Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

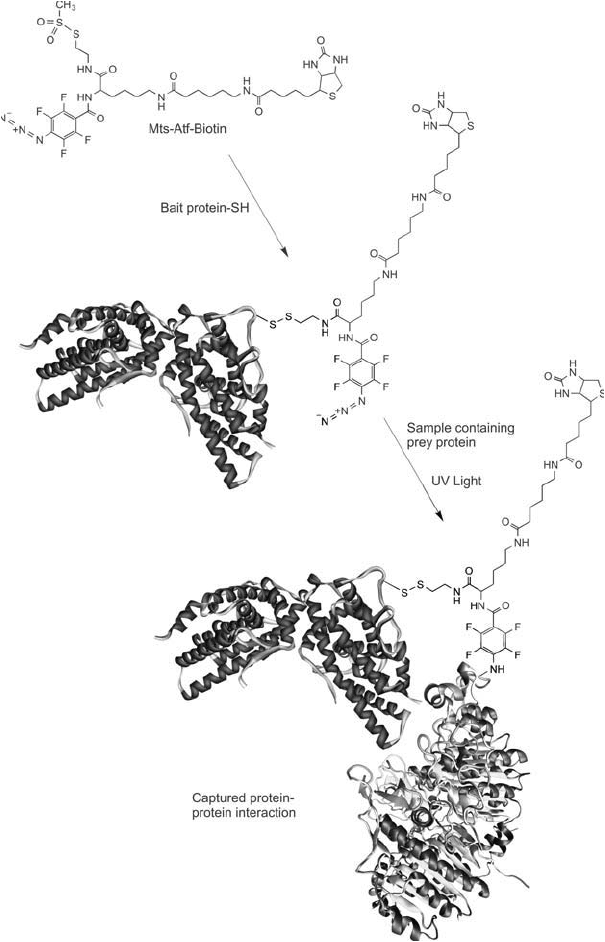
1030 28. Bioconjugation in the Study of Protein Interactions
Figure 28.16 Mts-Atf-biotin can be used to modify a bait protein using an available thiol group to form a
disulfi de bond. The labeled bait protein then is allowed to interact with a sample containing proteins that poten-
tially can interact with the bait. After an incubation period, the sample is exposed to UV light to photoactivate
the tetrafl uorophenyl azide group. This reaction causes any interacting prey proteins to be crosslinked with the
bait protein, forming a complex containing a biotin affi nity tag. Subsequent cleavage of the disulfi de bond to
the bait protein using DTT transfers the biotin group to the unknown interacting protein. The labeled prey pro-
tein then can be detected or isolated using (strept)avidin reagents.
disulfi des or through the use of a thiolation reagent. However, adding thiols by chemical deri-
vatization may not be advantageous, because this will create numerous surface modifi cations,
which may have detrimental effects on protein–protein interactions.
Protocol
1. Dissolve a thiol-containing bait protein in 10 mM HEPES, 0.15 M NaCl, pH 7.5 or in
0.1 M sodium phosphate, 0.15 M NaCl, pH 7.2. Other buffers around physiological pH
will work well; however avoid the presence of reducing agents containing thiols (i.e., DTT
or 2-mercaptoethanol). Typical concentrations that will work with the suggested protocol
range from 100 g/ml to 10 mg/ml, but higher concentrations are preferred to give high
reaction yields.
2. Dissolve MTS-ATF-Biotin or MTS-ATF-LC-Biotin in DMF or DMSO at a concentration
of at least 10 mg/ml. Protect from light.
3. Add a quantity of the crosslinker solution to the bait protein solution to provide a
1–5 molar excess of the crosslinker over the quantity of protein to be modifi ed. The addi-
tion of the organic solution containing the crosslinker should be done so that the fi nal
reaction contains no more than 10 percent organic solvent. Even lower concentrations of
solvent may be required for certain sensitive proteins.
4. React for at least 30 minutes at room temperature or 1 hour at 4°C. Continue to protect
the solution from light.
5. Remove excess crosslinker and reaction by-products by dialysis or size exclusion
chromatography.
6. Add the labeled bait protein to a sample containing the putative interacting prey proteins.
The quantity of bait protein to be added to a given sample should be within the same con-
centration level as the amount of prey proteins present. The optimal level of addition may
have to be determined by varying the amount of bait protein concentrations in a number
of sample solutions to decide which concentration results in the best interaction complexes
being formed.
7. Incubate the sample mixture for at least 1 hour at room temperature or under conditions
optimal for the interaction to be studied.
8. Expose the sample to UV light to photoactivate the aryl azide and cause the conju-
gation reaction to occur. For best results, use a UV lamp that irradiates in the range of
300–370 nm. Irradiate for 2–15 minutes, using briefer exposures when using UV lamps of
higher wattage.
9. Interaction complexes that are captured by the crosslinking reaction can be recovered or
analyzed using the biotin groups. For instance, immobilized streptavidin can be used to
purify the conjugates or a streptavidin–HRP conjugate can be used to probe a Western
blot after electrophoresis separation of the sample. Reduction of conjugates may be done
by cleavage with DTT of the disulfi de bond linking the bait protein. This can be done
using 50 mM DTT or 50 mM TCEP in aqueous solution to permit recovery of the bioti-
nylated prey proteins on immobilized streptavidin. Alternatively, the use of an affi nity
support having milder elution characteristics for biotinylated proteins, such as immobi-
lized monomeric avidin, would facilitate isolation of the biotinylated complexes or prey
proteins under non-denaturing conditions.
3. Trifunctional Label Transfer Reagents 1031
1032 28. Bioconjugation in the Study of Protein Interactions
4. Metal Chelates in the Study of Protein Interactions
Certain bifunctional metal chelating agents have been used to investigate protein interactions
by virtue of their ability to generate reactive oxygen species that affects protein structure in the
immediate vicinity of their modifi cation site. The following sections discuss two applications
of such chelate labels, one of which cleaves peptide bonds while the other one causes covalent
crosslinks to occur between interacting protein structures.
4.1. FeBABE for Protein Mapping Studies
Transition metals such as iron can catalyze oxidation reactions in aqueous solution, which
are known to cause modifi cation of amino acid side chains and damage to polypeptide back-
bones (see Chapter 1, Section 1.1; Halliwell and Gutteridge, 1984; Kim et al., 1985; Tabor and
Richardson, 1987). These reactions can oxidize thiols, create aldehydes and other carbonyls on
certain amino acids, and even cleave peptide bonds. The purposeful use of metal-catalyzed oxi-
dation in the study of protein interactions has been done to map interaction surfaces or identify
which regions of biomolecules are in contact during specifi c affi nity binding events.
Hydroxyl radical “footprinting”, as the technique is called, can produce a picture of where
ligand binding sites are located or map the interaction surfaces of protein complexes. An early
example of this application was used to map the binding site of protein–DNA interactions
(Tullius and Dombroski, 1986; Latham and Cech, 1989). Hydroxyl radicals are generated by
an EDTA chelated iron(II) complex using a redox reaction involving hydrogen peroxide and
ascorbic acid. In this case, the EDTA chelate is merely added to the solution of the interacting
protein and DNA. Nonselective cleavage of the DNA strand (and protein) then occurs every-
where except at the region in which the interaction takes place, thus leaving a “footprint” of
the interacting partners.
A second iteration of this reagent involved the attachment of affi nity ligands to the EDTA
chelator group to direct the hydroxyl radical generator to specifi c binding pockets on proteins
and receptors. Hoyer et al. (1990) created a biotin-EDTA derivative that was able to bind to the
biotin binding pocket on streptavidin and cause peptide cleavage in the region immediately sur-
rounding the binding site. Similarly, Schepartz and Cuenoud (1990) formed a trifl uoroperazine-
based affi nity reagent containing an EDTA group to map the binding site of the calcium
binding protein calmodulin. Once affi nity reagents of this sort bind to their receptor protein,
hydroxyl radical footprinting can identify exactly where in the protein ’s three-dimensional
structure the interaction took place.
Rana and Meares (1990b, 1991a, b) created a more versatile footprinting reagent by synthe-
sizing a bifunctional metal chelating agent with an EDTA group on one end and a bromoacetyl
group on the other end. This compound, Fe(III) (S)-1-( p -bromoacetamido-benzyl)ethylene
diamine tetraacetic acid (or FeBABE, pronounced “iron-babe”), resulted in a universal modi-
fi cation reagent for protein interaction footprinting, which could be used to study interacting
protein complexes or other biomolecules ( Figure 28.17 ). The bromoacetyl group of FeBABE
can react with a thiol to form a thioether bond ( Figure 28.18 ). Thus, proteins or other mol-
ecules containing a thiol can be covalently modifi ed with the FeBABE reagent forming a
hydroxyl radical probe to footprint interacting domains. Such modifi ed bait proteins are
termed “artifi cial proteases ”, because of their ability to chemically cleave peptide bonds.

Figure 28.17 FeBABE is a bifunctional chelating agent containing an EDTA group on one side and a thiol-
reactive bromoacetyl group on the other end. The EDTA group is coordinated with an iron ion.
Figure 28.18 FeBABE can be coupled to an available thiol group on a bait protein using the bromoacetyl reac-
tive group to form a thioether linkage.
The reactions involved in the cleavage of biological molecules by FeBABE and similar iron-
EDTA complexes have been theorized to involve two possible processes. Rana and Meares
(1991) speculated that the reaction proceeds by the coordination of peroxo oxygen to the iron
chelate group. They also found that O
2
might participate in this type of reaction, as evidenced
by the successful incorporation of
18
O into the carboxyl terminus of cleaved peptide chains of
human carbonic anhydrase. This peroxo-metal complex may facilitate subsequent nucleophilic
attack of oxygen on the carbonyl carbon of a peptide bond, which then results in cleavage of
the peptide bond through oxygen atom transfer to the carbonyl carbon, forming a C-terminal
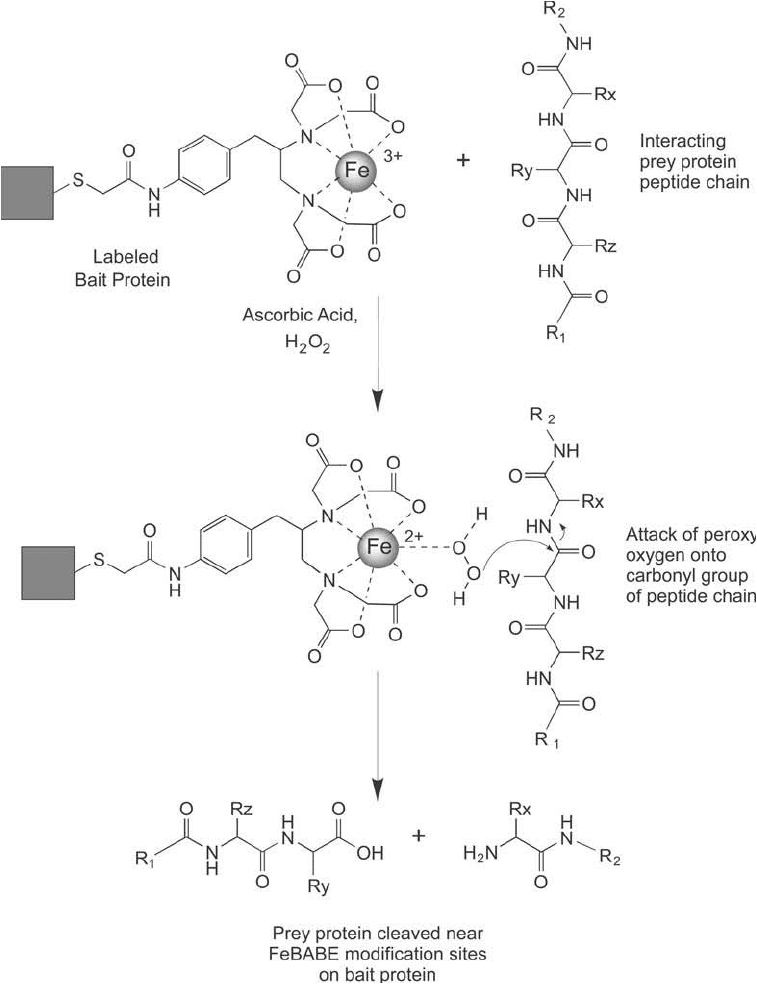
carboxylate. Figure 28.19 schematically shows part of this reaction sequence.
4. Metal Chelates in the Study of Protein Interactions 1033
1034 28. Bioconjugation in the Study of Protein Interactions
Figure 28.19 The cleavage reaction of FeBABE involves a catalytic process using peroxide and ascorbate to
form reactive oxygen species. Any protein structure in the immediate vicinity of the FeBABE label on the bait
protein will undergo peptide bond cleavage.

Another proposed mechanism involves the well-known Fenton reaction, which is catalyzed
effectively by Fe
2
-EDTA complexes. In this process, the ascorbate functions by reducing the
FeBABE Fe
3
complex to Fe
2
, producing the ascorbate radical (
Asc
). The reduced iron then
can form hydroxyl radicals through the Fenton reaction, which re-oxidizes the iron to the Fe
3
state. As additional Fe
3
is formed, ascorbate then regenerates the active Fe
2
chelate by reduc-
tion, resulting in the formation of the ascorbate radical. The entire process thus is catalytic and
may occur numerous times at the site of one FeBABE modifi cation, potentially resulting in a
number of peptide bond fragmentations. Notice also that the FeBABE reagent can be used in an
initial EDTA-Fe
3
form or in the reduced EDTA-Fe
2
form, as addition of the peroxide/ascor-
bate activators can catalyze hydroxyl radical formation from either metal oxidation state.
EDTA-Fe H O EDTA-Fe OH OH Fenton reaction
EDTA-Fe as
2
22
3
3
ccorbate EDTA-Fe Asc H Regeneration with ascorbate
2
The FeBABE modifying group is approximately 12 Å in length. Peptide bond cleavage can
occur anywhere on a protein surface that is within molecular distance of the iron chelate modi-
fying group. Cleavage probably doesn ’t occur at distant sites, because the hydroxyl radical is
extremely reactive and will quickly react with the neighboring peptide structure or be quenched
by ascorbate in solution. An interacting protein that is within the molecular distance of FeBABE ’s
reach therefore is susceptible to peptide bond cleavage.
In use, a bait protein labeled with FeBABE is bound to an interacting prey protein and the
peroxide/ascorbate cleavage reagents added to initiate the protein mapping reaction. After
the cleavage process is complete, the peptide fragments on each protein are analyzed to gleam
information about the interaction surfaces, which will be left relatively unaffected by the reac-
tion. Obviously, to utilize this technique the two interacting proteins must be highly pure and
their amino acid sequences known.
Typically, a bait protein is labeled with FeBABE at any available cysteine thiol group. If no
free thiols are available, disulfi des may be reduced to create sulfhydryls or a thiolation reagent
may be used to add them to the protein surface (see Chapter 1, Section 4.1). Care should be
taken when adding thiols through thiolation, however, because too high a substitution level
could affect the ability of the bait protein to bind to the prey protein. One to three thiol substi-
tutions on the surface of a bait protein should be suffi cient to modify it with FeBABE and study
protein interactions. A third option for adding a thiol is to change recombinantly one amino
acid in the bait protein ’s sequence to a cysteine. Site directed mutagenesis can create a thiol at a
known region of the protein ’s surface, thus permitting the study of protein interactions through
rational design of FeBABE modifi cations.
In order to facilitate analysis of FeBABE produced fragments, the prey protein or biomolecule
is labeled at one end with a tag that can be detected after electrophoresis, usually in a transfer
blot. The tag can be a fusion tag, such as 6 His, or any other group that can be targeted with
an antibody and detected. Alternatively, radiolabels and fl uorescent labels have been used with
prey molecules, including the use of end-labeled DNA to study where DNA binding proteins
dock onto the oligonucleotide sequence.
The fragments formed by FeBABE fragmentation are analyzed by comparing them to enzy-
matic or chemical cleavage patterns observed by treatment on the same prey protein. Since the
cleaved prey protein is detected by its end-labeled tag, the only fragments detected are those
4. Metal Chelates in the Study of Protein Interactions 1035
1036 28. Bioconjugation in the Study of Protein Interactions
that extend from the end labeled terminal to the point of cleavage. Comparison with known
fragmentation patterns using protease digestion or chemical cleavage provides information as
to the approximate size of the FeBABE fragment and its point of cleavage.
The following protocol assumes that the user has at least two pure proteins (or biomolecules)
of known sequences, which are able to interact specifi cally in solution. One of the two proteins
(the prey) is end labeled with a fusion tag or another detectable component and the other pro-
tein contains at least one thiol group. All buffers and reagents used in this protocol should be of
high purity and contain a very low metal content to prevent nonspecifi c cleavage reactions.
Protocol
This protocol is based on the procedure of Rana and Meares (1991) and the instruction book-
let provided by Thermo Fisher for use with the FeBABE kit.
1. Dissolve the bait protein to be modifi ed with the FeBABE reagent in 50 mM MOPS, 100 mM
NaCl, 1 mM EDTA, 5 percent glycerol, pH 8.2 (coupling buffer). The protein fi rst may be
treated by dialysis with EDTA to remove residual metals (30 mM MOPS, 4 mM EDTA, pH
8.2), and then dialyzed into the fi nal coupling buffer for the reaction with FeBABE.
2. Dissolve FeBABE in DMSO at a concentration of 5 mg/ml (8.5 nmol/ l) and add an aliq-
uot of this solution to the thiol-containing bait protein solution to obtain a 2- to 20-fold
molar excess of the reagent over the protein concentration. Protect the FeBABE solution
and the reaction mixture from light, as exposure to light will degrade the bromoacetyl
reactive group. The optimal molar excess of FeBABE over the bait protein may have to be
determined by experimentation. Since thiols are typically limited in number in most pro-
teins, an excess of FeBABE reagent will not result in more labels than the total number of
thiols present, provided the suggested reaction conditions are maintained.
3. Incubate the reaction mixture at room temperature or 37°C for 1 hour.
4. Purify the modifi ed bait protein from excess reactants by desalting or dialysis. During
this process buffer exchange the labeled bait protein into 50 mM MOPS, 120 mM NaCl,
0.1 mM EDTA, 10 mM MgCl
2
, 10 percent glycerol, pH 8.0. Components may be added
or removed from this buffer to promote the protein interaction being studied as long as
they don ’t interfere with the subsequent FeBABE cleavage reaction (i.e., they don ’t con-
tain transition metals like iron or copper, oxidants, or reducing agents).
5. Dissolve or buffer exchange the prey protein into 50 mM MOPS, 120 mM NaCl, 0.1 mM
EDTA, 10 mM MgCl
2
, 10 percent glycerol, pH 8.0.
6. Mix the labeled bait protein with the prey protein at equal molar ratios and incubate for
30 minutes at room temperature to create protein interaction complexes.
7. Initiate the cleavage reaction by the addition of an 8-fold molar excess of ascorbate (over
the concentration of protein) followed by an 8-fold molar excess of H
2
O
2
Mix well.
8. React for 10–30 seconds at room temperature.
9. Quench the cleavage reaction by the addition of an equal volume of SDS electrophoresis
sample buffer containing up to 40 percent glycerol. The SDS will denature the protein inter-
action and glycerol acts as a free radical scavenger, thus effectively quenching the reaction.
10. Analyze the cleaved proteins by SDS gel electrophoresis and Western blotting, followed by
specifi c detection of the prey protein label. Comparison of prey protein fragments formed
by chemical and enzymatic digestion can yield information as to the site of the resultant
FeBABE-mediated cleavage.
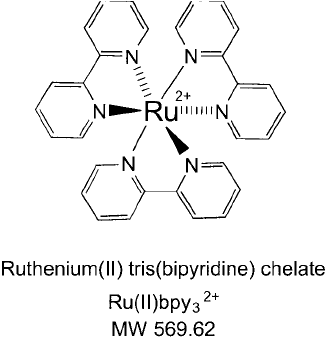
Figure 28.20 The structure of Ru(II)pby
3
2
a chelated ruthenium ion coordinated with three bipyridine groups.
4.2. Ru(II)bpy
3
2ⴙ
for Light-Triggered, Zero-Length Crosslinking of Protein Interactions
In many instances within cells or on cell membrane surfaces, large protein complexes are
formed containing many different proteins specifi cally interacting to effect biological processes.
Protein machines of this type are diffi cult to capture and study using traditional crosslinking
agents, because the resultant reactions may link some of the proteins, but not all of them in
the complex (Friedrichson and Kurzchalia, 1998; Simons et al., 1999). In addition, covalent
modifi cation of many nucleophilic groups in a protein by the electrophilic reactive groups of
standard crosslinking reagents can decorate the surface of proteins with these compounds and
affect nonspecifi c protein interactions.
Fancy and Kodadek (1999) developed a novel protein crosslinking process using a metal
chelating reagent that can be activated by visible light and effect zero-length crosslinks between
proteins. Photo-induced crosslinking of unmodifi ed proteins (PICUP) can capture protein inter-
actions without the use of modifying agents such as organic crosslinkers or fusion tags, thus
maintaining the interacting proteins in their native structure and conformation. The reaction is
mediated by the chelate ruthenium(II) tris(bipyridine) (Ru(II)bpy
3
2
; pronounced “ ru¯bippy ” ) in
a two-step reaction involving light absorption by the bipyridine groups followed by energy trans-
fer and excitation of the Ru(II) metal to an excited state intermediate. Subsequent reaction of
the excited state Ru(II)bpy
3
2
with persulfate anion results in the formation of a sulfate radi-
cal and Ru(III). The Ru(III) group then is postulated to undergo a one-electron transfer to tyro-
sine side chain phenolic groups in proteins, resulting in the formation of a tyrosine radical. This
highly reactive intermediate rapidly can react with neighboring nucleophilic groups in proteins
or with another nearby tyrosine phenolic group, resulting in the formation of tyrosine dimers or
a tyrosine heteroatom arene linkage with nearby nucleophilic groups. Either route of reaction
can result in the formation of covalent bonds between protein amino acids. If the linkages are
formed between interacting proteins, then these complexes are captured for subsequent analysis.
The proposed reactions of Ru(II)bpy
3
2
in the light-mediated zero-length crosslinking of interact-
ing proteins are shown in Figure 28.21 .
4. Metal Chelates in the Study of Protein Interactions 1037
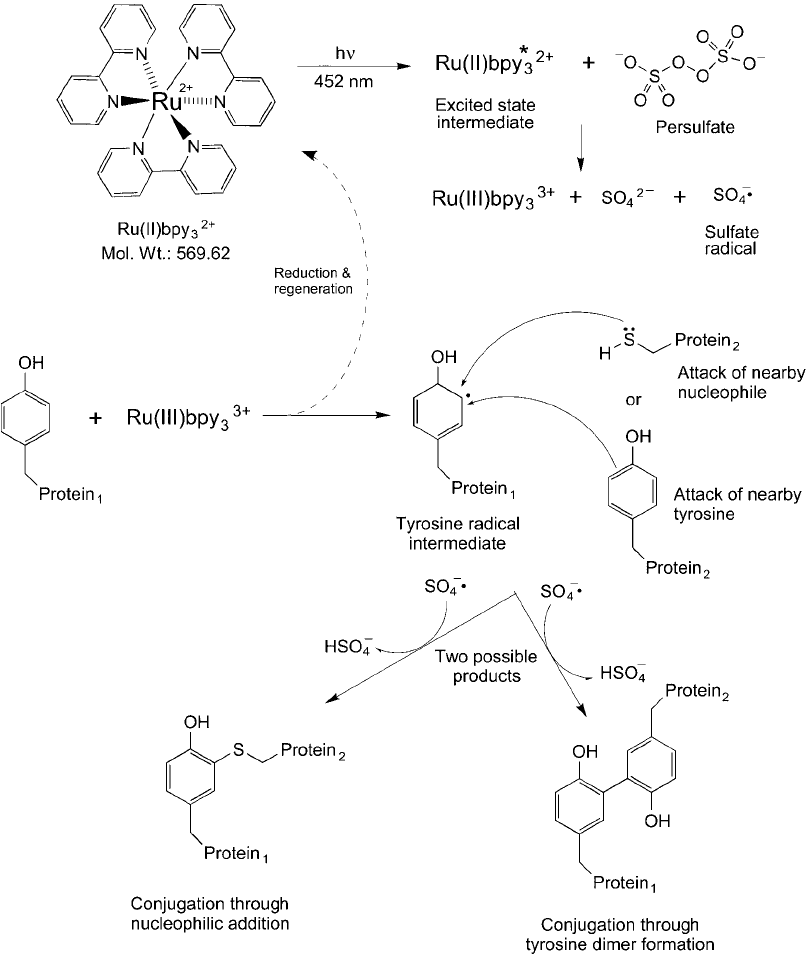
1038 28. Bioconjugation in the Study of Protein Interactions
Figure 28.21 The reactions of Ru(II)pby
3
2
are catalyzed by light at 452 nm that begins by forming an excited
state intermediate. In the presence of persulfate, a sulfate radical is formed concomitant with the oxidative
product Ru(III)bpy
3
3
. This form of the chelate is able to catalyze the formation of a radical on a tyrosine phe-
nolic ring that can react along with the sulfate radical either with a nucleophile, such as a cysteine thiol, or with
another tyrosine side chain to form a covalent linkage. The result of this reaction cascade is to cause protein
crosslinks to form when a sample containing these components is irradiated with light.
The following protocol successfully was used to study the interactions of the 180-amino acid
C-terminal domain of yeast TATA box binding protein (TBP), the Gal80 protein, and the Gal4
activation domain of the associated transcription factor complex (Fancy and Kodadek, 1999).
It also was used for the study of the amyloid -protein ( ) assembly (Bitan et al., 2003), to
characterize the tRNA-specifi c adenosine deaminase (tadA) from E. coli (Wolf et al., 2002) and
to understand the nature of the interaction between MIP-1a and proteoglycans (Ottersbach
and Graham, 2001). The reaction is very effi cient and typically can result in conjugation yields
of 60 percent or better between interacting proteins. In fact, the conjugation effi ciency may
yield crosslinks between proteins that are not specifi cally interacting. To limit the degree of
extraneous conjugation, the addition of a nucleophile in the reaction medium (e.g., histidine)
can help to restrict crosslinks only to proteins that are in close contact, as in those that are
forming interaction complexes at the time.
Protocol
1. Adjust the protein sample concentration to be between 0.01 M and 20 M by dilu-
tion in 20 l of reaction buffer: 15 mM sodium phosphate, 150 mM NaCl, 0.125 mM
Ru(bpy)
3
Cl
2
(pH 7.5). The protein sample should not contain any components that are
easily oxidized by the reaction, such as thiol reducing agents (e.g., DTT). The addition
of histidine to the reaction buffer (7.5 mM) can be done to modulate the crosslinking
reaction and limit the degree of protein conjugation between non-interacting proteins.
The presence of other nucleophiles also will inhibit the crosslinking reaction, such as Tris
buffer or DTT.
2. Add an aliquot of ammonium persulfate (APS) to make a fi nal concentration of 2.5 mM.
Protect from light until ready to start the reaction.
3. Illuminate the sample in a small open tube or the well of a microplate by placing a white
light source approximately 5–50 cm from the sample. If a weak light source is used, such
as a fl ashlight, move the light source closer to the sample, but if a strong photofl ood
lamp is used, then use the greater distance measurement to avoid heating the sample.
Illuminate the sample for 0.5–30 seconds, depending again on the intensity of the light
source.
4. Immediately after irradiation, stop the reaction by the addition of 7 l of 4 SDS elec-
trophoresis loading buffer or the equivalent (with a high concentration of reducing agent
present): 0.2 M Tris, 8 percent SDS, 2.88 M -mercaptoethanol, 40 percent glycerol,
0.4 percent xylene cyanol, 0.4 percent bromophenol blue. Heat the sample at 95°C for
5 minutes and analyze the complexes formed by electrophoresis.
4. Metal Chelates in the Study of Protein Interactions 1039
