Heard D.E. (editor) Analytical Techniques for Atmospheric Measurement
Подождите немного. Документ загружается.

388 Analytical Techniques for Atmospheric Measurement
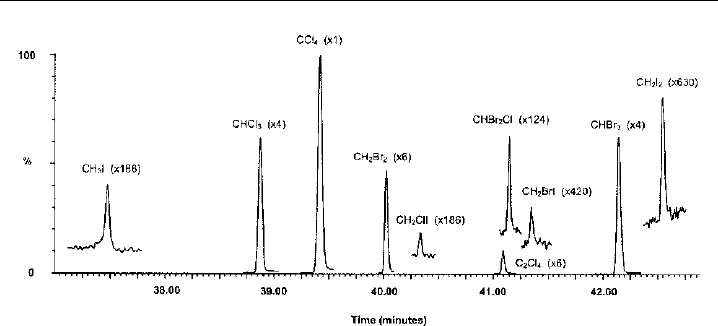
Figure 8.9 Typicalionchromatogramobtained attheMaceHead AtmosphericResearchStationin selective
recording mode. Identities are (1) CH
3
I(m/z 127 and 142), (2) CHCl
3
(m/z 83), (3) C
2
H
5
I(m/z 127 and 156),
(5) 2-C
3
H
7
I(m/z 127 and 170), (6) CH
2
Br
2
(m/z 172 and 174), (7) 1-C
3
H
7
I(m/z 127 and 170), (8) CH
2
ClI
(m/z 176 and 178), (9) CHBr
2
Cl (m/z 127 and 208), (10) CH
2
BrI (m/z 220 and 222), (11) CHBr
3
(m/z 171 and
173), (12) CH
2
I
2
(m/z 254 and 268). Separation was obtained using a SGE (BPX5) 50 m ×032 mm ×3 m
(film) capillary column. (By courtesy of L.J. Carpenter, University of York, UK.)
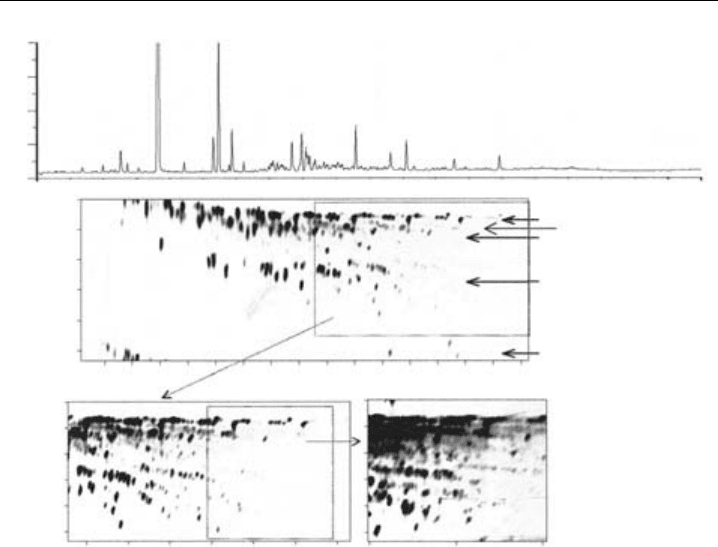
AGC×GC contour plot is shown in Figure 8.10, where each spot represents an individual
analyte. The improvement in resolution between the one-dimensional separation (shown
above) and the two-dimensional separation is apparent. The GC ×GC chromatogram has
been successively expanded to show the greater numbers of isomers at higher molecular
weights.
The composition of organic aerosol is even more complex than the gas phase, with
considerably more organic compounds in the diesel range. The analysis of this type of
sample can prove extremely difficult even with the large separating power of GC ×GC.
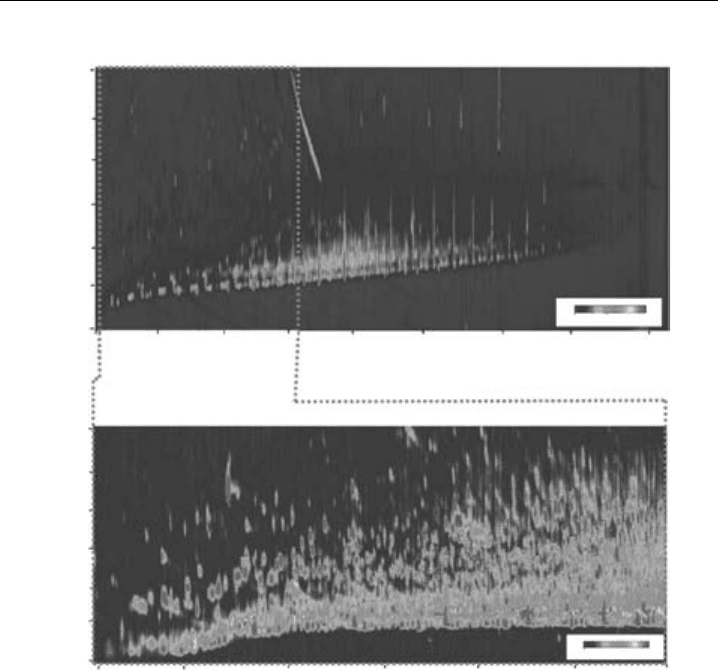
Coupling to a TOF/MS can add an additional separation mechanism, in this case, the
m/z ratio. Figure 8.11 shows a GC ×GC-TOF/MS chromatogram of a PM2.5 particulate
samples collected in Augsburg, Germany (Welthagen et al., 2003). In a typical sample,
more than 15 000 peaks could be detected. Using an MS as a detector allowed struc-
tural information and peak identifications to be obtained, which would have required
numerous standards if an FID was used.
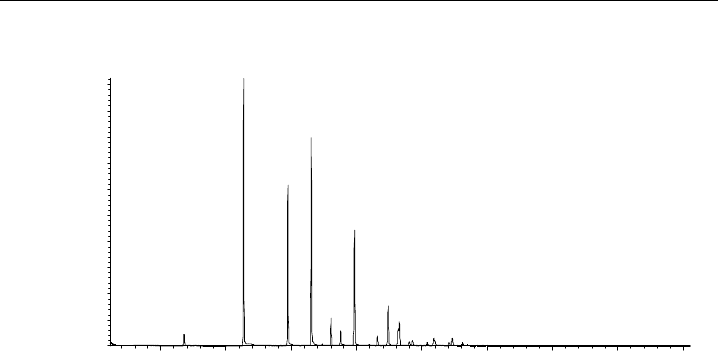
The composition of alkyl nitrate compounds has been studied during the TORCH
campaign (Tropospheric Organic Chemistry Experiment). This site was situated outside of
Chelmsford, UK, and during August 2003 was chosen as a site due to its position relative
to the London plume. Air samples were analysed in negative ion chemical ionisation
mode (NCI) with methane as the ionisation gas and a sample chromatogram is shown
in Figure 8.12.
The use of aircraft in atmospheric measurements is becoming increasingly popular as
more research aircraft become available. An on-board GC system has been developed,
which uses a cooled absorbant sampling trap followed by GC with HID, which is suitable
for use in an aircraft (Whalley et al., 2004). The time resolution of this system is
much quicker than conventional GC systems giving a chromatogram every 5 minutes.
Figure 8.13 shows a chromatogram collected on 26 March, 2004, on the UK research
aircraft, a BAE 146-301 (http://www.faam.ac.uk) during the initial test-flying period. The
Chromatographic Methods 389
A
A
B
B
C
C
D
D
E
F
G
H
1
2
3
4
5
3
3
4
5
5
6
7
8
8
9
3
4
5
7
8
9
9
11
50 55 61 66
66
72 77 82
75 84
400
300
200
100
0
0 10 20 30 40 50 60 70 80 90 100
51015202530354045505560 7065 75 80
Retention time (min)
1st retention time (min)
2nd column RT / secs
2nd column RT / secs
Figure 8.10 Comparison of single column (upper) and GC×GC separations (lower) of a Leeds urban air
sample. Areas of the full chromatogram are successively extracted at higher gain to illustrate increasing
isomeric complexity at higher boiling points. GC ×GC chromatograms are annotated with start of
individual C
x
isomer band (running right to left) where A = C
2
B = C
3
C = C
4
D = C
5
E = C
6
F = C
7
G = C
8
,H= naphthalene. Chemical banding assignments, 1; aliphatics, 2; olefinics, 3; oxygenated,
4; mono aromatics, 5; polyaromatics. (From Hamilton & Lewis, 2003, reproduced with permission from
Elsevier.) (Reproduced in colour as Plate 4 after page 264.)
aircraft was flying at an altitude of 214.8 m over the Bristol channel (Lat 5140
N, Lon
378
W).
Fieldwork is an integral part of atmospheric analysis and the ability to move equipment
from the laboratory to the field site is important. Mobile laboratories housed in vans and
shipping containers allow GC instruments to be moved without the need for permanent
laboratories facilities at the field site. The University of York, UK, has a GC laboratory
fitted within a shipping container, which can be delivered to the site and set-up within a
few days. This container was shipped to Mace Head as part of the NAMBLEX experiment
described in Section 1.8.2 and Figure 8.14 shows the container on-site and the GC system
housed in the interior. Six weeks of almost continuous measurements were made of
NMHCs and selected o-VOCs using the dual channel GC-FID system used in Figure 8.6.
A region of the concentration versus time series, from 30 July to 8 August, 2002, for
ethane, acetylene and benzene is shown in Figure 8.15. The directions marked on the
graph indicate the origin of the air mass, as calculated by the five-day back trajectory
(model used from the European Centre for Medium-Range Weather Forecasts, ECMWF).
In the figure, W indicates an air mass that has travelled over the Atlantic Ocean for most
390 Analytical Techniques for Atmospheric Measurement
GC × GC plot of Aerosol sample
(A)
(B)
4.0
3.5
3.0
2.5
2.0
1.5
1.0
4.0
3.5
3.0
2.5
2.0
1.5
1.0
Polarity 2nd dimension (seconds)Polarity 2nd dimension (seconds)
1000 1500 2000
2500 3000 3500 4000
600
1250 2500 3750 5000
6250 7500 8750 10
000
Volatility 1st dimension (seconds)
Volatility 1st dimension (seconds)
0
20 000
40 000
0
20 000 40 000
Figure 8.11 Two-dimensional GC ×GC-TOFMS total ion current (tic)-plot of an aerosol sample in
2D-contour plot: (A) showing the full chromatogram of the analysed aerosol with (B) the extraction of
the selected section for data analysis. (From Welthagen et al., 2003, reproduced with permission from
Elsevier.) (Reproduced in colour as Plate 5 after page 264.)
of the last five days; NW indicates an air mass which originated in the northern polar
region; and NE five indicates an air mass which has spent the previous five days travelling
over northern Europe. From the time series, it is clear to see that the most polluted
period is during the NE trajectories. The air mass during this period is travelling over
industrial and urban areas of Europe, where the emission of pollution is high. The W and
NW air masses are considerably cleaner, having not travelled over any major pollution
sources in the previous five days.
In comparison to the short period of measurements in the previous example,
the AGAGE network (previously ALE/GAGE) has been operating since 1978
(http://agage.eas.gatech.edu/). One of the primary objectives is to optimally determine
from observations the rate of emission and/or chemical destruction (i.e. lifetime) of
Chromatographic Methods 391
10.00 15.00 20.00 25.00 30.00 35.00 40.00 45.00
0
2 000
4 000
6 000
8 000
10 000
12 000
14 000
16 000
18 000
20 000
Time-->
Abundance
Ion 46.00 (45.70 to 46.70): 000236.D
Methyl nitrate
Ethyl nitrate
Propyl nitrates
Butyl nitrates
Pentyl nitrates
Hexyl nitrates
Figure 8.12 Sample alkyl nitrate chromatogram obtained during TORCH 1, at Writtle College, near
Chelmsford, UK in August 2003. Agilent Technologies GC (6890)-MS (5973N) running in negative ion
chemical ionisation (NCI) mode with methane as chemical ionisation gas. (By courtesy of D. Worton,
University of East Anglia, UK.)
the anthropogenic chemicals that contribute most of the reactive chlorine and bromine
released into the stratosphere. There are currently five stations around the world
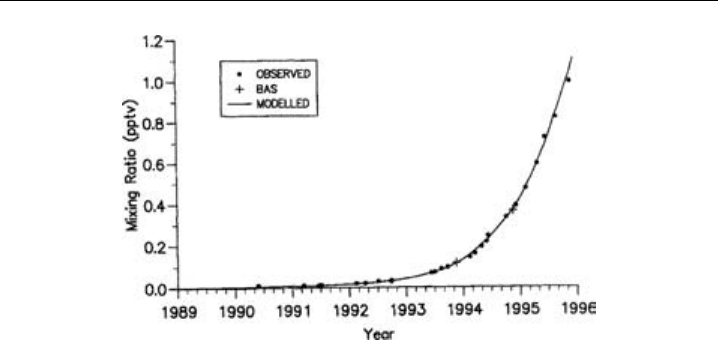
measuring a range of important halocarbons. Data collected for the growth of HFC-
134a, a CFC replacement, at the Cape Grim site, Tasmania, and over the Atlantic
(40
S, 50
W British Antarctic Survey) from 1991 to 1996, is shown in Figure 8.16.
The solid data represents the results obtained by a two-dimensional model with 3%
of global emissions occurring in the southern hemisphere. Long time-scale continuous
measurement networks are of vital importance to determine the concentrations of long-
lived species and in understanding their impact on the atmosphere.
8.3 Liquid chromatography
The analyses of the majority of organic compounds in the atmosphere are primarily
carried out using GC. However, the complete speciation of organics in the atmosphere is
not possible by GC alone. Liquid chromatography (LC) is a complementary technique and
is primarily used for the separation of non-volatile substances and highly polar or ionic
compounds. High performance liquid chromatography (HPLC) has its origins in classical
column chromatography, such as that used extensively in organic synthesis laboratories.
However, in theory and practice, it is more similar to GC. Particles as small as 1 min
diameter can be used as the stationary phase, packed into columns of about 10–25 cm
in length. The mobile phase is forced through the packed column by combinations of
high pressure pumps (up to 200 bar) to achieve volumetric flow rates of 1–5 cm
3
min
−1
.
Since the mobile phase affects selectivity in HPLC (rather than being inert as in GC),
combinations of solvents are dynamically combined to provide binary, ternary or even
quaternary mixtures of mobile phase.
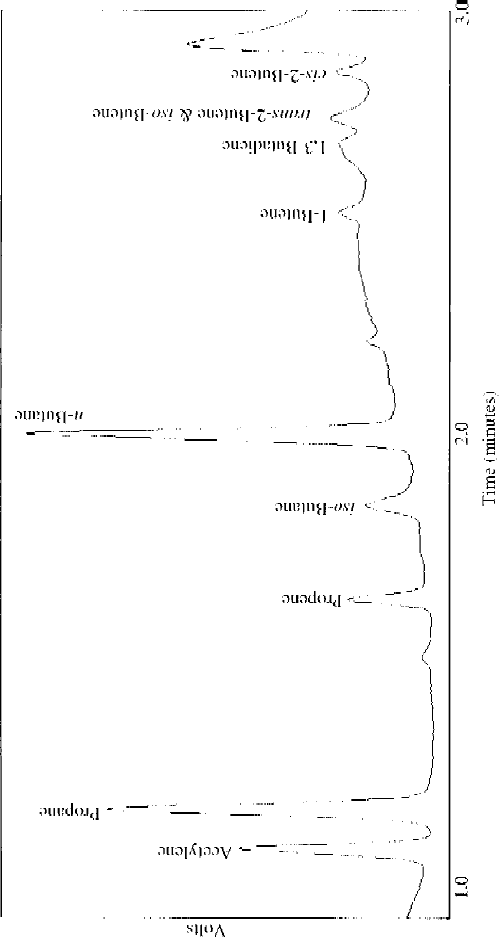
Figure 8.13 ORAC chromatogram collected on 26 March 2004 on the UK research aircraft, a BAE 146–301 during the initial test flying period, altitude of 214.8 m
over the Bristol channel (Lat 5140
, Lon −378
). Analyte assignments are given on the chromatogram. (By courtesy of J.B. McQuaid, University of Leeds, UK.)
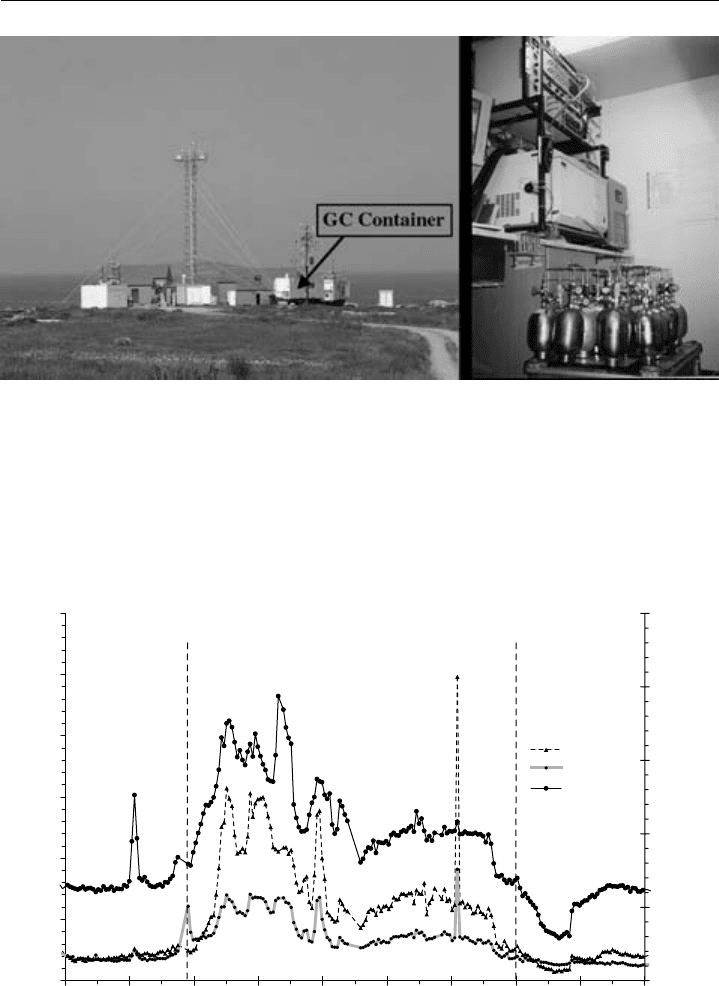
Chromatographic Methods 393
Figure 8.14 Left – Mace Head Atmospheric Research Station during NAMBLEX, July–September 2002.
The GC container is marked. Right – inside of the University of York GC container, showing the GC,
sampling and PTV system and a series of canisters.)
0
100
200
300
400
500
600
30/Jul 31/Jul 01/Aug 02/Aug 03/Aug 04/Aug 05/Aug 06/Aug 07/Aug 08/Aug
Date
Concentration (pptV)
0
500
1000
1500
2000
2500
Ethane (pptV)
Acetylene
Benzene
Ethane
NW NE W
Figure 8.15 Concentrations of acetylene, benzene and ethane observed in air samples at Mace Head
during NAMBLEX. NW, NE and W indicate the origin of the air mass arriving at the site as calculated
by the 5-day back trajectory. Dotted lines indicate a change in trajectory direction. (By courtesy of
J.R. Hopkins, University of York, UK.)
394 Analytical Techniques for Atmospheric Measurement
Figure 8.16 HFC-134a concentrations (pptv) observed in air samples collected at Cape Grim
(41
S 145
E) and over the Atlantic Ocean (40
S 50
W), compared to results from the 2-D model with 3%
of global emissions in the Southern Hemisphere. (From Oram et al., 1996, © 1996 American Geophysical
Union. Reproduced by permission of American Geophysical Union.)
8.3.1 Sample acquisition and preparation
In atmospheric chemistry the analytes amenable to HPLC are found in the gas and aerosol
phase or in atmospheric water, such as fog water. Samples must be introduced to the LC
inlet in a liquid solution and a number of trapping methodologies are in use. The primary
method of sample acquisition and preparation in atmospheric HPLC analysis uses
derivatisation techniques and is one of the most common methods of carbonyl analysis.
In situ derivatisation is commonly used where analytes are derivatised during the
collection process. Derivatisation can be carried out in the liquid phase, where air is
drawn through an impinger containing the derivatising agent in organic solution (such as
iso-octane). After collection, the organic fraction is evaporated to dryness and the residue
is dissolved in a polar solvent such as methanol. Alternatively, analytes can be derivatised
on solid phase absorbents such as SPME, via diffusion or a pumped flow. Derivatised
analytes are released from the absorbent using solvent extraction, which can be achieved
on-line (Sanchez et al., 2003). Post-acquisition derivatisation is less common in HPLC,
although it is used in aerosol analysis and for the detection of atmospheric peroxides (see
Chapter 7) (Kok et al., 1995).
Solvent extraction of filters is used extensively in particulate analysis. Much of the
aerosol compositional work since 2000 has involved the study of the highly polar organic
fraction, which is thought to be the result of numerous heterogeneous reactions in the
particulate phase. Solvent extraction using water yields the water-soluble organic content
(WSOC), a highly polar mixture that is most suited to LC analysis.
8.3.2 Separation
Early HPLC used columns packed with silica giving a highly polar stationary phase,
requiring the use of non-polar mobile phase solvents. This type of separation is known

Chromatographic Methods 395
as normal phase high performance liquid chromatography (NP-HPLC) and is limited to
the separation of non-polar compounds. In reverse phase (RP-HPLC), the addition of
functionalities to the stationary phase has increased the range of polarities that can be
analysed by HPLC. The addition of a hydrocarbon chain to the silica, most commonly
octadecylsiloxane (C
18
, ODS), changes the surface characteristics of the packing material
to a non-polar stationary phase. Polar mobile phases such as water and acetonitrile can be
used to separate hydrocarbon oxidation products, such as carboxylic acids and peroxides,
not amenable to GC.
Ion exchange chromatography (IEC), also a form of HPLC, is used for the analysis of
extremely polar organic compounds and ionic species, such as organic acids and HONO.
Stationary phases in IEC are generally resins, such as PS-DVB, a copolymer of styrene
and divinyl-benzene. The polymer is a three-dimensional cross-linked structure that is
rigid, porous and highly insoluble. Cation- or anion-exchange properties are introduced
to the resin by chemical modification after polymerisation. Metal ions, for example Ca
2+
,
K
+
,Mg
2+
,Na
+
, have been measured in aerosol particles using IEC (Lee et al., 2003).
Liquid chromatography can be used as a preparative step in atmospheric analysis to
simplify complex mixtures. WSOC in aerosol can be fractionated into three classes of
compounds using IEC: (1) neutral/basic compounds; (2) mono- and di-carboxylic acids;
and (3) polyacidic compounds. The functional group composition of fractions can then
be investigated using proton nuclear magnetic resonance spectroscopy (HNMR).
Multi-dimensional chromatography combinations of HPLC with GC are possible and
have been reported where an on-line sample preparation step is desirable. Since the
mobile phase from the LC stage must be interfaced to the GC, non-polar solvents that
may be easily evaporated are used (e.g. pentane). This limits the technique to normal
phase HPLC and water content in the sample becomes a serious problem in achieving
full automation.
8.3.3 Detection
The most common detector in routine HPLC analysis is the UV absorbance photometer
due to its wide linear range and good sensitivity. Photometers are operated at one or more
fixed wavelengths only and require the presence of a suitable chromophore within the
molecule. In many atmospheric samples, the molecules of interest have weak absorptions
in the UV region. The use of derivatisation to form analytes with strong UV absorbances
is widely used in HPLC, and DNPH-carbonyl adducts have maximum UV absorbances
at approximately 340–380 nm (Druzik et al., 1990). Diode array detectors can also be
coupled to HPLC, offering an extended detection range (190–950 nm), with high spectral
resolution and peak identification via UV spectral libraries. Ultraviolet detectors are
robust and non-destructive, allowing detected compounds to be collected or further
analysed.
Fluorescence detectors are highly selective and among the most sensitive of detectors.
Chromatographic analysis of hydrogen and organic peroxides are carried out on a C
18
-
RP-HPLC column followed by post-column reaction with horseradish peroxidase (Kok
et al., 1995). One of the reaction by-products is a dimer of p-hydroxyphenyl ethanoic
acid that has a strong fluorescence at an excitation of 301 nm and an emission wavelength

396 Analytical Techniques for Atmospheric Measurement
of 414 nm, giving typical atmospheric detection limits of H
2
O
2
and the organic peroxides
of 30 pptV.
One of the developments in HPLC is the routine coupling of the column to a mass
spectrometer. This is considerably more difficult than in GC-MS, where the eluent from
the end of the column flows directly into the ion source. The major difficulty in LC-MS
is the removal of the liquid mobile phase, whilst allowing only the analytes to pass into
the detector. Several interfaces have been designed for this purpose, but for air analysis
the most common are atmospheric pressure chemical ionisation (APCI) and electrospray
ionisation (ESI). The former uses a reagent gas such as nitrogen as a nebulising gas.
A heated nebuliser is used to vapourise the mobile phase and form reactant ions via a
corona discharge. The ions and analyte molecules are accelerated through skimmers into
a low-pressure region where the solvent is pumped away. Chemical ionisation (positive
or negative) of the analyte molecules occurs via collisions with the excited reagent ions,
which then pass into the mass spectrometer, typically a quadrupole-MS, for analysis.
LC-APCI/MS is particularly suited to moderately polar and non-polar analytes and has
been used to measure aldehydes in both aerosol particles and the gas phase (Grosjean
et al., 1999; Van den Bergh et al., 2002). It has also been used to measure nitroaromatic
compounds in the atmosphere as an indication of concealed land mines (Sanchez et al.,
2003).
In ESI, the eluent from the column flows through a metal capillary, which is at a
potential of several kilovolts relative to the surrounding chamber walls. The surfaces
of the emerging liquid become charged and the liquid is dispersed in a fine spray via
repulsive forces. In this case the analytes are ionised by the makeup gas before the solvent
is evaporated and swept away, along with any other non-charged material. Electrospray
ionisation is particularly suited to the analysis of charged, polar, and basic compounds,
making it a complimentary technique to APCI-MS. An example is the use of both ESI−
and APCI+ in terpene aerosol studies to identify the organic acids and carbonyl/alcohol
content respectively (Winterhalter et al., 2003).
8.3.4 Examples of the application of liquid chromatography
in air analysis
The importance of oxygenated compounds in the atmosphere has already been discussed.
Carbonyl species can be emitted from biogenic and anthropogenic sources, such as vehicle
emissions, and are also produced via photo-oxidation of hydrocarbons. Using DNPH to
convert carbonyls to their 2,4-dinitrophenyl hydrazones produces an analyte ideally suited
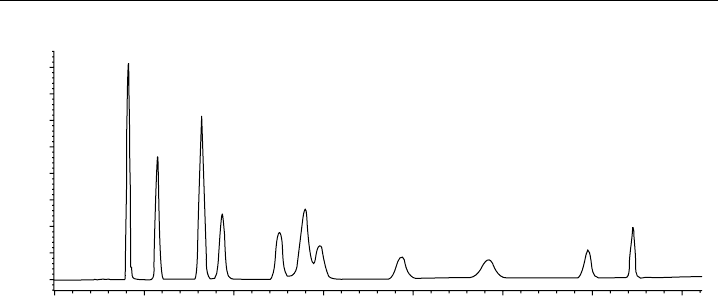
to HPLC. Figure 8.17 shows a DNPH–carbonyl standard mixture that has been analysed
using RP-HPLC column (150 mm ×46 mm C18 ODS, 5 m particle size) with a diode
array UV-visible detector (Grosjean et al., 1999). This method has been extended to the
analysis of carbonyls in both the gas and particulate phase of the atmosphere. The DNPH
derivatives of carbonyls found in a PM sample collected during the Pacific 2001 field
study in Fraser Valley, Canada, were analysed by HPLC-UV and a sample chromatogram
is shown in Figure 8.18 (Liggio & McLaren, 2003). Simplification of the complex aerosol
sample is achieved using a simultaneous extraction/derivatisation technique and an initial
guard column is used to trap the hydrazones before flushing onto the analytical column.
Chromatographic Methods 397
Time (min)
mAU
80
C1
0 5 10 15 20 25 30 35
C2
C3
CR
C4
BZ
C5
C6
TOL
C3K+ACR
MEK+MTH
70
60
50
40
30
20
10
0
Figure 8.17 Liquid chromatography analysis of a mixture of the DNPH derivatives of 13 carbonyls
by ultraviolet absorption at 360 nm (diode array detector). C1, formaldehyde; C2, acetaldehyde; C3K,
acetone; ACR, acrolein; C3, propanal; CR, crotonaldehyde; MEK, 2-butanone; MTH, methacrolein; C4,
butanal; BZ, benzaldehyde; C5, pentanal; TOL, m-tolualdehyde; C6, hexanal. (Reprinted with permission
from Grosjean et al., 1999. © 1999 American Chemical Society.)
An important aspect of atmospheric chemistry is the use of smog chambers to simulate
the reactions of compounds of interest under controlled pseudo-atmospheric conditions.
The production of reaction intermediates can be simulated and the analytes identified,
without any background interferences, which would make this process extremely difficult
in real air samples. A number of the reaction products of -pinene ozonolysis have been
identified at the EUPHORE smog chamber, Valencia, Spain, using LC-MS (Winterhalter
et al., 2003). An example of the ESI− ionisation mode chromatogram is shown in
Figure 8.19, with the Total Ion Count (TIC) at the top, followed by a series of single ion
chromatograms. In the SIM modes, identification and increased sensitivity and resolution
are possible. The identification of reaction intermediates can give important information
about degradation mechanisms and is vital for accurate modelling studies.
The complexity of compounds in aerosol can be simplified using LC as a fractionation
tool. Lewis et al. (1995) measured PAH in dust particles using SFE to remove the organic
compounds from particulate samples followed by online LC-GC analysis. The initial LC
analysis separates the organic content with respect to polarity and removes the aliphatic
and polar compounds. Portions of the aromatic fraction were then transferred to the GC
for analysis. Figure 8.20 shows both the LC and subsequent GC chromatograms obtained
using this technique, with the PAHs indicated by numbers.
8.4 Future work
The use of chromatographic methods in the future for atmospheric measurements
seems certain. No other technique can deal so effectively with such a wide range of
chemical functionalities and trace species contained within complex mixtures. The science
benefits greatly from having increasingly sophisticated and reliable, commercially available
