Heitjans P., Karger J. (Eds.). Diffusion in Condensed Matter: Methods, Materials, Models
Подождите немного. Документ загружается.

5 Diffusion in Oxides 221
.
.
.
.
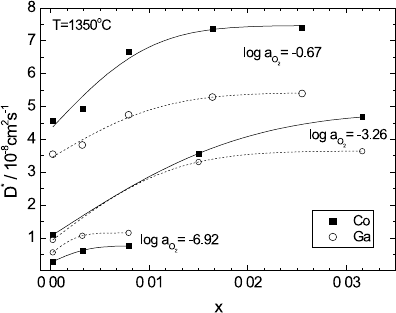
Fig. 5.8. Tracer diffusion
coefficients of
58
Co and
67
Ga in (Co
1−x
Ga
x
)
1−δ
O
as a function of the dopant
fraction, x,atT = 1350
◦
C
and different oxygen activi-
ties [21].
The modelling of both measured diffusion coefficients, D
∗
Co
and D
∗
Ga
,as
a function of the oxygen partial pressure, p
O
2
, and the dopant fraction, x,
yields the equilibrium constants K
P
1
and K
P
2
(see (5.16) and (5.17)) and
the diffusion coefficients D
Co,i
and D
Ga,i
(i =1, 2) [21]. The results show
that there is essentially no binding between the dopant, Ga
•
Co
, and singly
ionised vacancies, V
Co
, while there is strong binding between Ga
•
Co
and V
Co
.
As a consequence, D
Co,1
and D
Co,P
1
are identical. D
Co,P
2
is two orders of
magnitude smaller than D
Co,1
and D
Co,2
and can therefore be neglected. In
summary, Ga is mobile by means of strongly bound pairs, {Ga
•
Co
, V
Co
}
,and
weakly bound pairs, {Ga
•
Co
, V
Co
}. Co is mobile by means of free vacancies,
V
Co
and V
Co
, and also by exchange with vacancies which are strongly bound
in pairs {Ga
•
Co
, V
Co
}. In the latter mechanism the vacancy performs jumps
around the Ga ion, thereby moving the Co ion but without dissociating the
pair.
The binding energy E
P
2
determined from the equilibrium constant K
P
2
=
z · exp(E
P
2
/RT ) turns out to depend slightly on temperature. Assuming
nearest-neighbour pairs (z = 12) we obtain a binding energy of about
40 kJ/mol, while we obtain values of about 50 kJ/mol if we assume next-
nearest neighbour pairs (z = 6). The corresponding Coulomb energies (as-
suming point charges) are 72 kJ/mol (z = 12) and 50 kJ/mol (z =6)which
might be an indication for the next-nearest neighbour pairs, as proposed by
theoretical calculations [23].
A detailed analysis of the diffusion coefficients, D
Ga,i
and D
Co,i
, obtained
from the fitting in terms of defect mobilities and correlation factors is impos-
sible because the (physical) correlation factors are not known. However, we
can draw some general conclusions.
– The diffusion coefficients D
Co,1
and D
Co,2
describe the motion of the
solvent Co via free vacancies V
Co
and V
Co
, and can both be written
222 Manfred Martin
as a product of a vacancy diffusion coefficient and a correlation factor.
If we assume that these correlation factors are identical to the geometric
correlation factor in an fcc lattice, f
0
=0.781, we obtain different vacancy
diffusion coefficients, D
V
and D
V
in Ga-doped CoO. This result is in
agreement with measurements of the charge of transport
4
in pure CoO
[24], and the modelling of these data in terms of the Onsager-Fuoss-theory
of liquid electrolytes shows also that the average mobility of the vacancies
changes with the oxygen activity.
– The diffusion coefficients D
Ga,1
and D
Ga,2
describe the motion of the
solute Ga by means of pairs P
1
and P
2
, and both can be written in the
form D
Ga,i
=(1/6) ·s
2
·f
B,i
·ω
B,i
(i =1, 2), where s is the jump distance,
f
B,i
the correlation factor and ω
B,i
the impurity-vacancy exchange rate.
The experimentally obtained ratio D
Ga,1
/D
Ga,2
is about ten. If we assume
that the exchange rates ω
Ga,1
and ω
Ga,2
are about the same for both
mechanisms we can conclude that the correlation factor f
Ga,2
must be
much smaller than f
Ga,1
. This is in qualitative agreement with strong
binding between Ga
•
Co
and V
Co
, compared to weak binding between Ga
•
Co
and V
Co
.
5.3.2 Diffusion in Oxides with Dominating Oxygen Disorder
Oxygen defects are now the majority defects, while cation defects are only
minority defects. Thus, oxygen diffusion will be much faster than cation dif-
fusion.
Oxygen Self-Diffusion
The determination of the self-diffusion coefficient of oxygen is accomplished
by using one of the two stable oxygen isotopes,
17
Oor
18
O. The sample
is annealed in an isotope-enriched atmosphere and the diffusion profile is
generally determined by secondary ion mass spectrometry (SIMS) [25] (see
Chap. 1, Sect. 1.4.1). Mathematically the experimental setup corresponds
to the well-known infinite source solution [26]. However, if exchange of the
oxygen isotope between the atmosphere and the oxide is not sufficiently fast
there will be no equilibrium for the isotope at the surface. Most often, the
rate of isotope exchange at the oxide surface is assumed to be proportional
to the isotope concentrations in the gas and the solid, c
g
and c
s
, resulting in
−D
∗
O
·
∂c
∂x
x=0
= k · (c
s
− c
g
) (5.22)
where D
∗
O
is the oxygen tracer diffusion coefficient and k the surface ex-
change coefficient. The solution of the diffusion equation for a semi-infinite
4
The charge of transport will be discussed in more detail in Sect. 5.5.2

5 Diffusion in Oxides 223
medium subject to the boundary condition in (5.22) and having an initial
concentration c
0
is [26]
c(x, t)=c
0
+(c
g
− c
0
) ·
erfc
x
√
4D
∗
t
− exp
kx
D
∗
+
k
2
t
D
∗
· erfc
%
x
√
4D
∗
t
+ k
&
t
D
∗
'
. (5.23)
The parameters D
∗
O
and k are then obtained by fitting (5.23) to the experi-
mental profile. A detailed analysis of the conditions where D
∗
O
and k can be
determined unambiguously can be found in [27].
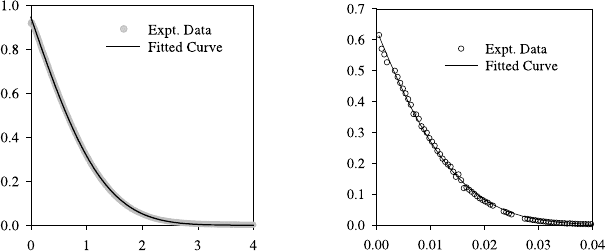
Two typical examples of oxygen diffusion in oxides of the ABO
3
per-
ovskite structure are shown in Fig. 5.9 [28]. Both examples belong to sam-
ples in the solid solution series between Sr-doped lanthanum cobaltate,
La
0.8
Sr
0.2
CoO
3−δ
, and Sr-doped lanthanum manganate, La
0.8
Sr
0.2
MnO
3−δ
.
The oxides are doped with SrO to increase the fraction of oxygen vacan-
cies (see (5.18)). In the manganese-rich sample, La
0.8
Sr
0.2
Mn
0.8
Co
0.2
O
3−δ
,
the oxygen diffusion profile extends over a few microns and can be deter-
mined by SIMS depth profiling (Fig. 5.9a). In Sr-doped lanthanum cobaltate,
La
0.8
Sr
0.2
CoO
3−δ
, however, oxygen diffusion is much faster and the penetra-
tion depth is about two orders of magnitude larger (Fig. 5.9b). For such large
penetration depths the SIMS depth profiling technique is no longer applica-
ble; the SIMS line-scanning technique [25] is used instead. Here the SIMS
analysis is performed on a section perpendicular to the sample surface. By
fitting (5.23) to the profiles, the oxygen diffusion coefficient and the surface
exchange coefficient in both materials were determined. It turns out that in
La
0.8
Sr
0.2
CoO
3−δ
the oxygen diffusion coefficient is about five orders of mag-
nitude larger than in La
0.8
Sr
0.2
Mn
0.8
Co
0.2
O
3−δ
, while the surface exchange
coefficient is about 2 orders of magnitude larger in the former oxide than in
the latter.
In general, oxygen diffusion can proceed in the same way as described for
cation diffusion in (5.19), namely by means of oxygen vacancies and oxygen
interstitials. As discussed in [28] there is, however, no evidence for oxygen
interstitials in ABO
3
perovskite oxides, mainly for geometrical reasons. Thus
the oxygen tracer diffusion coefficient is simply given by
D
∗
O
= f
0
· D
V
· [V
••
O
] (5.24)
where f
0
=0.69 is the geometrical correlation factor for oxygen tracer dif-
fusion in the oxygen sublattice of the ABO
3
structure [29] and D
V
the
self-diffusion coefficient of oxygen vacancies. In several other perovskites
the combination of oxygen diffusion coefficients and measured vacancy frac-
tions shows that the self-diffusion coefficient of oxygen vacancies, D
V
,ex-
hibits only small variations between different perovskites [29]. Thus, the
224 Manfred Martin
(a) (b)
Depth ( m)
c’(x)
Depth ( m)
c’(x)
Fig. 5.9. Typical
18
O diffusion profiles, showing the normalized isotope frac-
tion, c
(x)=(c(x) − c
0
)/(c
g
− c
0
), against depth, together with the fitted
curves according to (5.23) [28]. (a) determined by SIMS depth profiling of a
La
0.8
Sr
0.2
Mn
0.8
Co
0.2
O
3−δ
sample (
18
O anneal at 1000
◦
C for 3840 s). (b) deter-
mined by SIMS linescanning of a La
0.8
Sr
0.2
CoO
3−δ
sample (
18
O anneal at 1000
◦
C
for 1675 s).
large difference of the observed oxygen diffusion coefficients is predomi-
nantly due to the difference in the oxygen vacancy concentrations between
La
0.8
Sr
0.2
Mn
0.8
Co
0.2
O
3−δ
and La
0.8
Sr
0.2
CoO
3−δ
.
Cation Diffusion
In oxides with dominating oxygen disorder cation defects are only minor-
ity defects and consequently cation diffusion is much slower than oxygen
diffusion. Cation diffusion is nevertheless important since the slowest mov-
ing species determine many fundamental processes, such as sintering [30],
creep [31] or internal friction [32].
An important example is yttria-stabilized zirconia, (Zr
1−x
Y
x
)O
2−x/2
(YSZ), which exhibits very high oxygen ion conductivity and is therefore used
as electrolyte material in high-temperature applications [33]. While there is a
considerable amount of data available on the oxygen transport (e.g. [34,35]),
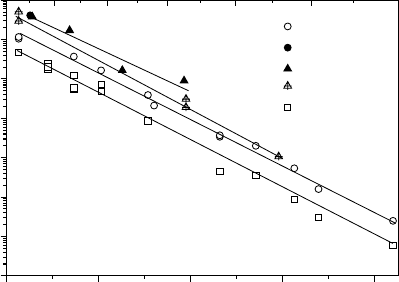
only little is known about the cation transport in YSZ [36, 37]. Figure 5.10
shows recent results for the diffusion coefficients of Y and Zr in single crys-
talline YSZ [38]. The diffusion coefficient of yttrium was measured using the
radioactive isotope
88
Y, while the diffusion coefficient of Zr was obtained by
implanting the stable isotope
96
Zr, annealing at elevated temperatures and
subsequent SIMS analysis.
From Fig. 5.10 it can be seen that Zr diffusion becomes slower with in-
creasing Y-content. This is due to the fact that the dopant yttrium deter-
mines the fraction of oxygen vacancies, [Y
Zr
]=2[V
••
O
], which again deter-
mines via the Schottky equilibrium (see (5.1)) the fraction of cation vacan-
cies. Thus cation diffusion should be slower the higher the dopant fraction, as
5 Diffusion in Oxides 225
5.00 5.50 6.00 6.50 7.00
10
-19
10
-18
10
-17
10
-16
10
-15
10
-14
10
-13
10
-12
96
Zr in YSZ-10
88
Y in YSZ-10
88
Y in YSZ-11
Sc in YSZ-11
96
Zr in YSZ-32
D
[c
m
²/
s
]
1/T [10000 K
-1
]
2000 1900 1800 1700 1600 1500 1400
T [K]
Fig. 5.10. Temperature dependence of the tracer diffusion coefficients of Zr, Y and
Sc in yttria-doped zirconia, YSZ, containing 10, 11 and 32 mol% Y
2
O
3
[38].
observed. As expected, comparison of the self-diffusion coefficients of oxygen
and cations in YSZ shows that D
O
is about 5 orders of magnitude larger than
D
cation
.
Similar results were found for doped lanthanum gallate,
La
1−x
Sr
x
Ga
1−y
Mg
y
O
3−(x+y)/2
(LSGM), which has a higher oxygen ion con-
ductivity than YSZ and is therefore a candidate for solid oxide fuel cells work-
ing at intermediate temperatures [39]. Here oxygen diffusion coefficients [40]
and cation self- and impurity diffusion coefficients [41, 42] have been mea-
sured. In contrast to YSZ there are two cation sublattices in the ABO
3
-
perovskite LSGM, an A- and a B-sublattice. In the perovskite structure (see
Fig. 5.11), a direct jump of A-cations within the A-sublattice is possible,
while a direct jump of B-cations within the B-sublattice is impossible due
to the oxygen ion located between two nearest neighbour B-sites. Thus, for
diffusion of B-cations curved diffusion pathways or jumps to next-nearest
neighbour sites must be considered. Atomistic simulations of migration en-
ergies in lanthanum gallate [43] yield much higher migration energies for
diffusion of B-cations than for diffusion of A-cations. The measured tracer
diffusion coefficients of La, Sr and Mg [41] and the impurity diffusion coef-
ficients for Fe, Cr and Y [41] are, however, very similar and show identical
activation energies, although Mg, Fe and Cr should occupy B-sites while La,
Sr and Y occupy A-sites. These observations may be an indication for anti-
site disorder in the perovskite LSGM, i.e. a small fraction of B-cations may
occupy A-sites. This would be sufficient to enable diffusion of B-cations with
similar diffusion coefficients as A-cations. A more detailled diffusion model

226 Manfred Martin
Fig. 5.11. Structure of the perovskite
ABO
3
.
considers a defect cluster consisting of an A-site vacancy, a B-site vacancy
and at least one oxygen vacancy [41]. This cluster is mobile as an entity, i.e.
without dissociation by means of four, correlated jumps of A- and B-cations
and oxygen ions. As a result, A- and B-cations have identical diffusivities.
5.4 Chemical Diffusion
So far we have considered self- and impurity diffusion processes in chemi-
cally homogeneous oxides without any concentration gradients. We will now
consider diffusion in concentration gradients, which is called chemical diffu-
sion. It is well known, from irreversible thermodynamics, that the real driving
force for isothermal mass transport of a component i is not its concentration
gradient but the gradient of its electrochemical potential, η
i
= µ
i
+ z
i
·F ·Φ,
where µ
i
is the chemical potential and Φ the electric potential [44] (z
i
is the
charge number and F, Faraday’s constant)
5
. The resulting flux equation for
component i is
j
i
= −L
ii
·∇(µ
i
+ z
i
· F · Φ) (5.25)
where L
ii
is the so-called Onsager transport coefficient
6
. For the sake of sim-
plicity, we will consider only a binary oxide AO with dominating cation dis-
order. The results can easily be transferred to oxides with dominating oxygen
disorder. We start with an oxide which is equilibrated at elevated tempera-
tures and at a certain oxygen partial pressure, p
(1)
O
2
. If the partial pressure of
the surrounding atmosphere is increased to p
(2)
O
2
an oxidation process takes
place. Oxygen is incorporated into the crystal by adding new lattice sites to
the crystal and producing cation vacancies and electron holes (see (5.8)). In
the case of reduction, lattice planes are annihilated and oxygen is released
5
For neutral particles (z
i
= 0) the driving force for mass transport is the gradient
of the chemical potential, as already discussed for metals in Sect. 1.3.2 of Chap. 1.
6
Cross coefficients L
ij
are neglected in this section but will be considered in
Sect. 5.5.

5 Diffusion in Oxides 227
to the atmosphere. To preserve local electrical neutrality during the result-
ing transport processes of cations and electronic defects their fluxes must be
coupled:
2j
A
2+
+ j
h
•
=0 . (5.26)
From (5.26) we can calculate the internal electrical potential gradient, ∇Φ
(the so-called Nernst field), and insert the resulting expression into the flux
equation for the cations in (5.25). With the equilibrium A + 2h
•
A
2+
between metal, A, cations, A
2+
, and electron holes, h
•
, the flux of cations
can be written as
j
A
2+
= −L
AA
· t
el
·∇µ
A
(5.27)
where t
el
= L
hh
/(4L
AA
+ L
hh
) is the electronic transference number. Equa-
tion (5.27) is the so-called Wagner formula for chemical or ambipolar diffu-
sion [45]. If the thermodynamic driving force, ∇µ
A
, is written in terms of the
concentration gradient, ∇µ
A
=(∂µ
A
/∂c
A
) ·∇c
A
,weobtain
j
A
2+
= −L
AA
· t
el
· (∂µ
A
/∂c
A
) ·∇c
A
= −
˜
D ·∇c
A
(5.28)
which defines the chemical diffusion coefficient,
˜
D. Due to the flux coupling,
j
A
2+
+ j
V
= 0, the vacancy flux can also be written in terms of the chemical
diffusion coefficient:
j
V
= −
˜
D ·∇c
V
. (5.29)
Finally, we write L
AA
in terms of the diffusion coefficient D
A
and the con-
centration c
A
, L
AA
= D
A
· c
A
/RT (Einstein relation) and make use of
µ
A
= µ
0
A
+ RT ln a
A
,wherea
A
is the activity of A. The result for
˜
D is
˜
D = D
A
· t
el
·
∂ ln a
A
∂ ln x
A
. (5.30)
Thus, the chemical diffusion coefficient,
˜
D, is the product of the cation self-
diffusion coefficient, D
A
, the electronic transference number, t
el
, and the ther-
modynamic factor, ∂ ln a
A
/∂ ln x
A
. Equation (5.30) applies also to oxides with
dominating oxygen disorder if D
A
, a
A
and x
A
are substituted by D
O
, a
O
and
x
O
.
The chemical diffusion coefficient,
˜
D,ofanoxideA
1−δ
O determines the
equilibration kinetics of δ after a change of the external oxygen partial pres-
sure. It can be determined by measuring δ directly, e.g. via thermogravimetry
or by measuring a quantity which is proportional to δ, such as the electronic
conductivity. These so-called relaxation experiments have been used to mea-
sure
˜
D in various oxides such as CoO with dominating cation disorder [46],
or (La,Mn)CoO
3
[47] and (La,Sr)CrO
3
[48] with dominating oxygen disorder.
If the oxide is a good semiconductor (t
el
= 1) and the majority defects are
cation vacancies, V
α
A
(with negative excess charge α =2,1,0),andelectron
holes, h
•
, (or oxygen vacancies, V
α
•
O
, and electrons, e
) the formula for
˜
D in
(5.30) simplifies to (see appendix)

228 Manfred Martin
˜
D = D
V
· (1 + α) . (5.31)
Thus, from the measured chemical diffusion coefficient,
˜
D, the self-diffusion
coefficient, D
V
, of the dominating vacancies can be calculated, if their excess
charge is known. This is the normal procedure, e.g. mostly adopted in oxides
with oxygen disorder where vacancies V
••
O
(α = 2) dominate. If, on the other
hand, the vacancy self-diffusion coefficient is known the excess charge of the
vacancies can be calculated. As will be shown in Sect. 5.5.1, both the vacancy
self-diffusion coefficient, D
V
, and the chemical diffusion coefficient,
˜
D,can
be obtained simultaneously by performing tracer self-diffusion experiments
during chemical diffusion. Then, from both diffusion coefficients the excess
charge α can be obtained via (5.31).
In the general case of a mixed conductor, t
el
= 1, the chemical diffusion
coefficient may show a strong dependence on the oxygen partial pressure for
two reasons: (i) the electronic transference number, t
el
, depends on the oxygen
partial pressure. (ii) if the stability field of the oxide contains the stoichio-
metric point, δ(p
∗
O
2
) = 0, the thermodynamic factor and also the chemical
diffusion coefficient exhibit a maximum at this oxygen partial pressure. This
case is found, e.g., in BaTiO
3
[49].
Another technique that has been used recently for the measurement of
chemical diffusion coefficients in Fe-doped SrTiO
3
, which is a mixed conduc-
tor, uses the optical absorption of the sample [50]. In this way, time- and
position-resolved concentration profiles of oxygen can be determined from
which the chemical diffusion coefficient is evaluated.
5.5 Diffusion in Oxides Exposed to External Forces
If an oxide is exposed to external thermodynamic forces, e.g. an oxygen po-
tential gradient or an electric potential gradient, defect fluxes are induced
which again cause fluxes of the chemical components. As before, it is rea-
sonable to distinguish between dominating oxygen disorder and dominating
cation disorder.
In oxides where the oxygen ions are much more mobile than the cations,
essentially only oxygen is driven through the oxide
7
. For pure oxygen ion
conductors this situation corresponds to an electrolyte in a solid oxide fuel
cell (applied oxygen potential gradient) or an electrochemical oxygen pump
(applied electric potential gradient). For mixed conductors this situation cor-
responds to oxygen permeation cells. A detailed analysis of these cases is,
however, beyond the scope of this chapter and can be found, e.g., in [51].
7
The (driven) motion of the slower cations is, however, a possible origin of
long-term degradation processes, such as creep or kinetic demixing (see also
Sect. 5.5.1).
5 Diffusion in Oxides 229
In oxides with dominating cation disorder external forces act on the mobile
cations. The implications will be considered in more detail in the following
sections.
5.5.1 Diffusion in an Oxygen Potential Gradient
Chemical Diffusion in an Oxygen Potential Gradient
If an oxide A
1−δ
O of thickness ∆z is exposed to an oxygen potential gradient,
established, e.g., by different gas mixtures on both sides of the disc, different
concentrations of cation vacancies, c
(1)
V
and c
(2)
V
, are established on both sides
of the disc (according to (5.9)). As a consequence, a vacancy flux, j
V
=
−
˜
D·∇c
V
(see (5.29)), occurs from the high- to the low-oxygen potential side.
Due to the flux coupling, j
A
2+
+ j
V
= 0, cations are driven in the opposite
direction. When vacancies and cations arrive at the oxide surfaces, reduction
and oxidation of the oxide occur at the low- and high-oxygen potential side,
respectively:
V
A
+2h
•
+AO
reduction
−−−−−−→
←−−−−−−
oxidation
A
x
A
+
1
2
O
2
(g) . (5.32)
Thus, lattice planes are removed from the low oxygen potential side and
added to the high oxygen potential side. As a result, the crystal surfaces move
relatively to the immobile oxygen sublattice to the side of the higher oxygen
activity. The crystal displacement and the vacancy concentration profile can
be calculated by solving the diffusion equation [52]. After a short transient
period the crystal moves with a constant velocity. A steady-state solution
can be calculated by transforming from the laboratory reference frame
8
to a
moving coordinate system (coordinate z) which is fixed at one surface. The
steady-state vacancy fraction profile in the moving system, x
V
(z), is linear
in position, z, to a very good approximation, and the steady state velocity,
v,isgivenby
x
V
= a + b · z, v=
˜
D · b (5.33)
with
a = x
(1)
V
,b=
x
(2)
V
− x
(1)
V
∆z
. (5.34)
Experiments with the model system CoO exposed to an oxygen potential
gradient confirm the shift of the crystal surfaces relatively to the immobile
oxygen sublattice [52].
8
Since oxygen is essentially immobile, the laboratory reference frame is identical
to the lattice reference frame.

230 Manfred Martin
Tracer Diffusion in an Oxygen Potential Gradient
The motion of cation tracers (being chemically identical to the cation A or
being an impurity) in an oxide which is exposed to an oxygen potential gradi-
ent is influenced by the directed vacancy flux in (5.29) in two respects: Firstly,
the tracer diffusion coefficient is proportional to the vacancy fraction which
varies linearly with position. Thus, tracer diffusion takes place with a linearly
position dependent tracer diffusion coefficient. Secondly, the tracer ions are
moved by the drifting vacancies. This drift flux of the tracer particles, which
is over and above their normal Brownian motion, reflects the interaction of
the tracer ions with the vacancies. In summary, the tracer flux consists of
two parts: the first part, j
diff
tracer
, describes Brownian motion, as in the case
of a homogeneous crystal, but with the difference that the diffusion coeffi-
cient is position dependent. The second part is a drift term, j
drift
tracer
,whichis
proportional to the vacancy flux, as will be shown below.
Tracer Self-Diffusion in an Oxygen Potential Gradient
In this case the tracer ions A
∗
are chemically identical to the normal cations
A, but in contrast to them they are distinguishable. Thus, the tracer diffusion
coefficient, D
A
∗
= f
0
· D
V
· x
V
, contains the geometrical tracer correlation
factor, f
0
, and the first part of the flux of the tracer particles has the form
j
diff
A
∗
= −f
0
· D
V
· x
V
·∇c
A
∗
. (5.35)
Since the tracer particles are chemically identical to the normal cations they
are moved by the directed vacancy flux in the same way as the normal cations.
However, only a fraction x
A
∗
of the total amount of A exists as tracer. There-
fore the drift flux of the tracer has the form:
j
drift
A
∗
= −x
A
∗
· j
V
. (5.36)
The source solution for this diffusion problem is given by [53]
c
A
∗
(z,t)=
M
D
V
· b · t
· exp
−
2a + b · (z + z
0
)
D
V
· f
0
· b
2
· t
· I
0
(a + bz)
1/2
· (a + bz
0
)
1/2
D
V
· f
0
· b
2
· t
(5.37)
where M is the total amount of tracer per unit area, z
0
the initial position of
the tracer source, and I
0
a Bessel function of order zero. In contrast to the
source solution for a constant diffusion coefficient (Gaussian) the maximum
of the curve shifts with increasing time to the side of higher oxygen potential,
and the profile becomes more and more asymmetric. The initial tracer source
position can be marked by inert markers. Its position in the moving system
is z
marker
= z
0
+ v · t. The position of the maximum, z
max
(t), relative to the
marker position is then given by
