Heitjans P., Karger J. (Eds.). Diffusion in Condensed Matter: Methods, Materials, Models
Подождите немного. Документ загружается.

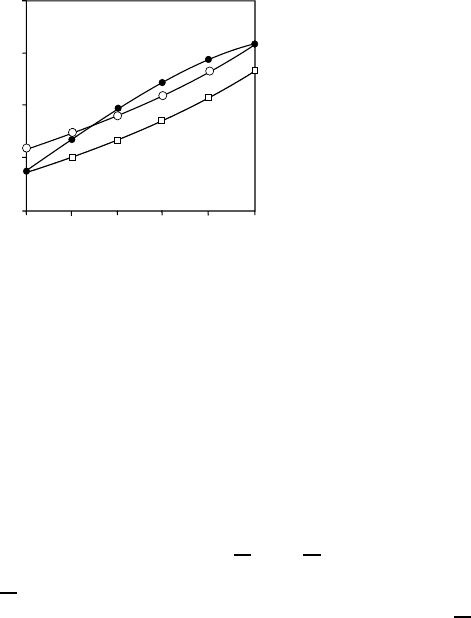
560 Hermann Weing¨artner
2
1.75
1.25
1.50
1
1
0 0.2
0.4
0.6
0.8
10 D/m s
9
2 1
x
1
Fig. 14.1. Composition dependence of diffusion coefficients in the system mesithy-
lene (1) + carbon tetrachloride (2) at 298.15 K: D
1
(open circles), D
2
(squares),
D
12
(filled circles).
frame of choice for ternary and multi-component systems in the presence of a
large excess of one of the components. Moreover, when describing diffusion in
electrolyte solutions, it is also quite natural to express mass flows in aqueous
electrolyte solutions relative to the solvent water at rest (see Chap. 17).
Presuming that experimental fluxes are indeed measured in the volume-
fixed frame, one can show that the two mass flows are not independent. The
special choice of the coordinate system imposes the condition [1]
J
1
V
1
+ J
2
V
2
=0. (14.5)
The
V
1
are the partial specific volumes of the components a =1, 2. Using the
thermodynamic definition of the partial specific volumes,
V
a
=(∂V/∂m
i
)
p,T
,
where m
a
is the mass of component a in the total volume V ,andtheir
interconnection via the Gibbs-Duhem equation, one can rigorously show that
D
12
= D
21
. (14.6)
Thus, the mutual-diffusion coefficient is one and the same value, regardless
of which of the components is monitored in the diffusion experiment.
Fig. 14.1 shows as an example the composition dependence of the mutual-
diffusion coefficient in the system mesithylene + carbon tetrachloride, which
is a standard example for systems with specific interactions between dislike
components [26]. This interaction between mesithylene and carbon tetrachlo-
ride is well established from thermodynamic data. The figure also contains
the self-diffusion coefficients of both components. It is seen that the concen-
tration dependence of D
12
differs markedly in shape from those of D
1
and D
2
.
It is also not correlated with the concentration dependence of other transport
coefficients such as the viscosity.
One may however note that at both ends of the concentration range self-
and mutual-diffusion coefficients approach one another. In fact, at trace con-
centration of a component the self-diffusion coefficient of this component

14 Mutual Diffusion in Mixtures 561
approaches the mutual-diffusion coefficient. This limiting behavior evolves
from rigorous theory, and is well established experimentally.
One attempt to bring some order into this confusing behavior is related
to a more detailed analysis of the driving force of diffusion. From the point of
view of thermodynamics, as described later in Sect. 14.4, the correct driving
force of diffusion is the gradient of the chemical potential rather than the
concentration gradient. Noting that
grad c
a
=
∂c
a
∂µ
a
grad µ
a
, (14.7)
and rewriting the concentration in terms of mole fractions leads to a trans-
formation factor of the form
Q =
dlna
1
dlnx
1
=
dlna
2
dlnx
2
, (14.8)
where a
i
is the activity of component i. Q is denoted as thermodynamic
factor. Therefore, one often considers the experimental diffusion coefficient
D
12
to consist of a product of a thermodynamic diffusion coefficient D
∗
12
which refers to the correct driving force and the correction term Q, i.e.
D
12
= D
∗
12
Q. (14.9)
From this line of arguing it is more convenient to compare and interpret data
for the quotient D
12
/Q rather than D
12
itself . In practice, the concentration
dependence of Q is often stronger than that of D
∗
12
, and the superposition
of the two terms often leads to quite complex concentration dependencies
of D
12
[1]. It is shown below that such a correction, often introduced by
qualitative reasoning, has indeed a theoretical basis.
Depending on the system, the magnitude of such corrections may be
largely different. Fig. 14.2 illustrates the behavior of Q for systems exhibiting
different thermodynamic situations. Thermodynamic ideality may be defined
by Raoult’s law, which implies that Q = 1. Examples for this type are binary
mixtures of rare gases, or hydrocarbons. Fig. 14.2 shows data for n-hexane +
n-dodecane mixtures, where Q is indeed very close to unity. Model calcula-
tions show that, to a first approximation, binary mixtures of Lennard-Jones
fluids are ideal as well [6] . Thus, for ideal mixtures the correction does not
play a role at all. The same is true for sufficiently dilute solutions, because
for x
1
→ 0andx
2
→ 0 we have by definition Q → 1.
Systems with aggregation between dislike components show negative de-
viations from Raoult’s law and one finds Q>1. This behavior is illustrated in
Fig. 14.2 for mesithylene + carbon tetrachloride. In contrast, self-aggregation
of the components reflects itself by positive deviations and 0 <Q<1. Ex-
amples are solutions of alcohols or carboxylic acids in comparatively inert
solvents such as carbon tetrachloride. Fig. 14.2 shows, as an example, data
for methanol + carbon tetrachloride, where in parts of the concentration

562 Hermann Weing¨artner
1.50
1
1
0 0.2
0.4
0.6
0.8
x
0.50
0
(1)
(2)
(3)
1
Q
Fig. 14.2. Composition dependence of the thermodynamic factor Q in the systems
mesithylene + carbon tetrachloride (1), n-hexane + n-dodecane (2), and methanol
+ CCl
4
(3) at 298.15 K. The first-mentioned components are denoted as compo-
nent 1.
range Q differs by more than an order of magnitude from unity. Thus, ther-
modynamic non-ideality enhances diffusion in the case of negative deviations
of Raoult’s law and slows down diffusion for positive deviations of Raoult’s
law.
In view of these reasonings one often reformulates the Darken relation (2)
by
D
12
=(x
2
D
1
+ x
1
D
2
) Q. (14.10)
In the case of thermodynamically non-ideal systems, an incorporation of the
thermodynamic factor often leads to an improved estimate of D
12
in compar-
ison with Darken’s original relation (14.2). In fact, in most cases the Darken
relation is applied in the form of (14.10) rather than (14.2). We show in
2
1
1
0 0.2
0.4
0.6
0.8
x
0
3
4
5
10 D/m s
9
2
1
1
Fig. 14.3. Composition dependence of diffusion coefficients in the system n-hexane
(1) + n-dodecane (2) at 298.15 K: D
1
(open circles), D
2
(squares), D
12
(filled
circles). n-hexane is component 1.

14 Mutual Diffusion in Mixtures 563
2
1.75
1.25
1.50
1
1
0 0.2
0.4
0.6
0.8
x
1
1
10 D/m s
9
2 1
Fig. 14.4. Composition dependence of diffusion coefficients in the system methanol
(1) + carbon tetrachloride (2) at 298.15 K: D
1
(squares), D
2
(open circles), D
12
(filled circles).
Fig. 14.3 diffusion data for the near-ideal system n-hexane + n-dodecane [4].
Equation (14.10) represents the experimental data for D
12
practically within
the limits of experimental uncertainty.
However, even in this improved form, the Darken equation is of little use
for predicting diffusion data for systems with comparatively strong specific
interactions between the components. We show in Fig. 14.4 experimental
data for methanol + carbon tetrachloride [27] with strong self-aggregation
of methanol molecules. Fig. 14.5 shows that (14.2) overestimates D
12
by
more than a factor of two in parts of the concentration range. If (14.10)
is used instead, D
12
is largely underestimated. Near the minimum of D
12
experimental and predicted data differ by an order of magnitude.
(14.2)
(14.10)
Fig. 14.5. Estimate of mutual-diffusion coefficients in the system methanol +
carbon tetrachloride by (14.2) and (14.20), respectively. Methanol is component 1.

564 Hermann Weing¨artner
Finally, we note that in thermodynamics (14.8) is just a measure for the
thermodynamic stability of mixtures, because the thermodynamic stability
condition for mixtures can be rewritten in terms of Q, leading to Q>0. In
other words, the spinodal line for liquid-liquid phase separation is defined
by Q = 0. This stability criterion is therefore often denoted as condition for
diffusional stability. As a consequence, when approaching the spinodal line,
mutual diffusion slows down, because the driving force vanishes. Very slow
diffusion in systems of comparatively low viscosity may therefore imply that
one is close to phase separation or to a critical point. This observation is well
established experimentally for systems with liquid-liquid phase separations
like triethylamine + water [28] or n-hexane + nitrobenzene (see Fig. 15.14
of Chap. 15).
14.4 Thermodynamics of Mutual Diffusion
By using the thermodynamics of irreversible processes, transport phenomena
can be described in a unified formalism [1, 29, 30]. This type of approach is
particularly adequate, when seeking for a starting point for a microscopic
description of transport phenomena. Again, it is necessary to define a ref-
erence frame to which the fluxes are referred to. Although, in principle, the
equations of the thermodynamics of irreversible processes can be set up in
any of these frames, the desired interconnection with statistical mechanics
suggests to do this analysis in the barycentric frame with the center-of-mass
at rest.
To transform Fick’s law to the barycentric frame we first introduce the
partial mass densities of the components as !
i
= m
i
/V . Moreover, we consider
specific chemical potentials defined by the mass derivative of the Gibbs free
energy, µ
∗
1
, rather than chemical potentials defined as partial molar quantities.
Then, Fick’s law may be formulated as
−J
m
1
= D
m
12
grad !
1
and
−J
m
2
= D
m
21
grad !
2
. (14.11)
Superscript m indicates quantities defined in the barycentric frame. Pro-
cedures for reference-frame transformation are extensively discussed in the
literature [1, 29, 30]. Detailed analysis shows that the diffusion coefficients
D
m
12
and D
m
21
are not equal, but interrelated by
D
m
12
V
2
= D
m
21
V
1
. (14.12)
However, within the framework of thermodynamics of irreversible processes,
the gradient of the partial mass density is not a suitable driving force. To
derive the correct force we consider linear equations between fluxes J
i
and
forces X
i
of the form

14 Mutual Diffusion in Mixtures 565
−J
1
=L
11
X
1
+L
12
X
2
+L
13
X
3
+···
−J
2
=L
21
X
1
+L
22
X
2
+L
23
X
3
+···
−J
3
=L
31
X
1
+L
32
X
2
+L
33
X
3
+···
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
(14.13)
Thermodynamics of irreversible processes says that the fluxes and forces have
to be chosen in such a way that their product is equal to the entropy pro-
duction Tσ of the system. In the latter case the coefficients L
ij
in (14.13) are
called phenomenological coefficients. One can show that in the barycentric
system the entropy production is given by [1, 29, 30]
Tσ = −J
m
1
grad (µ
∗
1
− µ
∗
2
) (14.14)
or an analogous expression for the flow of component 2. Thus, the correct
driving force is the gradient of the specific chemical potential. In a binary
system the phenomenological equations are then given by
−J
m
1
= L
11
grad µ
∗
1
+ L
12
grad µ
∗
2
,
−J
m
2
= L
21
grad µ
∗
1
+ L
22
grad µ
∗
2
. (14.15)
There are, however, two restrictions among the fluxes and forces. First, the
fluxes are not independent. If the local center-of-mass is at rest we have
J
m
1
+ J
m
2
= 0 (14.16)
which represents the analogue to (14.5). Furthermore, the Gibbs-Duhem
equation interrelates the specific chemical potentials through
!
1
grad µ
∗
1
+ !
2
grad µ
∗
2
=0. (14.17)
It can be shown [1, 29, 30] that these restrictions lead to the condition
L
12
= L
21
= −L
11
= −L
22
. (14.18)
Thus, information on the mutual-diffusion coefficient is sufficient to determine
all four phenomenological coefficients. This simplicity is, however, lost in
systems composed of more than two components. In detail, one finds
D
12
= −
(x
1
M
1
+ x
2
M
2
)
2
x
1
x
2
M
1
M
2
dlna
1
dlnx
1
L
12
. (14.19)
M
i
is the molecular mass of component i.
Let us briefly consider this situation from a more general point of view.
In a system of r components there are r forces and r fluxes described by
the phenomenological relations (14.13). Accordingly, one obtains a matrix of
phenomenological coefficients
566 Hermann Weing¨artner
⎛
⎜
⎜
⎜
⎝
L
11
L
12
···L
1r
L
21
L
22
···L
2r
.
.
.
.
.
.
.
.
.
.
.
.
L
r1
L
r2
···L
rr
⎞
⎟
⎟
⎟
⎠
. (14.20)
The coefficients of this matrix are not independent, because the gradients of
the chemical potentials are interrelated by the Gibbs-Duhem relation and the
sum of the fluxes has to be zero. For a binary system these restrictions lead
directly to (14.18). For ternary and higher-order systems these conditions
are not sufficient to determine the matrix. There is, however, an additional
postulate, saying that the matrix of the coefficients has to be symmetric, i.e.
L
ij
= L
ji
. (14.21)
This is Onsager’s well-known reciprocity relation which, in a molecular pic-
ture, reflects microscopic reversibility. Thus, in general, in a system of r
components there are only r · (r − 1)/2 independent coefficients.
Now, for a binary system with r = 2 we are back to a single diffusion
coefficient. However, for a ternary system, presuming the reciprocity relation,
we have already three independent diffusion coefficients. If, however, one
aims at proving the Onsager relation rather than implementing this relation
in data analysis, one has to extract four coefficients from the experimental
concentration distribution [1, 20].
One of the pressing questions is of course, whether the cross terms of the
matrix with i = j are sufficiently different from zero to be of importance in
data analysis. Otherwise, the analysis is largely simplified. In fact, in many
models for diffusion in complex systems, approximations are made which
implicitly imply that the cross terms are negligible. However, in this regard
the answer by accurate experiments is desillusioning: The cross terms may
be even larger in magnitude than the main terms [22,23].
Finally, we consider briefly systems with charged species such as elec-
trolyte solutions or molten salt mixtures. Thermodynamically, an electrolyte
solution consisting of the salt (s) and water (w) is a binary system with a
single mutual-diffusion coefficient D
sw
, because diffusion arises from the gra-
dients of the neutral salt and water. There cannot be gradients of single ions,
because the concentrations of the ions are not independent variables, but are
interrelated by the constraint of overall charge neutrality. Then, with regard
to mutual diffusion there is a set of four Onsager coefficients, which according
to (14.18) are interrelated by
L
sw
= L
ws
= −L
ss
= −L
ww
(14.22)
However, one can also try to apply the formalism of the thermodynam-
ics of irreversible processes to a ternary system consisting of the cation (c),
anion (a) and the solvent water (w). (Note that the three self-diffusion co-
efficients of the ionic constituents can indeed be measured separately.) Such
14 Mutual Diffusion in Mixtures 567
a formalism yields a 3 × 3 matrix of Onsager coefficients. We denote these
coefficients by l
ij
(i, j = c,a,w). It is an intriguing problem to relate the l
ij
of the ternary model system to the L
ij
of the salt-water system obtained by
direct thermodynamical analysis.
By symmetry, only three of the nine l
ij
of the 3×3 matrix are independent.
Obviously, in addition to the mutual-diffusion coefficient, two other exper-
imental quantities are needed for determining the l
ij
. The solution of this
problem is given by the fact, that thermodynamics of irreversible processes
relates two other transport coefficients to the l
ij
[29, 30], namely the ionic
mobilities of the cations and anions in the electric field. These can be deter-
mined directly, for example, by the new technique of electrophoretic NMR
discussed in Chap. 17. Traditionally, one measures, however, the electrical
conductance of the solution and the transference numbers that allow to sep-
arate the conductance into single-ion contributions. The resulting formalism
to determine the Onsager coefficients l
ij
has been worked out in detail long
time ago by Miller [31].
14.5 Linear Response Theory and Time Correlation
Functions
In statistical mechanics the time dependence of the dynamical variables of
N-particle systems are described by Hamilton’s equations of motion. These
can, for example, be solved for some hundreds or thousands of particles by
numerical solution in molecular dynamics simulations ([2] and Chap. 23). Self-
diffusion coefficients can then be derived from the simulated trajectories, e.g.
via (14.1).
For an analytical description it is only possible to consider certain time
and ensemble averages. For this purpose one considers the time dependence
of the N-particle correlation function ( [6,32], see also Chap. 23)
F
N
(r
N
, p
N
),
where r
N
and p
N
stand symbolically for the generalized position and mo-
mentum vectors of the N-particle system. At equilibrium, this correlation
function is given by
F
0
N
=const· exp(−H
0
/kT ), (14.23)
where H
0
is the classical Hamiltonian of the N-particle system. If there is
a weak perturbation due to an external force, the Hamiltonian contains an
additional contribution H
ext
, i.e. H
0
is replaced by
H = H
0
+ H
ext
. (14.24)
In this case, the time dependence of the correlation function is given by
Liouville’s equation

568 Hermann Weing¨artner
∂F
N
∂t
= iLF
N
, (14.25)
where
L=i
N
k
∂H
∂p
k
∂
∂r
k
−
∂H
∂r
k
∂
∂p
k
(14.26)
is the Liouville-Operator. If H
ext
is known, one can calculate expectation
values of the dynamical variables due to this perturbation. In the case of
diffusion the perturbation is related to a local gradient in the specific chemical
potential.
In the regime of linear response due to sufficiently weak perturbations,
the application of this formalism shows that any transport coefficient A can
be written as an integral over a time correlation function of the form [6,32]
A ∝
∞
0
J
A
(0) · J
A
(t)dt, (14.27)
where J
A
(τ) is the flux associated with A at time τ =0andt, respectively.
Expressions of this type are denoted as Green-Kubo relations.
Probably the most well-known and most simple example of a Green-Kubo
relation is the expression for the self-diffusion coefficient D
i
in terms of the
velocity auto-correlation function
D
a
=
1
3
∞
0
v
a,α
(0) · v
a,α
(t)dt, (14.28)
where the velocity of a particle α of component a at time t = 0 is correlated
with that of the same particle at time t. These velocities refer to the barycen-
tric reference frame. Because self-diffusion does not lead to macroscopic gra-
dients in the system, the barycentric frame coincides with the volume- and
laboratory-fixed frames. In other words, the self-diffusion coefficient is inde-
pendent of the reference frame.
By using the proper relation between position vectors and velocities one
can show that (14.28) is fully equivalent to the relation for the self-diffusion
coefficient in terms of the mean square displacement, (14.1). For example,
when determining the self-diffusion coefficient in molecular dynamics simu-
lations it is only a technical problem, whether the self-diffusion coefficient is
extracted via (14.1) or (14.28) [2] (see also Chap. 23).
Moreover, another equivalent expression may be mentioned which is par-
ticularly suitable when monitoring diffusion processes in neutron scattering
experiments (see Chaps. 2, 3, and 13). In this case, the quantity of inter-
est is the Van Hove dynamic structure factor S(κ, ω), where κ and ω refer
to the momentum and energy transfer in the scattering experiment. If one
rewrites (14.1) in terms of S(κ, ω) one finds the self-diffusion coefficient to be

14 Mutual Diffusion in Mixtures 569
related to the hydrodynamic (κ → 0,ω→ 0) limit of the self-part of the dy-
namical structure factor S
S
(κ, ω) which is available by incoherent scattering
experiments [6]
D
a
= π lim
ω→0
lim
κ→0
ω
2
κ
2
S
S
a
(κ, ω) . (14.29)
In fact, it is well-known that in the hydrodynamic limit incoherent neutron
scattering leads to a Lorentzian spectrum with a half-width determined by
the self-diffusion coefficient [6] (see also Chap. 3).
14.6 The Time Correlation Function for Mutual
Diffusion
In order to obtain the equivalents to (14.1), (14.28), and (14.29) for mutual
diffusion, the Green-Kubo theory has to be applied to the phenomenological
coefficients L
ab
. The result is [32]
L
ab
=(3kTV )
−1
∞
0
J
a
(0) · J
b
(t)dt, (14.30)
where J
i
is the microscopic mass flow of species i = a or b in the barycentric
frame. This mass flow has to be summed up over the velocities of all particles
of component i:
J
i
(t)=
N
i
k=1
m
i
V
v
i,k
(t); i = a, b. (14.31)
We consider first the expression for L
12
in the case of which a =1and
b = 2. Then, according to (14.30) the velocities of the species of component 1
are coupled to those of the species of component 2. If (14.31) is inserted
into (14.30) one obtains an expression involving velocity cross-correlations for
particles of different components, in contrast to the velocity auto-correlation
function entering into the expression for the self-diffusion coefficient.
Alternatively, we may consider the coefficient L
11
(or, by interchange of
indices, L
22
), instead of L
12
. Then, the situation turns out to be different.
After inserting (14.31) into (14.30) and expanding the sums, we can sort out
two different types of terms: In the first type, the velocity of a particle α of
component 1 (or 2) at time zero is correlated with that of the same particle α
at time t. However, there are also contributions, which correlate the velocity
of a particle α of component 1 (or 2) with that of a distinct particle β of the
same component. This suggests to split up L
11
(and L
22
) into a contribution
from a velocity auto-correlation function plus a contribution from velocity
cross-correlations of distinct particles of the same component. We note that
