Heard D.E. (editor) Analytical Techniques for Atmospheric Measurement
Подождите немного. Документ загружается.


418 Analytical Techniques for Atmospheric Measurement
intervals over the wavelength range that contributes to the photolysis process of
interest. Photolysis frequencies are then calculated from the measured radiation spectra
F
and the relevant molecular cross section and quantum yield , according to
Equation 9.12. The main advantage of spectroradiometry is the possibility to determine
photolysis frequencies for a whole set of different molecules at the same time.
•
Actinic-flux filter radiometers have a broad spectral coverage and measure the actinic
flux integrated over all wavelengths simultaneously. The optical wavelength selection is
chosen to mimic the spectral function × of the relevant photolysis frequency by
means of an optical transmission filter and a photoelectric sensor. In a first approxi-
mation, the filter radiometer signal is proportional to the photolysis frequency and can
be calibrated against an absolute reference (e.g. a chemical actinometer). In order to
obtain more accurate results, the dependence of the calibration on ambient conditions
(e.g. solar zenith angle, temperature) must be taken into account. Filter radiometers
are generally useful because of their compact size and fast time response.
•
Irradiance radiometers (spectroradiometers and broadband radiometers) can be used for
the determination of photolysis frequencies in principle like actinic-flux instruments.
However, the measured irradiances must be converted into actinic flux data, a step
that introduces additional errors in the derived photolysis frequencies. The conversion
requires knowledge of the angular distribution of the radiance in the atmosphere.
The latter information is difficult to obtain in the field, especially in the presence
of clouds and aerosols, and is usually estimated by empirical rules or by numerical
models. Irradiance radiometers have the advantage that they have a wide distribution
as meteorological instruments and are readily available as standard equipment from
various manufacturers. For details, see Section 9.6.
9.2.3 Actinic-flux sensing techniques
For practical purposes, most important are the measurement techniques that are directly
sensitive to the actinic flux, that is have the ability to collect radiation with equal
sensitivity independent of the angle of incidence (isotropic behaviour). To this class
of techniques belong the abovementioned chemical actinometers, actinic-flux spectrora-
diometers and actinic-flux filter radiometers. The measurement of actinic flux requires
the integral detection of the incident radiation from all directions over a solid angle of
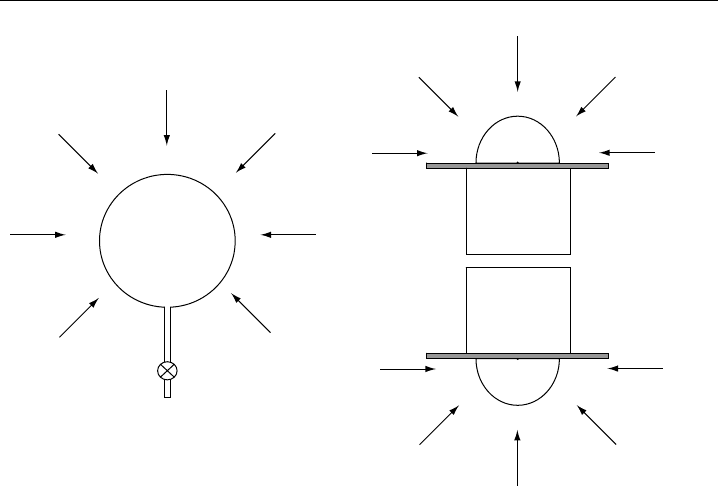
4 sr. Spherical or tubular photolysis reactors used in chemical actinometry closely fulfil
this requirement (Figure 9.8A). Actinic flux radiometers mostly use hemispherical input
optics, such that two instruments must be combined to cover 4 sr (Figure 9.8B). The
setup of two radiometers pointing into opposite directions has the additional advantage
of providing separate information about the up- and down-welling components of the
actinic radiation.
The basic features of actinic-flux sensing techniques are summarized in Table 9.2, while
detailed descriptions follow in later sections. It may be noted that chemical actinometry
is the oldest actinic-flux method and has been used since the mid-1970s to measure
photolysis frequencies in the atmosphere, mainly for NO
2
and O
3
(see Section 9.3).
Actinic-flux radiometry was developed later, starting at the end of the 1980s, and is now
widely applied in atmospheric chemistry for measurement of photolysis frequencies of
Measurement of Photolysis Frequencies in the Atmosphere 419
Gas inlet
(a) (b)
Quartz bulb
Artificial horizon
Photoelectric
detector
unit
Opt.
receiver
Figure 9.8 Receiver geometries for solar actinic flux. (a) Photolysis reactor of a chemical actinometer
with a spherical response. (b) Combination of two hemispheric radiometers facing into opposite directions.
various gases (see Sections 9.4, 9.5, 9.8). The performance and reliability of actinic-flux
techniques have been thoroughly tested in several assessments. Examples of results are
given in Section 9.8.3.
9.2.4 Radiative transfer models
For completeness, theoretical modelling should be mentioned as a method to determine
photolysis frequencies.
Radiative transfer (RT) models calculate first and foremost the spectral actinic flux in
the atmosphere. The models use the extraterrestrial solar spectrum as input, as well as
optical properties of the atmosphere and the earth’s surface albedo. In a second step,
photolysis frequencies can be calculated from the simulated radiation spectra F
and
the relevant molecular data and , using Equation 9.12.
Radiative transfer models play an important role for the prediction of photolysis
frequencies in atmospheric chemistry. In particular, they provide a broad temporal
and spatial coverage that currently cannot be achieved by measurements alone. On
the other hand models have difficulties to calculate accurate j-values at local sites
where the field-of-view is shadowed by trees, buildings or mountains, or when complex
cloudiness prevails. Model results are therefore no good substitute for in situ measure-
ments in local photochemistry field campaigns. However, models are useful to assist in
420 Analytical Techniques for Atmospheric Measurement
Table 9.2 In situ measurement techniques for atmospheric photolysis frequencies
a
Feature Chemical
actinometer
Actinic flux
spectroradiometer
Actinic flux filter
radiometer
Main components Spherical or
cylindrical quartz
reactor + gas
analysis
Optical receiver +
scanning
monochromator or
spectrograph +
photoelectric
detection system
Optical receiver +
optical filter +
photoelectric detector
Measured quantity Concentration [AB] F
U =
D
0
F
d
b
Signal evaluation j =−
1
t
AB
AB
c
j =
F
d
j = AU
e
Versatility Single specific
photolysis reaction
Various photolysis
reactions at the
same time
Single specific
photolysis reaction
Reported applications O
3
,NO
2
, RONO
2
O
3
NO
2
HONO
HCHO
O
3
,NO
2
Calibration reference Gas standard Calibration lamp Reference instrument
f
or calibration lamp
Sensitivity Medium to good Very good Good
Time resolution Flow reactors:
1–60 s; Static
reactors: 1–60 min
Scanning
monochromator
systems: 15–120 s;
Spectrograph
systems: 1–10 s
∼ 1s
Size Large to medium;
requires
pressurised gases
Medium to small Small
a
Listed are techniques that collect radiation with isotropic sensitivity.
b
U is a voltage signal; D
0
represents the wavelength-dependent sensitivity of the filter radiometer; the spectral design
of the filter radiometer aims for D
0
∝ and requires knowledge of and .
c
Equation 9.1, or alternatively equation 9.3 may be applied.
d
Equation 9.12 is applied; it requires knowledge of T and T , and, if necessary, of ambient temperature T .
e
See equation 9.45; the calibration factor A usually depends on ambient parameters such as solar zenith angle,
temperature etc.
f
Reference instrument can be a chemical actinometer, a spectroradiometer, or another calibrated filter radiometer.
the determination of photolysis frequencies by certain techniques, like filter radiometry
or irradiance radiometry. See Section 9.7 for more information about RT modelling.
9.3 Chemical actinometry
Chemical actinometry in its original sense means the quantification of an absolute photon
flux by measuring the rate of photoinduced change in a chemical system (Calvert & Pitts,
1966; Pravilov, 1987; Kuhn et al., 2004). In general the chemical system can be a solid,

Measurement of Photolysis Frequencies in the Atmosphere 421
liquid or gas and is called chemical actinometer. Actinometry is then based on the principle
that the photolytical conversion rate of molecules in an actinometer cell is equal to the
absorption rate of photons times the quantum yield of the actinometer. The absorption
rate of photons in the actinometer is related to the incident photon flux by Lambert–
Beer’s law. Application of these fundamental principles allows the calculation of absolute
incident photon-fluxes from measured chemical-conversion rates, if the quantum yield
of the actinometer is well known (for details, see Calvert & Pitts, 1966).
While classical chemical actinometry has been used in laboratories by photochemists
for almost a century (e.g. Warburg, 1912; Vaughan & Noyes, Jr., 1930), the general
concept has been adapted, beginning in the 1970s, by atmospheric chemists for the
measurement of photolysis frequencies in the atmosphere. Applications have been
reported for O
3
,NO
2
and alkyl nitrates (Tables 9.3 and 9.4). Corresponding techniques for
the photolysis of O
3
and NO
2
will be presented in some detail in Sections 9.3.4 and 9.3.5,
respectively.
9.3.1 Principle of chemical actinometry
Chemical actinometers for atmospheric measurements are realised by (1) filling the gas
of interest into a photolysis reactor, (2) exposing the actinometer to solar radiation,
and (3) measuring the photochemical conversion rate. Here, it is important that the
actinometer gas is exposed to the ambient actinic flux without altering the spectral
composition and intensity of the radiation significantly. This is achieved by the usage
of a transparent quartz cell with a suitable geometrical shape (spherical or cylindrical)
and by applying gas concentrations with small optical absorbances (see more about this
aspect in Section 9.3.3 and Appendix A.2). If these conditions are fulfilled, the photolysis
frequency can be evaluated according to Equation 9.1, which requires measurements of
gas concentrations and time but which does not need the knowledge of the absorption
spectrum and quantum yield of the primary photodissociation.
There are two possible modes of actinometer operation. In the static batch mode the
actinometer gas is filled into the photolysis reactor which is then sealed off by a gas
valve and is covered with an opaque hood to exclude sunlight. For measurement, the
actinometer is uncovered and exposed to solar radiation for a fixed time interval t.
Thereafter, the actinometer is covered again and is analysed for change in its chemical
composition.
The other mode of operation involves flowing gas. The actinometer gas is passed
continuously through a photolysis reactor which is permanently exposed to ambient solar
radiation. In this case t is equal to the mean residence time of the gas in the illuminated
photolysis cell. After having passed the reactor, the gas composition is analysed by an
online gas detector. This operational mode allows a continuous monitoring of photolysis
frequencies.
9.3.2 Chemical kinetics considerations
The rate of photochemical change for a general reaction AB +h → A +B can be
determined from either the loss of reactant AB or the increase of its product A (or B).
Table 9.3 O
3
→ O
1
D chemical actinometers used in atmospheric field studies
Reference Photolysis reactor
a
Gas mixtures
b
Exposure time Detection technique k
a
/k
b
c
Platform
Bahe and
Schurath (1978);
Bahe et al.
(1979)
Static and flow
types, spher.
O
3
/N
2
O 0.5–2 h N
2
: gas chromatography 062 Ground + balloon
h =26 km
Dickerson et al.
(1979, 1982)
Flow type, cyl. O
3
/O
2
/N
2
O10s N
2
O
5
: conversion into
NO + detection by
chemiluminescence
08 Ground + aircraft
h =01–68km
Junkermann
et al. (1989)
Static type, spher. O
3
/N
2
O <30 min N
2
: gas chromatography 075 Ground
Blackburn et al.
(1992)
Flow type, cyl. O
3
/O
2
/N
2
O34s NO
3
+N
2
O
5
: transfer into
liquid methanol + detection
by electrical conductivity
079 Ground
Bairai and
Stedman (1992)
Flow type, cyl. O
3
/O
2
/N
2
O/He 7 s NO
2
: transfer into luminol
solution + fluorescence
detection
08 Ground
Müller et al.
(1995)
Static type, spher. O
3
/O
2
/N
2
O 0.8–2 h N
2
: gas chromatography 075 Ground
Shetter et al.
(1996)
Flow type, cyl. O
3
/O
2
/N
2
O40s N
2
O
5
: transfer into liquid
water + detection by
electrical conductivity
072 Ground
a
Cyl., cylindrical tube; spher., spherical bulb. Typical reactor volumes are 50–250cm
3
. Walls are made of transparent quartz.
b
N
2
OO
3
>100; total pressure is about 1 atm in flow systems, and 1–2 atm in static systems.
c
Branching ratio of the O
1
D +N
2
O reaction channels producing (a) N
2
+O
2
and (b) NO+NO, used for the calculation of the jO
1
D-values.
Table 9.4 NO
2
→ NO +O and RONO
2
→ RO +NO
2
chemical actinometers used in atmospheric field studies
Reference Photolysis reactor
a
Gas mixtures
b
Exposure time Detection technique
c
Platform
NO
2
→ NO +O actinometer
Jackson et al.
(1975); Harvey
et al. (1977)
Flow type, cyl. NO
2
/Air 1–4 s NO, NO
2
:
chemiluminescence
Ground
Zafonte et al.
(1977)
Flow type, cyl. NO
2
/N
2
7 s NO, NO
2
:
chemiluminescence
Ground
Bahe et al. (1980) Flow type, spher. NO
2
/N
2
10 s NO: chemiluminescence Ground
Static type, cyl. NO
2
30 s NO
2
: spectrophotometry Ground
Dickerson et al.
(1982)
Flow type, cyl. NO
2
/O
2
1 s NO, NO
2
:
chemiluminescence
Ground + aircraft
h =01–55km
Parrish et al. (1983) Flow type, cyl. NO
2
/N
2
(60 hPa, total)
5 s NO, NO
2
:
chemiluminescence
Ground
Madronich et al.
(1983, 1985)
Static type, cyl. NO
2
5–10 min Pressure increase Ground + balloon
(h =24, 32 km)
Shetter et al.
(1992); Lantz et al.
(1996)
Flow type, cyl. NO
2
/O
2
(67 hPa, total)
0.3–0.5 s NO, NO
2
:
chemiluminescence
Ground
Schultz et al.
(1995)
Static type, cyl. NO
2
1–2 min NO
2
: photometry Ground
Table 9.4 (Continued)
Reference Photolysis reactor
a
Gas mixtures
b
Exposure time Detection technique
c
Platform
Kelley et al. (1995);
Dickerson et al.
(1997)
Flow type, cyl. (dual
system, upward and
downward looking)
NO
2
/Air 1 s NO, NO
2
:
chemiluminescence
Aircraft
h =02–76km
Castro et al. (1995,
1997)
Flow type, cyl. NO
2
/N
2
1 s NO, NO
2
:
chemiluminescence
Ground
Vuilleumier et al.
(2001)
Flow type, cyl. NO
2
/N
2
1 min NO, NO
2
:
chemiluminescence
Ground
Kraus et al. (2000) Flow type, cyl. NO
2
/Air 1 min NO, NO
2
:
chemiluminescence
Ground
RONO
2
→ RO +NO
2
actinometer
Luke et al. (1989) Flow type, cyl. RONO
2
/Air
d
6–11 s NO
2
: transfer into luminol
solution + fluorescence
detection
Ground
a
Cyl., cylindrical tube; spher., spherical bulb. Typical reactor volumes are 7 to 380 cm
3
. Walls are made from transparent quartz.
b
In NO
2
flow actinometers the mixing ratio of NO
2
is typically between 1 and 80 ppmv at total pressures around 1 atm, unless otherwise indicated. In static systems pure NO
2
is used
at pressures of 1–5 hPa.
c
NO
2
can be detected by chemiluminescence after chemical conversion into NO.
d
R = ethyl, n-propyl, n-butyl, 2-butyl; mixing ratios of RONO
2
are between 23–52 ppmv.

Measurement of Photolysis Frequencies in the Atmosphere 425
If the exposure time t is held at less than 1% of the reciprocal j-value, the concentration
of AB will change very little and AB can be assumed to have a constant concentration
AB ≈ AB
0
. It is then reasonable to measure the increase of the product A, which can
be related to the photolysis frequency as follows:
dA =jABdt (9.19)
Integration over the time interval t yields
A = AB
0
t
0
jt d t (9.20)
In principle the photolysis frequency may vary during the exposure time t. It is then
useful to define a mean value
j for this time interval:
j =
1
t
t
0
jt d t (9.21)
Combining Equations 9.20 and 9.21 results in
j =
1
AB
0
A
t
(9.22)
This demonstrates that the photolysis frequency derived from the measured increase A
is a true mean value over the respective time interval.
Another approach is to let the initial concentration AB
0
noticeably decay and measure
the decrease AB during t. In this case we get
dAB =−jAB dt (9.23)
which after integration yields
j =−
1
t
ln
AB
0
−AB
AB
0
(9.24)
Here again, a mean j-value is obtained for the time of exposure.
It should be noted that the photochemistry and its kinetics in real chemical actinometers
is often complicated by secondary chemistry. The reason is that the photolytical products,
atoms and radicals, are highly reactive. Consecutive reactions in the gase phase and on
wall surfaces lead to losses of the products, and possibly also of the initial reactant. For an
accurate evaluation of the required photolysis frequency the effect of secondary chemistry
must be taken into account. Sometimes scavenger gases are added to the actinometer gas
in order to capture the reactive primary photofragments and convert them into species
which are more stable or easier to measure. Examples will be given in Sections 9.3.4
and 9.3.5.

426 Analytical Techniques for Atmospheric Measurement
9.3.3 Optical and photochemical considerations
The advantage of a properly designed chemical actinometer is that the gas molecules
inside the photolysis reactor will photodissociate with the same rate coefficient as would
molecules do in the surrounding atmosphere. This will only happen if the conditions that
have an influence on the photolysis rate are the same inside and outside of the chemical
actinometer.
Gas temperature and pressure have a possible influence on the absorption cross section
and quantum yield of photolysis reactions. For example, the photodissociation of O
3
→
O
1
D exhibits a strong temperature dependence (Dickerson et al., 1982; Bohn et al.,
2004), while the NO
2
photolysis is only weakly temperature dependent (Dickerson et al.,
1982; Shetter et al., 1988). In few cases, a notable pressure dependence has been observed,
such as in the formation of H
2
+CO in the photolysis of HCHO (Moortgat et al., 1983).
Whenever a temperature or pressure dependence exists, the operating conditions in the
actinometer must be chosen similar to the corresponding ambient conditions in order to
provide representative measurements.
Optical effects caused by the design of the chemical actinometer can modify the actinic
flux inside the photolysis reactor. Potentially-interfering processes are:
•
Strong absorption by the actinometer gas.
•
Absorption by the photolysis-cell wall-material.
•
Optical reflections at the outer and inner wall surfaces.
•
Shielding of solar radiation by the gas inlet/outlet of the photolysis cell.
Gas phase absorption can be a problem at high concentrations of the actinometer
gas. If the gas phase is optically thick, for example the absorbance exceeds 1%, an
appreciable fraction of radiation is absorbed in the photolysis cell. Correspondingly,
the absorption protects some of the actinometer gas from photolysis and the resulting
photolysis frequency will be systematically lower than in the surrounding atmosphere.
Optically thin conditions can be achieved by a sufficiently low actinometer-gas concen-
tration, which in turn may require the use of highly sensitive analytical instruments for
the measurement of correspondingly low reactant and product concentrations.
Chemical-actinometer walls are usually made of quartz and have a typical thickness
of a few millimeters. At this thickness the material is highly transparent and has an
absorbance of less than 1% between 250 and 1000 nm. Thus, losses of light due to wall
absorption are negligible. A considerable amount of incident solar radiation is, however,
lost by reflection I
R
when the light enters the photolysis reactor (Figure 9.9; for details,
see Appendix A.2). The reflectance of the quartz wall depends on the angle of incidence
and varies for unpolarized UV radiation between ∼8% at normal incidence = 0
to 100% at grazing incidence = 90
. The attenuated radiation I
1
= I
0
−I
R
entering
the photolysis cell is subsequently reflected multiple times at the inner surface of the
photolysis cell. These reflections enhance the photon flux inside the chemical actinometer.
For a photolysis reactor that has a spherical or infinitely long cylindrical shape and
non-absorbing walls, it has been shown in a theoretical study by Zafonte et al. (1977)
that the reflections at the exterior and interior wall surfaces cancel each other exactly,
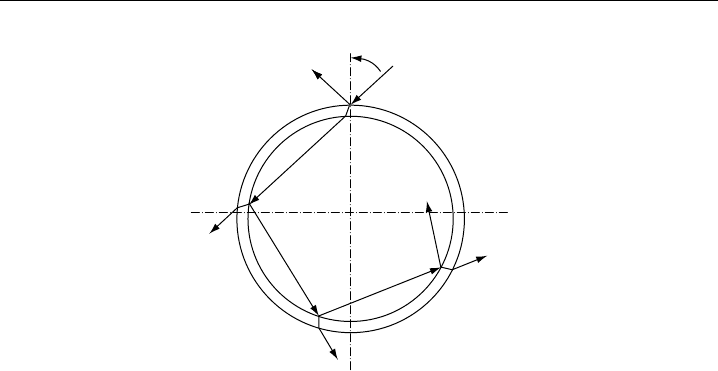
Measurement of Photolysis Frequencies in the Atmosphere 427
I
0
I
1
I
2
I
3
I
4
I
R
ϑ
0
Figure 9.9 Illustration of multiple reflections inside a spherical or cylindrical (infinite length) photolysis
reactor. I
0
is the intensity of the incident solar radiation. I
R
is the intensity lost by reflection at the outer
wall and I
1
is the transmitted intensity. I
2
I
3
I
4
denote the intensities of the internally reflected light.
that is I
1
+I
2
+I
3
+···=I
R
(see Appendix A.2). Therefore, the actinic flux inside the
actinometer is expected to be the same as in the surrounding atmosphere. The perfect
geometry of the photolysis cell is, however, perturbed by the gas inlet/outlet (usually the
ends, in case of cylindrical tubes) which act as a sink for reflected radiation and shield
some of the incident solar radiation. It is generally assumed that the corresponding loss
of light is small and equal to the ratio of the area of the inlet/outlet to the total surface
area of the photolysis reactor (Zafonte et al., 1977).
The theoretical prediction of the cancelling effects of external and internal reflections
has been confirmed experimentally (Dickerson & Stedman, 1980; Madronich et al., 1983).
In one study, one to three additional cylindrical tubes were slid over the initial photolysis
tube in which the photolysis frequency of NO
2
was measured in a field experiment. Within
5% precision of the experiment, the additional tubes had no effect on the measured
j-value, suggesting that a single tube caused less than 1.7% loss of radiation inside the
actinometer (Dickerson & Stedman, 1980).
9.3.4 Ozone chemical actinometers
The photodissociation of O
3
in the UV and visible region has five energetically possible
pathways for which experimental evidence exists (Matsumi & Kawasaki, 2003):
O
3
+h < 310 nm −→ O
1
D +O
2
a
1
g
(R 9.4)
O
3
+h < 411 nm −→ O
1
D +O
2
X
3
−
g
(R 9.5)
O
3
+h < 463 nm −→ O
3
P +O
2
b
1
+
g
(R 9.8)
O
3
+h < 612 nm −→ O
3
P +O
2
a
1
g
(R 9.9)
O
3
+h < 1180 nm −→ O
3
P +O
2
X
3
−
g
(R 9.10)
