Heitjans P., Karger J. (Eds.). Diffusion in Condensed Matter: Methods, Materials, Models
Подождите немного. Документ загружается.

5 Diffusion in Oxides 231
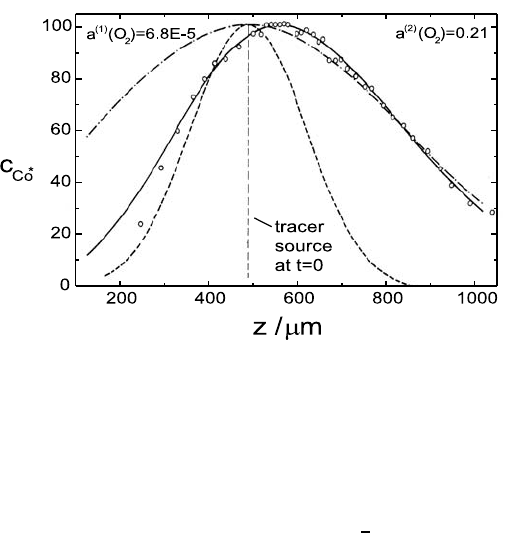
Fig. 5.12. Normalised tracer concentration profile of
60
Co in CoO in an oxygen
potential gradient (T = 1210
◦
C, a
(1)
O
2
=6.8 · 10
−5
, a
(2)
O
2
=0.21, ∆z = 1184 µm,
t = 15 h) [20]. ◦ experimental concentrations; — best fit using (5.37); – – – Gaussian
profile for a homogeneous crystal at a
(1)
O
2
;–·– Gaussian profile for a homogeneous
crystal at a
(2)
O
2
.
z
max
(t) − z
marker
=
˜
D −
1
2
· f
0
· D
V
· b · t. (5.38)
Figure 5.12 shows a typical tracer concentration profile of
60
Co in Co
1−δ
O
in an oxygen potential gradient. By fitting of (5.37) the vacancy diffusion
coefficient, D
V
, and the chemical diffusion coefficient,
˜
D,canbedetermined
simultaneously. Using (5.31) the average excess charge of the cation vacan-
cies was found to be α =0.86 [20]. This value is in reasonable agreement
with values calculated on the basis of equilibrium constants (see Sect. 5.2.1).
For comparison, the Gaussian profiles which one would obtain in a homoge-
neous crystal equilibrated at oxygen activities a
(1)
O
2
and a
(2)
O
2
are also shown in
Fig. 5.12. The actual profile lies between the two Gaussian profiles.
Similar experiments have been performed for tracer diffusion of Fe in
magnetite, Fe
3
O
4
, which was exposed to an oxygen potential gradient [54].
Due to the high electronic disorder in magnetite (Fe
2+
and Fe
3+
ions are
regular structure elements) the chemical diffusion coefficient,
˜
D,isidentical
to the vacancy diffusion coefficient, D
V
, in the vacancy regime, and identical
to the interstitial diffusion coefficient, D
I
, in the interstitial regime [20]. This
means, that the two parameters which can be determined simultaneously
are the self-diffusion coefficient of the dominating defect and the correlation
factor for tracer diffusion via this defect. In contrast to oxides with the NaCl-
structure, e.g. CoO, the effective correlation factor for a jump sequence in the
spinel Fe
3
O
4
cannot be specified a priori since several vacancy and interstitial
or interstitialcy mechanisms are possible in magnetite. This is due to the fact

232 Manfred Martin
that in the spinel structure two regular cation sites exist, an octahedrally
and a tetrahedrally coordinated site. Likewise, several interstitial positions
are possible.
In the vacancy regime, three basic vacancy mechanisms are discussed.
They are characterised by jumps only within the octahedral sublattice, only
within the tetetrahedral sublattice [55], or jumps between these two sub-
lattices [56]. The geometrical correlation factors for these mechanisms are
f
V
=0.50, f
V
=0.56, and f
V
=0.73, respectively. The correlation fac-
tor obtained from tracer experiments in an oxygen potential gradient is
f
V
=0.56±0.07 [54] indicating preferred diffusion of Fe within the octahedral
sublattice, in agreement with results of in situ M¨oßbauer spectroscopy [56].
In the interstitial regime, the correlation factor obtained from tracer ex-
periments in an oxygen potential gradient is f
I
=0.43 ± 0.1. Only a direct
interstitial mechanism (f
I
= 1) can be excluded. The experimental accu-
racy is, however, too small to distinguish between different interstitial or
interstitialcy mechanisms for which the theoretical correlation factors, f
I
,are
between0and1[55].
Impurity Diffusion in an Oxygen Potential Gradient
The flux of impurity ions, B, in an oxide which is exposed to an oxygen
potential gradient consists also of two parts: a pure, diffusion term, which is
characterized by the impurity diffusion coefficient, D
B
, and which describes
Brownian motion of the impurity ions, and a second part which is again a drift
term caused by the directed flux of vacancies which drift through the oxide.
However, in contrast to self-diffusion, where the drift flux was always opposite
to the vacancy flux and directly proportional to the fraction of tracers, now
the magnitude and direction of the drift flux have to be calculated with
the aid of linear transport theory. This means that transport coefficients L
ij
(i, j =A, B) have to be used which describe the coupled transport of A and
B under the influence of an applied oxygen potential gradient. The impurity
drift flux was calculated in detail in [20] and can be written as
j
drift
B
= −j
V
· x
B
·
D
B
D
A
· β. (5.39)
As expected, the impurity drift flux is proportional to the vacancy flux, j
V
,
and the fraction of impurities, x
B
. In addition it is proportional to the ra-
tio of the diffusion coefficients of the impurity, D
B
,andthesolvent,D
A
.
This ratio reflects the fact that impurities and solvent ions exchange with
different rates with vacancies. As a result they are moved differently by the
vacancies. In contrast to these first terms, which are model independent, the
last factor, β, depends on the microscopic model used. It describes essen-
tially the interaction of the impurity with its neighbours which can be small
(as is expected for homovalent impurities) or stronger (for aliovalent impuri-
ties). Particularly for higher valent impurities which possess a positive excess

5 Diffusion in Oxides 233
charge there can be strong interaction with the negatively charged vacancies.
Now the two possible limiting cases will be discussed: First, the situation
where the interaction is small. This means that impurities and vacancies
should drift in opposite directions, as in the case of self-diffusion. Second, the
case of attractive interaction between impurities and vacancies. Then impu-
rities and vacancies form bound pairs and the impurities should drift in the
same direction as the vacancies. This qualitative picture is confirmed by the
theoretical results for the constant β in (5.39) which was calculated in [57]
within the five-frequency model. Within this model the transport coefficients
L
ij
(i, j =A, B) are known exactly [22]. The five-frequency model (Fig. 1.18,
note also footnote 15 in Chap. 1) is a nearest-neighbour model and uses five
exchange rates of vacancies and ions: ω
0
and ω
1
for exchange of vacancies
with solvent ions in the pure crystal (i.e. far away from the impurity) and
in the next neighbourhood of the impurity; ω
3
for vacancy jumps, which dis-
sociate an impurity-vacancy pair; ω
4
which creates a new pair; and ω
2
for
exchange of a vacancy and an impurity. For strong binding the parameter β
is negative, yielding an impurity drift flux in the same direction as the vacan-
cies, while for weak binding β is positive, and the drift flux is opposite to the
vacancies. Thus, an impurity drift experiment in an oxygen potential gradient
performed in the same way as described for tracer self-diffusion shows first
bythedirectioninwhichtheprofilemoveswhetherstrongorweakimpurity-
vacancy binding prevails, and second allows the determination of ratios of
the exchange jump rates ω
i
(i =0, 1, 2, 3, 4).
An example is impurity diffusion of Fe in CoO in an oxygen potential
gradient. It was found, that the drift direction of the Fe-tracer profile depends
on the oxygen potential region [19]. In region I (log a
O
2
≈−2) the profile
drifts to the high oxygen potential side, i.e. opposite to the vacancies, while in
region II (log a
O
2
≈−8) the profile shifts to the low oxygen potential side, i.e.
in the same direction as the vacancies. The impurity diffusion coefficient, D
∗
Fe
,
and the constant β in (5.39) can be obtained from the profiles. To calculate
from these data the vacancy exchange rates ω
i
in the five-frequency model, or
at least ratios of them, two additional experimental parameters are needed.
These are: (i) the impurity correlation factor which was obtained from the
isotope effect [58] and which changes from 0.78 in region I to 0.87 in region
II. (ii) the impurity-vacancy binding energy, ∆g
pair
, which was obtained from
the p
O
2
-dependence of the Fe-tracer diffusion coefficient. It is small compared
to the thermal energy in region I and about 0.7 eV in region II [19]. All four
quantities are known exactly as functions of the five rates ω
i
(i =0,1,2,3,
4) within the five-frequency model [22]
9
. As a result four ratios of exchange
rates can be calculated in regions I and II, respectively:
9
The dependence of the impurity diffusion coefficient and the impurity correlation
factor on the exchange ratees ω
i
can be found in Sect. 1.9.1 in Chap. 1, the
expression for β in [19], and the relation for the impurity-vacancy binding energy
is given by ω
4
/ω
3
=exp(−∆g
pair
/k
B
T ).
234 Manfred Martin
region I: ω
3
/ω
1
=0.29, ω
2
/ω
1
=0.52, ω
4
/ω
0
=0.53, ω
4
/ω
3
∼
=1
region II: ω
3
/ω
1
=0.02, ω
2
/ω
1
=0.14, ω
4
/ω
0
=0.60, ω
4
/ω
3
∼
= 300
While the ratios ω
2
/ω
1
and ω
4
/ω
0
remain nearly unchanged in passing
from region I to region II, ω
4
/ω
3
increases drastically and ω
3
/ω
1
decreases.
This is mainly due to the fact that the escape rate, ω
3
, decreases in pass-
ing from region I to region II indicating the transition from weak to strong
binding between Fe and vacancies. If the binding is mainly due to Coulom-
bic interaction between oppositely charged structure elements, one cause for
the change in ∆g
pair
could be the change in the relative concentrations of
the differently charged cation vacancies. At high oxygen activities (region I)
vacancies V
Co
dominate, whereas vacancies V
Co
become more important at
lower oxygen activities (region II). As a result, there is stronger Coulombic
interaction with trivalent iron at lower oxygen activities. On the other hand,
iron exists as Fe
2+
and Fe
3+
, and the fraction of trivalent iron decreases with
decreasing oxygen activity, which results in a smaller degree of association.
Another possible cause for the increase of ∆g
pair
goes back to the different
sizes of di- and trivalent iron ions. The larger ionic radius of Fe
2+
would
result in displacements of neighbouring ions which may be compensated by
association of a vacancy.
Demixing in an Oxygen Potential Gradient
If, instead of a pure oxide, a mixed oxide, (A
1−x
B
x
)O, in which oxygen is im-
mobile, is exposed to a stationary oxygen potential gradient, again different
cation vacancy fractions are established at the high and low oxygen potential
sides. However, the resulting cation vacancy flux from the high to the low
oxygen potential side causes fluxes of both cations, A and B. In general, they
have different mobilities and the oxide will become enriched in the more mo-
bile cation at the high oxygen potential side, while it will become enriched in
the less mobile cation at the low oxygen potential side (see Fig. 5.13). This
kinetic demixing process was studied first by Schmalzried et al. [59,60] consid-
ering steady-state demixing and homovalent solid solutions, e.g. (Co,Mg)O,
where Co is the faster cation and becomes enriched at the high oxygen poten-
tial side of the oxide. The formal solution of the transient demixing problem
with moving boundaries and time-dependent boundary values can be found
in [61].
However, kinetic demixing may also be important in doped oxides
(A
1−x
B
x
)O, where the kinetic segregation of impurities is of interest, e.g.
during sintering or alloy corrosion [62]. The basis for the subsequent analysis
is given by the general transport equations [44]
j
i
= −
j
L
ij
·∇η
j
(5.40)
where L
ij
are the transport coefficients and η
j
is the electrochemical potential
(see Sect. 5.4). As shown in [63], in a dilute oxide, A
1−x
B
x
O(x 1)), the
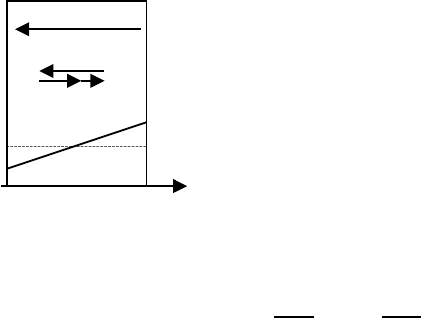
5 Diffusion in Oxides 235
2
O
plow
2
O
phigh
A
V
h
AB
x
A
(z)
0
A
x
0 zz
Fig. 5.13. Schematic representation of
the fluxes of electron holes, cation va-
cancies and cations A and B, and the
initial and steady state concentration
profile of A in a mixed oxide (A,B)O
exposed to an oxygen potential gradient
for the case that component A is faster
than B.
flux of the dopant, B, can be written as
j
B
= −D
B
·∇c
B
− j
V
·
L
BB
L
AA
·
γ +
L
BA
L
BB
. (5.41)
The dimensionless quantity γ contains all physical correlation effects and is of
the order of unity. Equation (5.41) shows that the dopant flux consists of two
terms, a pure diffusion term, −D
B
∇c
B
, characterized by the dopant diffusion
coefficient, D
B
(= L
BB
RT/c
B
), and a drift term, which is proportional to
the vacancy flux, j
V
. The direction of the drift term, which determines at
which side of the sample the dopant becomes enriched, depends crucially
on the sign and magnitude of the non-diagonal element L
BA
.Particularlyin
trivalent doped oxides, AO(+B
2
O
3
), solute-vacancy interactions might result
in a non-vanishing cross term L
AB
. Thus the demixing behaviour or the
kinetic segregation of the dopant B strongly depends on the strength of the
solute-vacancy binding energy.
Demixing experiments with Ga
2
O
3
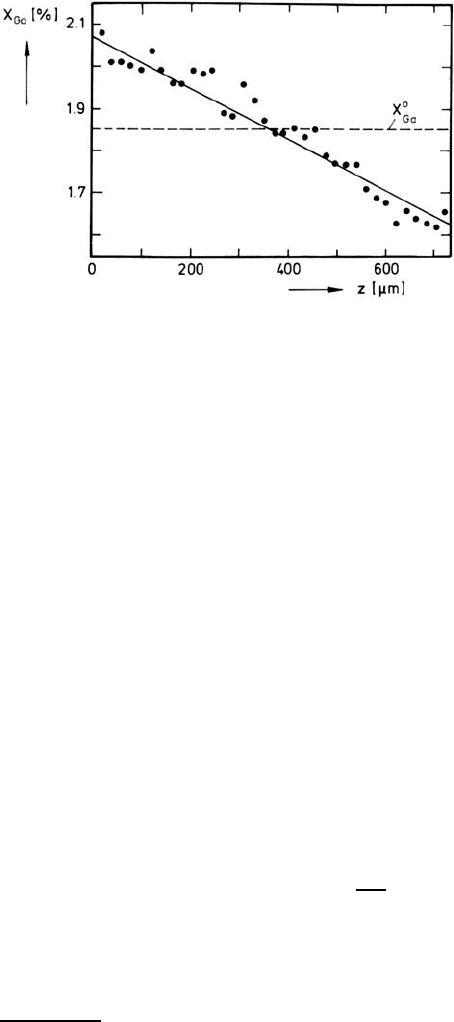
-doped CoO [64] clearly show demix-
ing of Co and Ga with enrichment of Ga at the low oxygen potential side
(see Fig. 5.14). Since the tracer diffusion coefficients of Co and Ga are also
known (see Sect. 5.3.1), the ratio L
GaCo
/L
GaGa
can be obtained from the
demixing profile using (5.41). The result is L
CoGa
/L
GaGa
= −1.7, leading to
the following interpretation. Due to the strong binding between the dopant
Ga and the vacancies the drift flux of the dopant is directed to the side of
lower oxygen potential (i.e. in the same direction as the vacancy flux), where
the dopant Ga therefore becomes enriched. As shown in detail in [64] the
result L
CoGa
/L
GaGa
= −1.7 can be explained adequately in terms of the
five-frequency model of impurity diffusion [22] and strong impurity-vacancy
binding, which was found independently in the tracer diffusion studies of this
system [21].
Finally it should be mentioned that demixing in an oxygen potential gra-
dient might be important also in oxygen ion conductors, such as yttria-doped
zirconia (YSZ) or doped lanthanum gallate (LSGM). When these oxides
are used as electrolytes, e.g. in solid oxide fuel cells (SOFCs), oxygen ions
are driven through the electrolyte and simultaneously electrons are flowing
through the external circuit. As soon as the cations, e.g. Zr
4+
and Y
3+
in
236 Manfred Martin
Fig. 5.14. Demixing profile of Ga in (Co
1−x
Ga
x
)O exposed to an oxygen potential
gradient (T = 1250
◦
C, a
(1)
O
2
=10
−6
(left), a
(2)
O
2
=10
−5
(right), ∆z = 725 µm,
x
0
Ga
=1.85%, t =38.5 h) [64].
YSZ, have different diffusion coefficients (see Sect. 5.3.2) there will be demix-
ing of the electrolyte. However, since cation diffusion is very slow, steady-
state demixing will be reached only after rather long times. If, for example,
the slowest diffusion coefficient is taken as D =10
−18
m
2
s
−1
,oneobtains
15 000 years for an electrolyte thickness of 1mm. However, for a thickness of
10 µm the time to reach the steady state is only 1.5 years which is comparable
to the desired operating times of SOFCs.
5.5.2 Diffusion in an Electric Potential Gradient
In this section, electro-transport in oxides, i.e. the motion of ions due to an
external electric potential gradient, ∇Φ, will be discussed. As in the previous
section, where diffusion in an applied oxygen potential gradient was analyzed,
the fluxes of the mobile components i (ions and electronic defects) can be
written as a sum of a diffusion and a drift flux, j
i
= j
diff
i
+ j
drift
i
.Ina
chemically homogeneous oxide without any gradients in chemical potentials,
the diffusion fluxes vanish, and the drift flux can be written as
j
drift
i
= −L
ii
%
k
z
k
·
L
ik
L
ii
'
· F ·∇Φ. (5.42)
The sum in parentheses is usually denoted as effective charge, z
i,eff
.Itis
identical to the formal charge, z
i
, only if the cross coefficients L
ik
(i = k)are
zero and, consequently, the fluxes are independent of each other
10
.
10
In metals, the effective charge is frequently denoted by the symbol z
∗
i
.

5 Diffusion in Oxides 237
E
ext
U
x
sample
anode
)x
tracer
j
h
.
j
Co
2+
cathode
Fig. 5.15. Schematic experimental
setup for the measurement of the ef-
fectivechargeinaconstantelectric
potential gradient (sandwich exper-
iment). The Gaussian curve shows
the broadening and shift of a tracer
concentration profile which has de-
veloped from a point source located
originally in the middle of the crys-
tal.
The effective charges are thus a measure of the cross coefficients which are
again indicating defect-defect interactions. In homogeneous oxides exposed to
an electric field, (radioactive) tracers can be used to measure the drift veloc-
ities of ions from which their effective charges can be calculated (Sect. 5.5.2).
During demixing experiments, however, the oxide becomes chemically inho-
mogeneous resulting in drift and diffusion fluxes as well. This situation will
be discussed in Sect. 5.5.2.
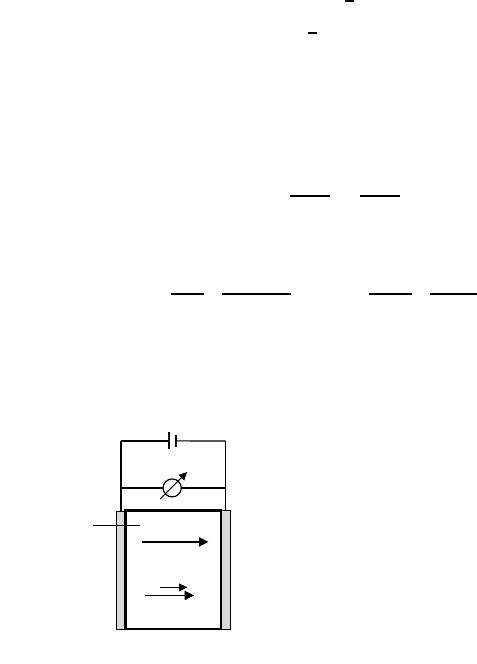
Tracer Diffusion in an Electric Potential Gradient
Figure 5.15 shows the typical experimental setup which can be used to mea-
sure the effective charges, z
i,eff
, of the host cation A or of impurity cations
B in an oxide AO using radioactive tracers, A
∗
and B
∗
. The tracer source is
located between two single crystals of the oxide in a sandwich arrangement,
and the electric potential gradient is applied by two reversible electrodes.
Diffusional broadening of the tracer source results in a Gaussian profile from
which the tracer diffusion coefficient can be obtained. Superimposed is a drift
of the tracer concentration profile due to the applied electric field. As shown
in detail in [18], the drift velocity of the tracer concentration profile, v
drift
A
∗
or
v
drift
B
∗
, allows the determination of the effective charges of the corresponding
cations, A or B. For self-diffusion, i.e. tracers A
∗
,weobtain
v
drift
A
∗
=
D
A
RT
· z
A,eff
· F ·∇Φ, z
A,eff
= z
A
+
L
Ah
L
AA
. (5.43)
The effective charge of cation A, z
A,eff
, contains the cross coefficient, L
Ah
,
which indicates the flux coupling between cations and electron holes. z
A,eff
is
often called ‘charge of transport’. For impurity tracer cations, B
∗
,theresult
is
v
drift
B
∗
=
D
B
RT
· z
B,eff
· F ·∇Φ, z
B,eff
= z
B
+
L
Bh
L
BB
+
L
BA
L
BB
. (5.44)
TheeffectivechargeofB,z
B,eff
, now contains two cross coefficients, L
Bh
and
L
BA
, which indicate flux coupling between B and h and between B and A.
238 Manfred Martin
.
.
.
.
.
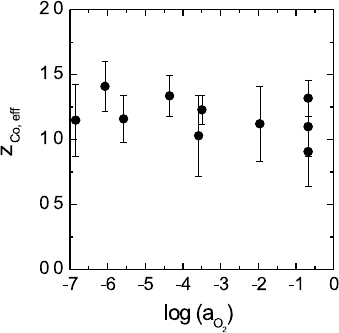
Fig. 5.16. Effective charge of cobalt
in Co
1−δ
O as a function of the oxy-
gen activity at 1100
◦
C [18].
Figure 5.16 shows results for the charge of transport of Co in CoO as
a function of the oxygen partial pressure [18]. z
Co,eff
has a value close to
+1.SincetheformalchargeofCoinCoOisz
Co
= 2 this result demon-
strates clearly that the cross coefficient L
Co h
is by no means negligible. The
cross effect may be understood in terms of vacancy-electron hole associates,
V
Co
+h
•
V
Co
, and long-range Coulomb interactions between the oppo-
sitely charged vacancies and electron holes. Application of the Onsager-Fuoss
theory to this system [65] has shown that long-range interactions are of minor
significance compared to association reactions. If the lifetime of the associate,
V
Co
, is long enough to move as an entity, a simple interpretation of the ef-
fective charge can be given: in an electric field, Co
2+
ions move towards the
cathode via lattice site exchange with vacancies. If the vacancy is associ-
ated with an electron hole, two positive charges (fixed on the cobalt ion)
movetowardsthecathodeand,atthesametime,onepositivecharge(the
hole associated with the vacancy) moves in the opposite direction during the
exchange step. Thus, the net charge which is moved towards the cathode
and which is the only quantity that can be measured is +1. Additional ev-
idence for vacancy-electron hole associates stems from measurements of the
electrical conductivity [66]. The data were modelled by two different elec-
tronic conductivity processes, by means of free electron holes and by means
of electron holes bound by vacancies. The lifetime of a vacancy-electron hole
associate was found to be 20 times larger than the residence time of a free
electron hole on a cation site.
The effective charges of the impurities indium and iron are shown in
Fig. 5.17. Whereas z
Fe,eff
remains nearly constant with changing oxygen ac-
tivity with a value of about +1, z
In,eff
decreases drastically with changing
a
O
2
and becomes even negative at a
O
2
< 10
−4
.Thisisequivalenttoare-
versal of the migration direction in the electric field. In the range of high
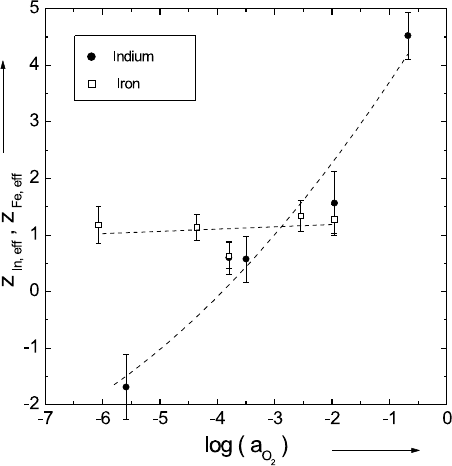
5 Diffusion in Oxides 239
Fig. 5.17. Effective charges of indium and iron in Co
1−δ
O as a function of the
oxygen activity at 1100
◦
C [18].
oxygen activities, the indium tracer moves towards the cathode, at low ac-
tivities it moves towards the anode, and therefore virtually behaves like an
anion. If we try to explain the observed behavior in the same way as for the
Co ions and assume that the impurity ions move via vacancy-electron hole
associates, V
Co
(majority defects), we expect z
In,eff
= +2 (with the formal
charge z
In
= +3) and z
Fe,eff
=+2or+1(withz
Fe
= +3 or +2, respectively).
It is obvious from Fig. 5.17 that the strong decrease of z
In,eff
and the ap-
pearance of negative values at low oxygen activities cannot be interpreted in
this manner. Furthermore, the experimentally obtained value of z
Fe,eff
≈ +1
at high oxygen activities is unexpected, because iron should have a formal
charge of about +3 in this region. This means that in (5.44) the second ratio
including the cross coefficient L
BA
is also important. It is well known that
this cross coefficient is due to the formation of impurity-vacancy pairs [22].
If the pair binding energy is strong enough to make the lifetime of the pair
much longer than the time required for an individual jump of the impurity
ion, the pair moves as an entity and the vacancy drags the impurity cation
towards the anode. As a result, we find a negative effective charge, as ob-
served experimentally. A more detailed formal analysis can be found in [67]
and [68].
240 Manfred Martin
Demixing in an Electric Potential Gradient
The external electric field can also cause demixing of an initially homoge-
neous oxide solid solution, e.g. (A
1−x
B
x
)O, if the cations have different mo-
bilities. In semiconducting oxides demixing due to an electric field was found
by several authors [69–72]. Demixing processes have important practical im-
plications. Local composition changes can severely alter the physical, chem-
ical and electrical properties and are therefore a source of high temperature
degradation of materials in electric fields.
We consider a semiconducting, ternary oxide, (A
1−x
B
x
)
1−δ
O, with an im-
mobile oxygen sublattice, where the cations are mobile via a vacancy mech-
anism. As in the previous section, the external electric field (see Fig. 5.18)
causes fluxes of the homovalent cations, A
2+
and B
2+
, and electron holes,
h
•
, which are given by (5.40).
At the reversible electrodes the cations are involved in chemical reactions,
e.g. at the cathode:
A
2+
+2e
−
(Pt) +
1
2
O
2
(g) → AO,
B
2+
+2e
−
+
1
2
O
2
(g) → BO (5.45)
This means that the oxide grows at the cathode. At the anode the opposite
reactions take place, i.e. here the oxide dissociates into cations, electrons and
oxygen molecules. Thus, both oxide surfaces move to the cathode side. In the
steady state both surfaces and also both cations move with the same velocity,
v:
v =
j
A
2+
c
A
=
j
B
2+
c
B
. (5.46)
Integration of (5.46) over the sample thickness yields
x
(1)
A
x
(2)
A
·
1 − x
(2)
A
1 − x
(1)
A
=exp
2FU
RT
·
γ − 1
γ
(5.47)
where x
(1)
A
and x
(2)
A
are the unknown molar fractions of A at the oxide sur-
faces and U is the applied voltage. γ = D
A
/D
B
is a constant, since both
semiconducting
oxide
(A
1-x
B
x
)O
U=const.
h
A
2+
B
2+
(1) (2)
cathodeanode
Fig. 5.18. Schematic representation
of the fluxes in a mixed oxide (A,B)O
exposed to an electric potential gra-
dient established by Pt-electodes.
