Heitjans P., Karger J. (Eds.). Diffusion in Condensed Matter: Methods, Materials, Models
Подождите немного. Документ загружается.

262 Franz Faupel and Klaus R¨atzke
simulations of the dense random packing of soft spheres of single component
glasses [64] indicate the existence of a high concentration of relatively large
octahedral and a portion of even larger interstices similar in size to a relaxed
vacancy in crystals. In multicomponent glasses the large voids may be filled by
the smaller atoms. Brandt [65] has demonstrated the stability of vacancies at
0 K in computer simulations of a one-component system. However, this result
does not allow one to draw conclusions on the stability of vacancies at high
temperatures. Bennet et al. [66] have shown vacancies to be unstable in a
Lennard-Jones computer glass at elevated temperatures, whereas a Keating
potential was found to stabilize a point defect. More recent simulations based
in realistic metallic interatomic potentials have shown vacancy-like defects to
be stable on the limited time scale below 1 ns [67].
So far we have focussed on irreversible structural relaxations. These can
be associated with long-range atomic transport. The aforementioned fast
reversible relaxation phenomena are most pronounced in multicomponent
glasses and have been attributed to local rearrangements of atoms within of
the general framework of β relaxation (Sect. 6.4, [68]). Anelastic coopera-
tive changes in the local structure, which may lead to a different chemical
short-range order, seem to be involved [69,70].
In addition to fast reversible relaxation, slow reversible changes are ex-
pected to occur for many properties near the glass transition temperature
T
g
[62]. As mentioned above the rapid crystallization of almost all conven-
tional glasses near T
g
, however, made it difficult to study this behavior.
Gerling et al. [71] utilizing an up-quenching technique and neutron irradia-
tion [72] have demonstrated the occurrence of reversible changes in ductility
and density in several amorphous alloys. In the new bulk glasses, of course,
reversibility of properties can always be achieved by annealing above T
g
and
subsequent cooling [59].
6.5.2 Possible Diffusion Mechanisms
We have seen that metallic glasses are prone to structural relaxation, phase
separation, and crystallization. Diffusion plays a mayor role in these processes
and is also important for solid-state amorphization alluded to in Sect. 6.5.1.
Nevertheless, despite of a considerable amount of research effort during the
last decades [4, 5, 45, 56, 73–76], the knowledge of diffusion in amorphous al-
loys has long been rather limited. Even after the discovery of the new bulk
metallic glasses with their stimulating effect on research several issues re-
main controversial. As for conventional metallic glasses, this is partly due to
experimental difficulties, such as the onset of crystallization, which restricts
the maximum annealing temperature and time to values corresponding to a
typical diffusion length of some ten nm. In order to circumvent the problems
concomitant with recording diffusion profiles on this length scale, conclusions
were often drawn from results of indirect methods like measurements of the
crystallization kinetics, viscosity, resistivity, magnetic anisotropy, and from
6 Diffusion in Metallic Glasses and Supercooled Melts 263
x-ray and M¨oßbauer techniques. Often, however, an unequivocal relationship
between the measured quantity and the diffusion coefficient in the amorphous
phase could not be established. Moreover, despite employing direct techniques
based on high-resolution ion-beam depth profiling in combination with a ra-
diotracer method, mass spectrometry, or Auger analysis, many investigators,
particularly in the early measurements, did not properly take into account
structural relaxation. We have seen in Sect. 6.5.1 that the diffusivity is very
sensitive to relaxation, and that reproducible results can only be obtained in
well relaxed samples.
While, in view of the disordered nature of amorphous alloys, one would ex-
pect a temperature-dependent effective activation energy (Sect. 6.4), it is now
well established that Arrhenius plots for diffusion in the relaxed metastable
amorphous state are linear, i.e. they exhibit a constant activation energy H.
This has often been interpreted as being indicative of a diffusion mechanism
similar to that in crystals, where vacancy-like defects in thermal equilibrium
are the carriers of diffusion, even though resulting D
0
values were sometimes
many orders of magnitude different from those typical of a vacancy mech-
anism. Moreover, numerous observations, e.g., the occurrence of anomalous
hydrogen diffusion [29] (cf. Sect. 6.4) and the difference in the activation en-
ergies for self-diffusion and magnetic relaxation, Kronm¨uller and Frank [27]
point to a broad spectrum of activation energies. Apparently, only a narrow
range of this spectrum is probed during long-range diffusion experiments in
the small accessible temperature interval. Furthermore, it has been shown
that compensation of side and saddle point disorder (see Fig. 6.2) may also
lead to a almost linear Arrhenius plot [26, 77]. An additional explanation of
the linear Arrhenius plots can be given in terms of a highly cooperative dif-
fusion mechanism, discussed below, which averages over local differences in
the barrier heights.
There are several experimental results that lend support to the idea of
defect-mediated diffusion in metallic glasses. For example, diffusion can be
enhanced by irradiation, which in crystalline materials produces additional
point defects. The annealing behavior of the radiation-induced excess volume
also resembles that of crystals [78,79]. Moreover, the crystallization kinetics
of amorphous (FeNi)
8
(PB)
2
were studied under hydrostatic pressure [76]. The
resulting activation volume of the order of one atomic volume was taken as
evidence of diffusion via point-defectlike entities in thermal equilibrium. It
should be noted, however, that the evaluation of diffusion coefficients from
measurements of the crystallization kinetics is a rather indirect approach
which has often been criticized [73,80]. Direct measurements of the pressure
dependence of diffusion will be discussed in detail in Sect. 6.5.4.
Tu and Chou [81] studied interdiffusion in electron-beam evaporated
amorphous NiZr trilayer films. Void formation was observed when both Ni
and Zr diffused. Since the voids formed on the side with the higher con-
centration of the slower species this was termed ‘opposite Kirkendall effect’.
264 Franz Faupel and Klaus R¨atzke
No voids were seen when Ni was the dominant diffusing species. The au-
thors concluded that self diffusion in the glass can be mediated by localized
vacancy-like defects, which may agglomerate like vacancies in crystals if their
concentration is out of equilibrium, as well as by non-localized ‘free-volume
defects’. A free-volume model of diffusion in metallic glasses has been pro-
posed by Spaepen [55]. The original theory for liquids (Sect. 6.3) was mod-
ified by introduction of an activation barrier for a diffusive jump into an
evolving hole. The free volume concept was corroborated by Chason and Mi-
zoguchi [82] who evaluated diffusion coefficients from the shift in position of
the modulated diffraction peak in compositionally modulated amorphous Fe-
Ti films as function of densification. The expected exponential dependence
of D on the free volume was indeed observed. Hahn and coworkers [83] mea-
sured the dependence of tracer diffusion on atomic size and alloy composition
in amorphous Ni-Zr. From similarities to α-zirconium they drew the conclu-
sion that small atoms, including Ni, diffuse by an interstitial-like mechanism,
using the interstices of the amorphous structure as jump positions. Such an
interstitial model of diffusion has been proposed by Ahmadzadeh and Can-
tor [84]. For larger atoms like Zr diffusion was explained in terms of the
coordinated motion of many atoms creating a localized free volume defect.
Cooperative diffusion mechanisms in metallic glasses have also been pro-
posed by others, [55, 73, 85–87], mostly in view of the extreme variety of
D
0
values alluded to above, which reflects a corresponding variability of the
entropy of diffusion (Chap. 1). Furthermore, the ln D
0
-vs-H relationship de-
viates strongly from that for single-jump diffusion in crystals. The envisioned
atomistic picture is often reminiscent of or directly related to the free-volume
approach. Highly collective thermally activated processes, such as chain-like
displacements of many atoms have been observed in computer simulations,
which will be discussed in more detail below [41,88, 89].
The new bulk metallic glasses have offered the opportunity to measure
diffusion in the supercooled liquid state and to investigate changes in the
transport mechanism concomitant with the glass transition and even in the
equilibrium melt. Several groups have carried out diffusion measurements
in bulk glasses during the last years by means of the radiotracer technique
[90,91] or secondary ion mass spectrometry [92] (see Chap. 1 for experimental
techniques). One generally observes a kink in the Arrhenius plot near the
caloric glass transition temperature as measured, e.g., by differential scanning
calorimetry (DSC). In accord with the expected time scale dependence of the
caloric glass transition temperature the kink is shifted to lower temperatures
for slower diffusing elements, and for Al no kink is observed in the investigated
temperature range, for instance. The kink in the Arrhenius plot has often been
interpreted as being due to a change in the diffusion mechanism [92, 93]. In
particular, a transition from single-atom hopping to liquid-like viscous flow
has been proposed at the caloric glass transition temperature T
g
[94,95].

6 Diffusion in Metallic Glasses and Supercooled Melts 265
The notion of a change in the mechanism of atomic transport at T
g
is in
conflict with predictions of the mode coupling theory (MCT) [96] discussed
in Sect. 6.4. According to MCT, a gradual transition in the atomic transport
mechanism from solid-like thermally activated local hopping – envisioned as
a highly collective process involving many atoms [25] – to liquid-like motion
occurs well above T
g
at a microscopic glass transition temperature T
c
. T
c
does not depend on the time scale of the experiment and is the temperature
where, upon cooling, due to the concomitant increase of density, the cage
formed by the neighboring atoms of a given atom is frozen in and can only
be overcome by hopping processes.
In the present light we are left with the following questions:
1. Is diffusion in fully relaxed metallic glasses generally mediated by thermal
equilibrium defects similar to vacancies in crystals as often suggested (see
review articles [18, 97])?
2. If thermal defects do not mediate diffusion, is diffusion in relaxed amor-
phous alloys a highly cooperative process as one would infer from com-
puter simulations and the concept of ‘medium assisted hopping’ discussed
in Sect. 6.4?
3. Which role do non-equilibrium defects play?
4. Do we have to consider different mechanisms for diffusion in relaxed and
as-quenched samples, in other words: how does quenched-in excess volume
affect diffusion?
5. Is there a change in the diffusion mechanism at the caloric glass transition
temperature or does the change occur above the critical temperature Tc
where, according to the mode coupling theory liquid-like atomic motion
sets in?
In the next sections we shall see that these questions can be answered
satisfactorily by critical experiments. Investigations of the isotope-mass de-
pendence of diffusion in relaxed samples (Sect. 6.5.3) are pertinent to the
second question. In Sect. 6.5.4 measurements of the pressure dependence of
diffusion will be discussed, which are related to the role of equilibrium defects.
The role of excess volume will be addressed in Sect. 6.5.5 mainly based on
measurements of the isotope effect during structural relaxation. Section 6.6
will be devoted to the 5th question. Here we will also discuss very recent
diffusion and isotope measurements in the equilibrium liquid.
6.5.3 Isotope Effect
A salient feature of diffusion in metallic glasses is the extremely small isotope
effect. The isotope effect E for diffusion of two isotopes with diffusivities D
i
and masses m
i
is defined as ( [98], see also Chap. 1, Sect. 1.7.2)
E =(D
α
/D
β
− 1)
)
*
m
β
/m
α
− 1
. (6.9)
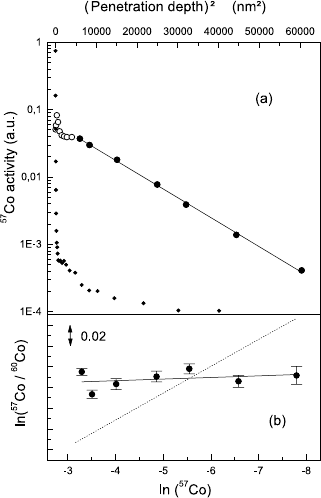
266 Franz Faupel and Klaus R¨atzke
Fig. 6.3. (a) Typical penetra-
tion profile of
57
Co diffusion in
Pd
40
Cu
30
Ni
10
P
20
at 603 K and 1 h
annealing time. The activity is
plotted vs. the square of penetra-
tion depth. (The very first data
points (open symbols)areaffected
by surface effects and were not
taken in to account.) The resolu-
tion function of the depth profil-
ing technique, obtained from a thin
tracer layer without annealing, is
shown in black. (b) Corresponding
isotope effect profile. The activity
ratio of
57
Co and
60
Co is plotted
vs. the
57
Co activity on a logarith-
mic scale. The dotted line indicates
an isotope effect of E =1andis
shown for comparison (from [60]).
For single jump diffusion in densely packed lattices E is generally of the order
of unity because of the m
−1/2
dependence of the attempt frequency [98],
although correlation effects may give rise to a significant reduction of E,
particularly in case of diffusion of diluted impurities due to impurity-vacancy
binding (Chap. 1) [99]. E can also be strongly reduced by relaxation of the
surrounding [100] and a broad distribution of activation energies [101].
Almost vanishing isotope effects have been observed for Co diffusion in
various metallic glasses [102–106]. E was determined by measuring the si-
multaneous diffusion of the radiotracers
57
Co and
60
Co employing the serial
sectioning technique in conjunction with ion-beam sputtering. For illustra-
tion of the technique, which is described in Chap. 1, Fig. 6.3 depicts a typical
radiotracer profile on a semilogarithmic scale. According to the thin film so-
lution of Fick’s 2nd law (Chap. 1, (1.9)) a straight line fitted to the profile
has the slope 1/(4Dt) and thus yields the tracer diffusivity D if the annealing
time t is known. In order to obtain the isotope effect one can easily derive
the following equation based on the thin film solution
ln[c
α
(x, t)/c
β
(x, t)] = const. − (D
β
/D
α
− 1) · ln[c
β
(x, t)] . (6.10)
Equation (6.10) shows that the slope of a straight line fitted to the data in
Fig. 6.3b is given by D
α
/D
β
−1. The isotope effect E then follows from (6.9)
and the isotope masses. Representative isotope effects for Co-Zr glasses are
shown in Fig. 6.4.
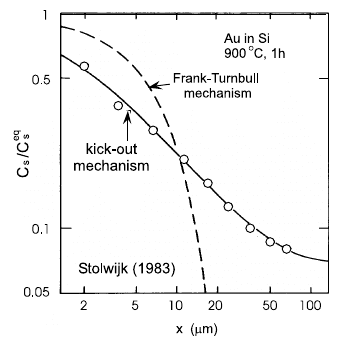
6 Diffusion in Metallic Glasses and Supercooled Melts 267
Fig. 6.4. Isotope effect in Co
x
Zr
1−x
glasses for 0.31 <x<0.86. One
notes that the isotope effect is very
close to zero in the whole concentra-
tion range [105].
The essentially vanishing isotope effects measured in metallic glasses were
taken as strong evidence of a highly collective hopping mechanism, as sug-
gested by the notion of highly cooperative medium assisted hopping (see
Sect. 6.4) and molecular dynamics (MD) simulations [107–109]. In these terms
the extremely small isotope effects are attributed to a strong dilution of the
mass dependence of diffusion due to the participation of a large number of
atoms in collective hopping processes.
In MD simulations mainly chain-like displacements have been observed
[88]. Both the computer simulations as well as the isotope effect measurements
suggest the number of atoms participating in a cooperative hopping process
to be very high, typically well above ten [109, 110].
Computer simulations also indicate a connection between the low-fre-
quency excitations in glasses and long-range diffusion [88]. These low-fre-
quency excitations appear to be characteristic of topologically disordered
solids [111–114]. They give rise to extra specific heat at low temperatures and
to additional contributions to the low-frequency part of the vibrational den-
sity of states as obtained by Raman and inelastic scattering. Close to absolute
zero temperature the excitations can be attributed to tunneling (two-level)
systems and at somewhat higher temperatures to quasi-localized vibrational
states [113]. In addition to the periodic low frequency excitations aperiodic
thermally activated relaxations have been observed [115]. Fits of an extended
soft-potential model to experimental data resulted in effective masses of 20 to
100 atomic masses for the entities moving in the effective soft potentials. In a
soft-sphere glass, which is a first approximation of a metallic glass, the local
relaxations turned out to be collective jumps of groups of atoms, predom-
inantly chains (sometimes with side-branches) along dense directions [115].
The effective mass and the total jump length of the atoms were found to
increase with increasing temperature, and the possibility of fusion of isolated

268 Franz Faupel and Klaus R¨atzke
relaxations at high temperatures was demonstrated. It is not unlikely that
such processes lead to long-range diffusion. We emphasize, however, that our
isotope effect measurements do not rule out other cooperative mechanisms,
e.g., those involving smeared out defects.
6.5.4 Pressure Dependence
Information on the role of thermal defects have been obtained from measure-
ments of the pressure dependence of diffusion. For a single-jump-type vacancy
mechanism in crystalline close packed metals the activation volume, given by
V
act
≈−k
B
T
∂ ln D
∂p
T
, (6.11)
is of the order of the size of the relaxed vacancy, i.e., somewhat smaller than
one atomic volume [98]. In (6.11) D, p, T ,andk
B
are diffusivity, pressure,
absolute temperature, and Boltzmann constant, respectively. For interstitial
diffusion, i.e. a direct mechanism without thermally generated defects, the
activation volume proved to be nearly vanishing [98]. Both observations sug-
gest the activation volume to be essentially given by the formation volume of
the defect and the migration volume for a single-jump mechanism to be very
small.
Several measurements, carried out by our group [116–118] and the M¨unster
group [119], have revealed an almost vanishing pressure dependence for
Co diffusion in the metallic glasses Co
76.7
Fe
2
Nb
14,3
B
7
,Fe
40
Ni
40
B
20
,and
Co
81
Zr
19
. A recent example is shown in Fig. 6.5 [118]. This in conjunction
with the very small isotope effects, measured in these systems, led us to the
conclusion that the migration volume can be close to zero even for a coop-
erative hopping process involving many atoms. Consequently, diffusion was
interpreted in terms of direct cooperative hopping process as observed in the
above mentioned MD simulations.
On the other hand, a pronounced pressure dependence, corresponding
to activation volumes between one half and two atomic volumes, expressed
in terms of the average atomic volume of the alloy, was reported for Au
diffusion in amorphous Pd
40
Ni
40
P
20
[120], Ni diffusion in Ni
x
Zr
100−x
(42 <
x<62) [121] and Co
42
Zr
58
glasses [122], Hf diffusion in Ni
54
Zr
46
[123], and
Zr diffusion in Co
91
Zr
9
[124]. Based on our aforementioned conclusion on
the possibility of very small migration volumes even for cooperative hopping
processes we tend to attribute the observed significant activation volumes to
formation of thermal defects, which, however, may not necessarily be localized
like a vacancy in a crystal but are expected to be spread out. On the other
hand, recent results from MD simulations indicate that collective hopping
processes may have a significant migration volume [109].
Of particular interest is the case of Co-rich Co-Zr glasses. Here Co seems
to diffuse by a cooperative hopping process without assistance of thermal
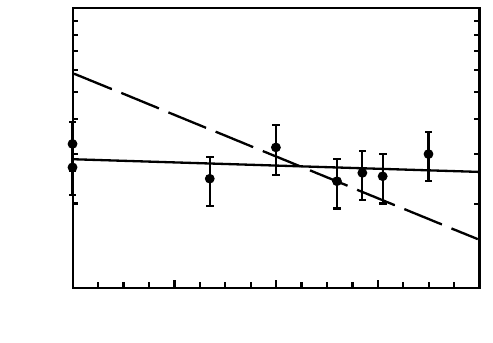
6 Diffusion in Metallic Glasses and Supercooled Melts 269
p (MPa)
0 200 400 600 800
D (m
2
s
-1
)
10
-21
10
-20
Fig. 6.5. Isothermal pressure dependence of
57
Co diffusion in structurally relaxed
amorphous Co
81
Zr
19
at 563 K [118]. The dashed line corresponds to an activation
volume of one atomic volume.
defects, judging from a vanishing isotope effect [104] and an almost vanishing
pressure dependence [118]. Diffusion of Zr, in contrast, is characterize by a
large activation volume of the order of an atomic volume, which we attributed
to diffusion via thermal defects [124]. This points to the existence of an op-
posite Kirkendall effect in interdiffusion in amorphous Co-Zr or Ni-Zr alloys
which should behave similarly. An opposite Kirkendall effect, evidenced by
void formation on the side with a higher concentration of the slower com-
ponent, was indeed reported under conditions where both Ni and Zr were
mobile in interdiffusion experiments involving amorphous Ni-Zr couples of
different composition. As expected no such effect was seen when only Ni was
mobile (see Sect. 6.5.1) [81]. These experiments lend support to the existence
of thermal defects that mediate diffusion.
Mechanisms based on delocalized thermal defect have been proposed be-
fore [55, 97, 125, 126]. A well known example is the spread out free vol-
ume within the free-volume approach modified for glasses (see Sect. 6.5.2)
[55,94, 127].
6.5.5 Effect of Excess Volume on Diffusion
So far, we have discussed diffusion in structurally relaxed metallic glasses.
Only in these systems the structure is stable during a diffusion experiment,
and reproducible diffusion measurements can be made, if the diffusion tem-
perature does not significantly exceed the relaxation temperature and the
diffusion time in not too long. On the other hand, conventional metallic
glasses, and to some extend also bulk glasses [128, 129], contain excess vol-
270 Franz Faupel and Klaus R¨atzke
ume quenched in from the liquid state. This excess volume affects nearly all
properties [130] and enhances diffusion [131,132]. Therefore, we have investi-
gated the influence of excess volume on the diffusion mechanism by measuring
both the diffusivity and the isotope effect during structural relaxation of as
quenched glasses. First measurements were carried out for Co diffusion in
a Co-rich metal-metalloid glass [133]. The isotope effect was found to be as
high as 0.5 in the as-quenched state and to drop to a very low value of 0.1 in
the relaxed state evidenced by a plateau region of the diffusivity. The high
isotope effects in an as quenched state corroborates the notion of quenched-in
quasi-vacancies, which migrate to the outer sample surface during structural
relaxation [56].
On the other hand, our recent isotope effect measurements in thin amor-
phous Co-Zr films do not show any change in the diffusion mechanism during
structural relaxation and point to a collective hopping mechanism in the
relaxed and in the as-quenched state [132,134]. As shown in Fig. 6.6a the dif-
fusivity drops substantially during relaxation and finally reaches the expected
plateau value. The course of D(t) is well described by the Kohlrausch law
given in (6.7). The isotope effect in very low in the whole range (Fig. 6.6b)
reflecting highly collective diffusion. Similar behavior was observed in thin
Co
81
Zr
19
films. Apparently, in the thin Co-Zr films excess volume appears to
annihilate intrinsically, e.g., by recombination of regions of higher and lower
density on the nanoscopic scale as first suggested by Egami et al. [130].
6.6 Diffusion in Supercooled and Equilibrium Melts
Most diffusion studies in the new bulk-glass formers where performed in the
amorphous ‘Johnson alloy’ Zr
47
Ti
8
Cu
7.5
Ni
10
Be
27.5
also termed Vitrelloy 4.
An Arrhenius plot summarizing the results for the is shown in Fig. 6.7, for
example. One notes the aforementioned kinks shifting to lower temperatures
for slower diffusing species, which have often been interpreted as a change
in the diffusion mechanism at the caloric glass transition temperature. The
diffusivities follow the expected size dependence (for a detailed discussion
see, e. g., Chap. 5 in [5]) in both the glassy and the supercooled liquid state
except for the Be data reported by Geyer et al. (dashed line in Fig. 6.7). The
intersection of the Be diffusivity with those of substantially larger elements
is quite unusual and contradicts expectations on the size dependence of dif-
fusion as well as more general concepts of the convergence of the diffusivities
at higher temperatures (above T
c
) as predicted by the Stokes-Einstein equa-
tion. While Geyer at al. performed chemical diffusion experiments, which are
influenced by the thermodynamic factor [99] recent radiotracer experiments
of our group in cooperation with the group of Geyer demonstrated that the
behavior of Be is by no means exceptional. As shown in Fig. 6.7, the radio-
tracer data of Rehmet et al. [135] (solid line) nicely fit in with the overall
picture. The differences between the tracer and the chemical diffusion data
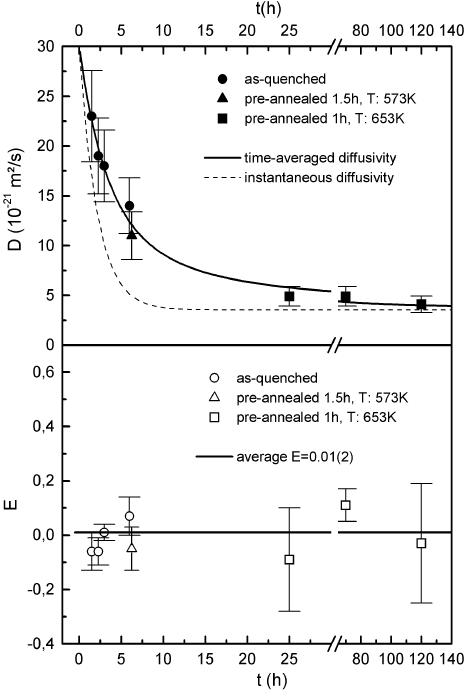
6 Diffusion in Metallic Glasses and Supercooled Melts 271
Fig. 6.6. (top) Time dependence of
57
Co diffusion in Co
51
Zr
49
during structural
relaxation at T = 300
◦
C. The squares indicate samples that have been preannealed
at T = 380
◦
C for 1 h. Circles indicate specimens that have not been preannealed
before diffusion treatment while the specimen described by a triangle was annealed
for 1.5 h and again for 4.75 h. The straight line represents the time-averaged dif-
fusivity, the dashed line shows the instantaneous diffusivity (for details see 132).
(bottom) Isotope effect E in Co
51
Zr
49
during structural relaxation. The squares
indicate the preannealed samples while circles display the specimens which have
not been preannealed before diffusion annealing. The specimen represented by a
triangle was annealed for 1.5 h and again for 4.75 h.
are not yet understood. They cannot be attributed to the thermodynamic
factor, which goes to unity at high temperatures.
Recently, we have also carried out isotope effect measurements involving
the isotopes
57
Co and
60
Co by means of the radiotracer technique in the
