Heitjans P., Karger J. (Eds.). Diffusion in Condensed Matter: Methods, Materials, Models
Подождите немного. Документ загружается.


5 Diffusion in Oxides 211
(a) (b)
Fig. 5.1. Formation of Schottky defects (a) and Frenkel defects (b) in an oxide AO
(• =cation, =anion).
constants for the three equilibria, depending on temperature
1
.Inthemass
action laws in (5.1)–(5.3) we have assumed that the site fractions of vacancies
and interstitials (defects), denoted by brackets, are small compared to the site
fractions of regular cations and anions, and that the structure elements in
each sublattice form an ideal mixture.
Incorporation of oxygen from the surrounding atmosphere into the crystal
or removal of oxygen from the crystal results in the formation of a non-
stoichiometric oxide with oxygen excess or oxygen deficit. It can also be
described by a quasi-chemical reaction:
1
2
O
2
(g) + V
••
O
O
x
O
+2h
•
K
O
=
[h
•
]
2
(p
O
2
)
1/2
· [V
••
O
]
. (5.4)
Oxygen from the gas phase,
1
2
O
2
(g), occupies an oxygen vacancy, V
••
O
,and
for charge compensation electron holes, h
•
, are produced (see also Fig. 5.2).
Finally, the electronic equilibrium, resulting in the formation of electrons, e
,
and electron holes, h
•
, has to be considered:
nil e
+h
•
K
e
=[e
] · [h
•
] . (5.5)
The fractions of all charged structure elements are coupled by the condition
of local electrical neutrality:
2[V
A
]+2[O
i
]+[e
]=2[V
••
O
]+2[A
••
i
]+[h
•
] . (5.6)
The set of equations is completed by the site balances for each sublattice, e.g.
[A
x
A
]+[V
A
] = 1. Provided the equilibrium constants are known, all defect
fractions and the non-stoichiometry
δ =[V
A
]+[O
i
] − [V
••
O
] − [A
••
i
] (5.7)
can be calculated as a function of the thermodynamic variables, p, T and
p
O
2
.
1
The temperature dependence of the equilibrium constants is given by K =
exp(−∆G
0
/RT )=exp(∆S
0
/R) · exp(−∆H
0
/RT ), where ∆G
0
, ∆S
0
and ∆H
0
are the standard Gibbs energy, entropy and enthalpy of the corresponding quasi-
chemical reaction.
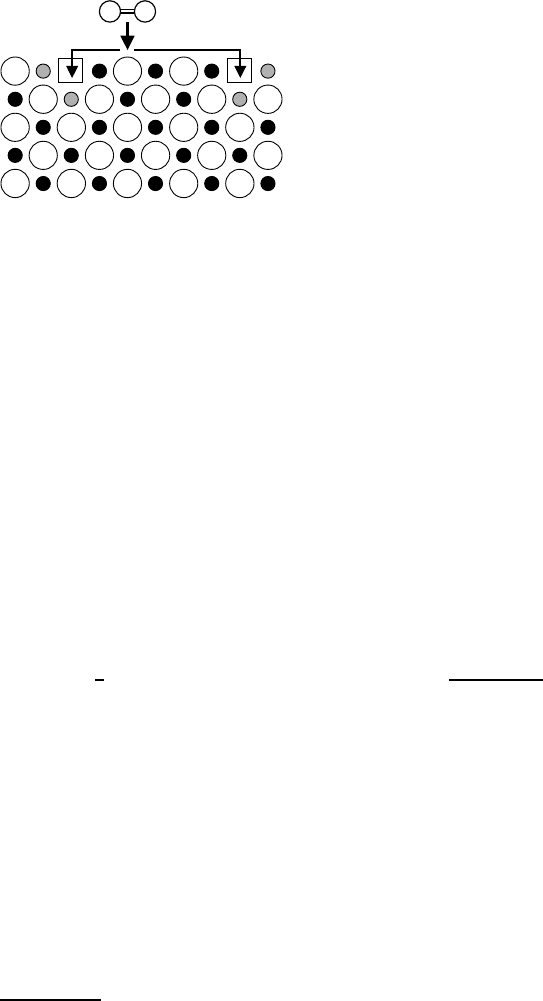
212 Manfred Martin
Fig. 5.2. Incorporation of an oxygen
molecule (=) from the gas phase
into an oxide AO with dominating oxy-
gen vacancies (see (5.4)). For each oxy-
gen atom two electron holes are pro-
duced. For the sake of simplicity they
are localized on cations (•).
5.2.1 Dominating Cation Disorder
Oxygen Activity Dependent Disorder
In oxides with a fcc oxygen sublattice, i.e. cubic close packing of the oxygen
ions, as in most transition metal oxides, the formation of oxygen vacancies
and oxygen interstitials is energetically unfavourable in comparison with the
formation of cation defects. Thus, we may regard the oxygen sublattice as
perfect compared to the cation sublattice. This means that in the exactly
stoichiometric oxide with δ = 0 the Frenkel equilibrium dominates
2
and (5.2)
and (5.6) simplify to [V
A
]=[A
••
i
]=(K
F
)
1/2
. At fixed temperature, T ,and
pressure, p, the so-called stoichiometric point, δ = 0, corresponds to a well
defined oxygen partial pressure, p
∗
O
2
. If we increase the oxygen partial pres-
sure relative to p
∗
O
2
, oxygen is incorporated into the crystal. Since oxygen
defects are only minority defects it is more convenient to formulate the incor-
poration of oxygen from the ambient atmosphere in terms of cation defects
which is possible by ‘adding’ (5.4) and (5.1):
1
2
O
2
(g) O
x
O
+V
A
+2h
•
,K
A
=
[V
A
] · [h
•
]
2
(p
O
2
)
1/2
. (5.8)
Oxidation of the oxide results in the formation of new lattice sites in both
the oxygen and the cation sublattices, i.e. now the crystal grows, in contrast
to the incorporation of oxygen described in (5.4). While the new oxygen
sublattice sites are occupied by oxygen ions the new cation lattice sites are
empty, i.e. cation vacancies are formed (see Fig. 5.3). They are electrically
compensated by the formation of electron holes.
Thus, for oxygen partial pressures that are sufficiently large compared to
p
∗
O
2
cation vacancies and electron holes will be the majority defects, which
results in a simplified condition of electrical neutrality, 2[V
A
]=[h
•
]. Us-
ing this relation, the p
O
2
-dependence of the majority type of defects can be
calculated from (5.8):
2
Here we have assumed that the fractions of the electronic defects are small com-
pared to the fractions of the Frenkel defects. The opposite case, dominating
electronic disorder, will not be considered.
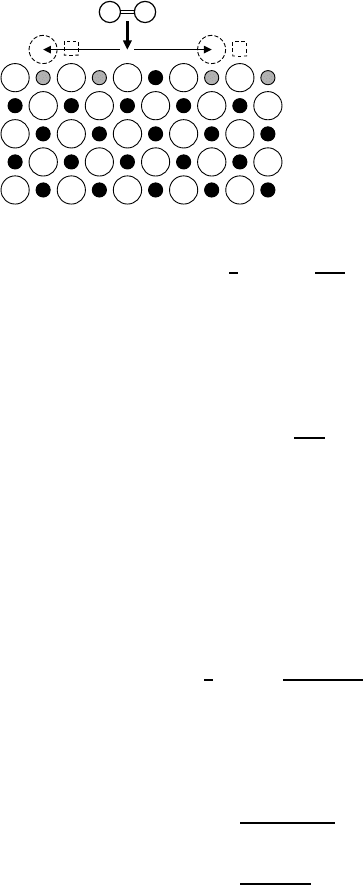
5 Diffusion in Oxides 213
Fig. 5.3. Incorporation of an oxygen
molecule (=) from the gas phase
into an oxide AO with dominating
cation vacancies. For each oxygen atom
a new occupied anion lattice site (),
a new unoccupied cation lattice site ()
and two electron holes (•) are produced.
For the sake of simplicity the electron
holes are localized on cations.
[V
A
]=
1
2
[h
•
]=
K
A
4
1/3
· (p
O
2
)
1/6
. (5.9)
On the other hand, cation interstitials, A
••
i
, and electrons, e
, are only mi-
nority defects, and their fractions decrease with increasing p
O
2
(see (5.2) and
(5.5)) according to
[A
••
i
]=K
F
A
·
K
A
4
−1/3
· (p
O
2
)
−1/6
,
[e
]=K
e
· (2K
A
)
−1/3
· (p
O
2
)
−1/6
. (5.10)
For oxygen partial pressures that are sufficiently small compared to p
∗
O
2
,
cation interstitials and electrons will be the majority defects, which results in
a simplified condition of electrical neutrality [e
]=2[A
••
i
]. With this relation,
the p
O
2
-dependence of the majority type of defects can be calculated from
(5.8) and (5.2) by
[A
••
i
]=
1
2
[e
]=
K
F
A
· K
e
4K
A
1/3
· (p
O
2
)
−1/6
. (5.11)
In this regime, cation vacancies, V
A
, and electron holes, h
•
, are the minority
defects and their fractions decrease with decreasing p
O
2
:
[V
A
]=
4K
A
· K
2
F
A
K
2
e
1/3
· (p
O
2
)
+1/6
,
[h
•
]=
K
A
· K
e
2K
F
A
1/3
· (p
O
2
)
+1/6
. (5.12)
Equations (5.9) to (5.12) show that all defect fractions follow a power law
dependence on p
O
2
with typical exponents 1/6 and −1/6 for oxygen par-
tial pressures which are sufficiently larger or smaller than p
∗
O
2
.Intheclose
vicinity of p
∗
O
2
the fractions of the Frenkel defects do not depend on p
O
2
,
[V
A
]=[A
••
i
]=(K
F
)
1/2
. In contrast, the fractions of the minority defects, e
and h
•
, exhibit p
O
2
-dependencies with exponents −1/4 and 1/4, respectively
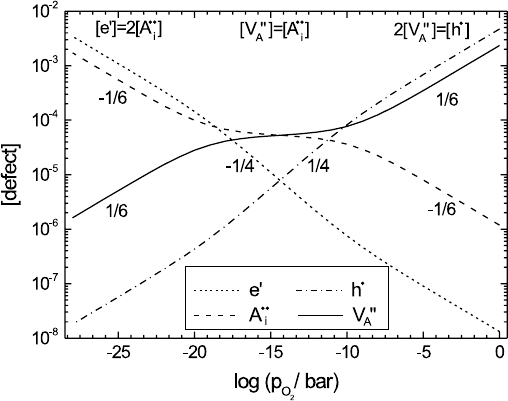
214 Manfred Martin
Fig. 5.4. Dependence of the defect fractions on the oxygen partial pressure p
O
2
(Kr¨oger-Vink-diagram) in an oxide AO with dominating Frenkel disorder at the
stoichiometric point (see (5.9)–(5.12)).
(see (5.5) and (5.8)). In a double-logarithmic plot (Kr¨oger-Vink diagram) we
therefore obtain straight lines (see Fig. 5.4) and three typical regions corre-
sponding to δ<0, δ ≈ 0andδ>0.
Without going into further details we note that other exponents are also
possible, mainly for two reasons:
i) Defects might form associates, e.g. a vacancy might trap an electron hole
resulting in a singly ionised vacancy:
V
A
+h
•
V
A
. (5.13)
If these vacancies become the majority defects they show a dependence
[V
A
] ∝ (p
O
2
)
1/4
. Similarly, cation interstitials might trap an electron,
A
••
i
+e
A
•
i
, resulting in a dependence [A
•
i
] ∝ (p
O
2
)
−1/4
.
ii) Often, there are also oxides of different stoichiometry, i.e. A
3
O
4
,A
2
O
3
or A
2
O. For the oxide of a monovalent metal, e. g. Cu
2
O, we obtain typ-
ical exponents 1/4 and −1/4, and in the mixed-valent oxide Fe
3
O
4
the
exponents are 2/3 and −2/3.
Doping
Doping an oxide A
1−δ
O with dominant cation disorder with an oxide of a
highervalentmetalB,e.g.B
2
O
3
can be described by

5 Diffusion in Oxides 215
B
2
O
3
→ 2B
•
A
+V
A
+3O
x
O
(5.14)
where we have assumed that the dopant B occupies regular cation lattice sites.
To conserve the lattice structure of the host oxide AO the incorporation of
two dopant cations, B
•
A
, and three oxygen ions, O
x
O
, demands the formation
of one cation vacancy, V
A
. Since the dopant is charged it must be considered
in the condition of electrical neutrality in (5.6), and the defect structure of
the doped oxide will be changed:
2[V
A
]+[e
]=2[A
••
i
]+[h
•
]+[B
•
A
] . (5.15)
i) For strong doping, the fraction of cation vacancies is directly given by the
dopant fraction, (see (5.14)), and is independent of the thermodynamic
variables p, T and p
O
2
, i.e. the exponent of the p
O
2
-dependence is zero.
ii) For small dopant fractions, however, the disorder of the host oxide cannot
be neglected, and the defect structure depends on the thermodynamic
variables p, T , p
O
2
and the dopant fraction as well.
iii) Coulombic interaction between the dopant ions, B
•
A
, and differently
charged cation vacancies, V
A
and V
A
, may lead to the formation of solute-
vacancy pairs:
B
•
A
+V
A
#
B
•
A
, V
A
$
x
,K
P
1
=
[P
1
]
[B
•
A
] · [V
A
]
, (5.16)
B
•
A
+V
A
#
B
•
A
, V
A
$
,K
P
2
=
[P
2
]
[B
•
A
] · [V
A
]
. (5.17)
Here we have denoted the solute-vacancy pairs containing singly and dou-
bly ionised vacancies by P
1
and P
2
.
5.2.2 Dominating Oxygen Disorder
In oxides where the oxygen ions do not form a cubic close packing but a
more open substructure, for example, in the perovskites or fluorites, oxygen
defects may be the dominating defects while cation defects are only minority
defects. As before, the defect fractions are determined by (5.1)–(5.7), and
typical exponents are obtained for the different disorder types. To increase
the fraction of oxygen vacancies, these oxides are often doped with oxides
of lower valent metals. Then, the condition of electrical neutrality in (5.6)
simplifies to
[B
A
]=2[V
••
O
] . (5.18)
Thus, the negative excess charge of the dopant cation, B
A
, is compensated
by oxygen vacancies, and these oxides are good oxygen ion conductors. Some
well know examples are
216 Manfred Martin
– yttria-stabilized zirconia, (Zr
1−x
Y
x
)O
2−x /2
(YSZ). Here, doping with
Y
2
O
3
increases the fraction of oxygen vacancies, [Y
Zr
]=2[V
••
O
], and sta-
bilizes the cubic fluorite structure. YSZ is a pure oxygen ion conductor
over many orders of magnitude in p
O
2
andisusedassuchinoxygen
sensors and solid oxygen fuel cells (SOFC).
– Sr- and Mg-doped lanthanum gallate, (La
1−x
Sr
x
)(Ga
1−y
Mg
y
)O
3−(x +y )/2
(LSGM), which belongs to the class of perovskites ABO
3
.InLSGM
oxygen vacancies are produced by co-doping in both cation sublattices,
[Sr
La
]+[Mg
Ga
]=2[V
••
O
], resulting also in a good oxygen ion conductor.
However, at very low or very high oxygen partial pressures the electronic
disorder can no longer be neglected and the oxide becomes a mixed ionic and
electronic conductor. Examples are
– YSZ and LSGM, both of which become mixed conductors (V
••
O
and e
)at
low oxygen partial pressures.
– strontium-doped lanthanum-chromate, (La
1−x
Sr
x
)CrO
3−δ
,whichisa
good semiconductor at high oxygen partial pressures and becomes a mixed
conductor at low oxygen partial pressures.
5.3 Self- and Impurity Diffusion in Oxides
For the definition of diffusion coefficients and flux equations the reader is
referred to Sects. 1.1 to 1.3 of Chap. 1.
5.3.1 Diffusion in Oxides with Dominating Cation Disorder
Since cations are mobile in the cation sublattice by means of cation vacancies
and in the interstitial sublattice as cation interstitials, the cation self-diffusion
coefficient, D
A
, can be written as
D
A
= D
V
A
· [V
A
]+D
A
i
· [A
i
] . (5.19)
Here D
V
A
and D
A
i
are the self-diffusion coefficients of cation vacancies and
cation interstitials, and [V
A
]and[A
i
] are the corresponding site fractions.
If the self-diffusion coefficients of vacancies and interstitials do not depend
on the oxygen partial pressure, the p
O
2
-dependence of the cation diffusion
coefficient is solely determined by the p
O
2
-dependence of the defect fractions
which has been calculated in the previous section. Then, the p
O
2
-dependence
of the diffusion coefficient can be used to identify the disorder type of the
oxide under investigation. At high p
O
2
cation vacancies dominate while at low
p
O
2
cation interstitials are the dominating defects (see Fig. 5.4). We therefore
obtain a minimum of the cation self-diffusion coefficient as a function of p
O
2
and typical exponents in the p
O
2
-dependence left and right of the minimum.
Since D
V
A
and D
A
i
are usually different from each other, the minimum in
the diffusion coefficient is shifted relative to the stoichiometric point (δ =0).

5 Diffusion in Oxides 217
Cation Self- and Impurity Diffusion in Spinels A
3−δ
O
4
The best-known oxide where the transition from a vacancy to an intersti-
tial regime was found experimentally is magnetite, Fe
3−δ
O
4
[10]. It crystal-
lizes in the spinel structure where the oxygen ions form a cubic close pack-
ing while the cations occupy well defined octahedral and tetrahedral sites.
The observed exponents of the iron diffusion coefficients are 2/3 at high
p
O
2
and −2/3 at low p
O
2
, as expected for dominating cation vacancies and
cation interstitials, respectively (see Sect. 5.2.1). This typical behaviour of
the cation diffusion coefficients remains the same if the spinel consists of
several cations, e.g. (Co,Fe,Mn)
3
O
4
[11, 12]. Another example is manganese-
zinc-ferrite, Mn
1−x
Zn
x
Fe
2
O
4
, where part of the Fe-ions in magnetite has
been replaced by Mn- and Zn-ions. Cation tracer diffusion coefficients have
been measured with radioactive isotopes in the thin-film geometry, using both
the sectioning method (described already in Sect. 1.4.1 in Chap. 1) and the
residual activity method [13]. In the sectioning method a thin layer of the
sample is ground off and its activity, A
sect
, is counted, while in the residual
activity method the residual activity, A
res
, of the sample after grinding off
a thin layer is counted. In the first case the activity profile is a Gaussian
curve, A
sect
∝ exp(−x
2
/4D
∗
t), while in the second case an error function is
obtained, A
res
∝ (1 −erf(x/
√
4D
∗
t)). Typical profiles from both methods are
shown in Fig. 5.5 for diffusion of the radioisotope
54
Mn in manganese-ferrite.
The diffusion coefficients obtained from both methods agree well. Figure 5.6
shows in a double-logarithmic plot results for the tracer diffusion coefficients
of Mn, Fe and Zn and the impurity diffusion coefficient of Co as a function of
the oxygen partial pressure [13]. All diffusion coefficients show a minimum as
a function of p
O
2
. The slopes +2/3 and −2/3 at high and low p
O
2
indicate that
diffusion proceeds via cation vacancies and cation interstitials, respectively.
While all diffusion coefficients are nearly the same in the vacancy regime,
the diffusion coefficient of zinc is higher in the interstitial regime, resulting in
a minimum of the Zn-diffusion coefficient which is shifted to higher oxygen
partial pressures compared to the other cations. A more detailed analysis,
considering that the cation sublattice in the spinel structure consists of two
sublattices with octahedral and tetrahedral sites and that iron, manganese
and cobalt cations exist in two charge states, +2 and +3, can be found in [13].
Cation Self- and Impurity Diffusion in Monoxides A
1−δ
O
In most transition metal monoxides, such as Co
1−δ
O, Ni
1−δ
OorMn
1−δ
O,
the oxide is reduced to the metal before the stoichiometric point, δ =0,is
reached. Thus, only a vacancy regime for cation diffusion is observed [14–16].
However, as mentioned before, the typical exponent in the p
O
2
-dependence
of the cation diffusion coefficient quite often differs from the value 1/6 that is
expected if cation vacancies V
A
would dominate. Subsequently, two examples
will be discussed, pure cobalt oxide and gallium-doped cobalt oxide.
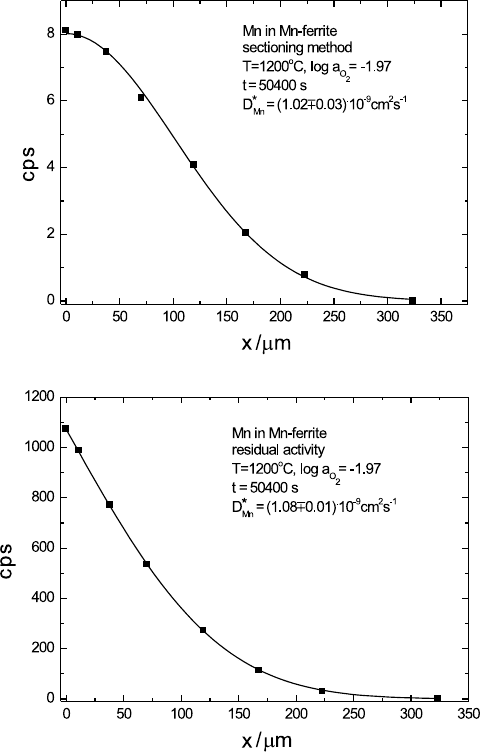
218 Manfred Martin
(a)
(b)
Fig. 5.5. Tracer sectioning profile (a) and residual activity profile (b) of
54
Mn in
manganese ferrite [13] and corresponding fits with a Gaussian (a) and an error-
function (b).

5 Diffusion in Oxides 219
Fig. 5.6. Tracer diffusion coefficients of
54
Mn,
59
Fe,
65
Zn, and
57
Co in
Mn
0.54
Zn
0.35
Fe
2.11
O
4
as a function of the oxygen activity at T = 1200
◦
C (symbols)
and fits (solid lines) [13].
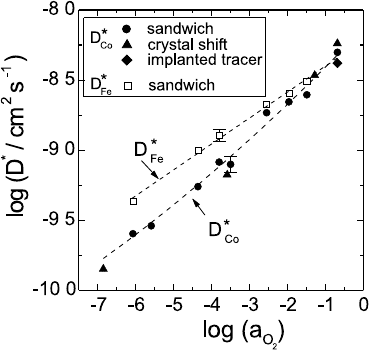
Cobalt Monoxide, Co
1−δ
O
Cobalt monoxide, Co
1−δ
O, exists only with a cation deficit and is a p-type
semiconductor. As shown by Dieckmann [17], the p
O
2
-dependence of three
defect-dependent quantities, the non-stoichiometry, δ, the cation tracer dif-
fusion coefficient, D
∗
Co
, and the electrical conductivity, σ, can be explained by
a defect structure, where differently charged cation vacancies, V
Co
and V
Co
,
and electron holes, h
•
, are the dominating defects (see (5.13)). At interme-
diate oxygen partial pressures singly ionised cation vacancies V
Co
dominate,
and all three quantities, δ =[V
Co
] (see (5.7)), σ, which is proportional to
[h
•
], as well as the cation tracer diffusion coefficient
3
, D
∗
Co
= f
0
· D
V
· [V
A
],
show the same dependence on p
O
2
with a typical exponent 1/4. Results for
the tracer diffusion coefficient of cobalt, D
∗
Co
, measured with the radiotracer
method [18] and demonstrating this p
O
2
-dependence are shown in Fig. 5.7
together with results for impurity diffusion of Fe in CoO. The smaller slope
can be explained by impurity-vacancy binding between iron ions and cation
vacancies (see (5.16) and (5.17)). In the exact analysis one must consider that
the charge states of both the iron ions and the cation vacancies change with
decreasing p
O
2
[19,20].
Ga-Doped Cobalt Monoxide, (Co
1−x
Ga
x
)
1−δ
O
In Co
1−δ
OdopedwithGa
2
O
3
, resulting in (Co
1−x
Ga
x
)
1−δ
O, both cation
tracer diffusion coefficients, D
∗
Co
and D
∗
Ga
, have been measured as a function
3
f
0
is the geometrical correlation factor appearing in tracer diffusion (see
Sect. 1.3.1 in Chap. 1).
220 Manfred Martin
.
.
.
.
.
Fig. 5.7. Tracer diffusion co-
efficients of
57
Co and
59
Fe in
Co
1−δ
O as function of the oxy-
gen activity at 1100
◦
C. Dashed
lines are guides to the eyes.
For the different experimental
techniques see [18].
of the oxygen partial pressure, p
O
2
, and the dopant fraction, x,usingra-
dioisotopes [21]. The results depicted in Fig. 5.8 for a temperature of 1350
◦
C
show two typical features: (i) The dependence of both diffusion coefficients
on the dopant fraction, x, is non-linear. (ii) At high oxygen partial pressures
Co is the faster moving species while at low oxygen partial pressures Ga is
faster than Co. To explain this behaviour we have to consider that the solute,
Ga, and the solvent, Co, move by means of different diffusion mechanisms.
– In the dilute system, x 1, the Ga ions are distinguishable, as tracer ions
in tracer self-diffusion. Therefore, Ga is only mobile via solute-vacancy
pairs, P
1
and P
2
(see (5.16) and (5.17)), and the solute diffusion coeffi-
cient, D
∗
Ga
, is proportional to the association degrees p
1
=[P
1
]/[Ga] and
p
2
=[P
2
]/[Ga] of solute ions bound in these pairs [22].
D
∗
Ga
= D
Ga,1
· p
1
+ D
Ga,2
· p
2
(5.20)
The diffusion coefficients per defect, D
Ga,1
and D
Ga,2
, depend only on
temperature and include the mobilities of the defects and all physical
correlation effects arising in solute tracer diffusion [22] (see also Sect. 1.9.1
in Chap. 1). The dependence of D
∗
Ga
on the defect concentrations, and
hence on x, T and p
O
2
is given by the association degrees p
1
and p
2
.
– In contrast, the solvent ion Co is mobile by means of free, i.e. unbound
vacancies, V
A
and V
A
, and conceivably by vacancies bound in pairs. Since
Co is the majority component, its tracer diffusion coefficient is propor-
tional to the appropriate defect fractions (and not to the association de-
grees, as for the solute):
D
∗
Co
= D
Co,1
·[V
Co
]+D
Co,2
·[V
O
]+D
Co,P
1
·[P
1
]+D
Co,P
2
·[P
2
] . (5.21)
Here we have permitted different mobilities for the vacancies V
A
and V
A
.
