
rated carbon–carbon bonds, and other structures in target molecules. By contrast, unsubsti-
tuted phenyl azides ring expand and react mainly with amines, and even then at low yield. The
perfl uorophenyl azide may be activated with UV light at 320 nm.
Sulfo-SFAD also contains a disulfi de bond in its cross-bridge, which provides subsequent
cleavability of any crosslinks formed. Thus, prey proteins can be recovered from complexes for
analysis by simple reduction of the conjugate with DTT. Once reduced, the perfl uorophenyl ring
group of Sulfo-SFAD is transferred to the interacting prey protein. This group can be identifi ed
by
19
F NMR, and it also provides a traceable label for identifi cation of peptide fragments by
mass spec. Figure 28.9 illustrates the reactions of Sulfo-SFAD in capturing a bait–prey complex.
The following references provide further information on the use of Sulfo-SFAD and per-
fl uorophenyl azide photoreactive groups: Pandurangi et al. (1995a, b, 1996, 1997a, b, 1998);
Yan et al . (1994).
A general protocol that can serve as a guide for the use of heterobifunctional crosslinkers in
the study of protein interactions is given below. Some optimization of concentrations may have
to be done depending on the particular type and properties of proteins being studied.
Protocol
1. Dissolve the photoreactive crosslinker in DMF or DMSO at a concentration of
10–25 mM (protect from light). Water-soluble crosslinkers can be added to water or
buffer, but should be used immediately.
2. Modify a purifi ed bait protein by adding an aliquot of crosslinker to achieve a molar
excess of 2–10 moles per mole of protein (protect from light). For NHS ester-containing
reagents, react in 0.1 M sodium phosphate buffer at pH 7.2–7.4. Avoid using any other
amine-containing buffer components, such as Tris or imidazole, which will interfere with
the reaction.
3. React for 30–60 minutes in the dark (room temperature or 4 C).
4. Remove excess crosslinker from the modifi ed bait protein by gel fi ltration or dialysis in
the dark.
Figure 28.9 Sulfo-SFAD is an advanced heterobifunctional photoreactive crosslinker that contains an amine-
reactive sulfo-NHS ester on one end and a tetrafl uorophenyl azide group on the other end. The fl uorine sub-
stitutions on the phenyl ring prevent the photoactivated nitrene from reacting with the ring, thereby providing
greater yields in capturing interacting proteins. The disulfi de-containing cross-bridge allows cleavage of conju-
gated molecules by reduction with DTT.
2. Use of Photoreactive Crosslinkers to Study Protein Interactions 1019
