Heard D.E. (editor) Analytical Techniques for Atmospheric Measurement
Подождите немного. Документ загружается.


328 Analytical Techniques for Atmospheric Measurement
and resulted in conversion efficiencies of about 0.50, with an instrumental uncertainty
range of 10–30%. These NO
2
measurements were used in combination with NO, O
3
,
ClO, and HO
2
measurements to make a comparison with calculated NO
2
values in the
sunlit lower stratosphere, with the finding of a 19% difference, which was well within the
combined uncertainties of 50–70%.
Around 2000 Ryerson et al. (2000) built an NO
2
converter using a high-pressure Hg arc
lamp. This design was driven by the need for high time resolution aircraft measurements,
which in turn requires a high NO
2
-to-NO conversion fraction during a relatively short
time in the photolysis cell. The Hg arc lamp makes this possible while at the same time
reducing the heat load, also a significant consideration. An ellipsoidal reflector is used
to direct the light toward the photolysis cell through a set of optical filters designed to
minimize sample heating and attendant interferences from thermally labile species, as well
as to restrict the light to longer wavelengths. This results in the loss of 35% of the useful
lamp output, but this is compensated for by increased specificity due to reduced photolysis
of potential interferent species (HNO
3
,HO
2
NO
2
,N
2
O
5
; see Figure 7.7). Interferences
from PAN and HNO
3
were measured in the laboratory and found to be negligible. The
long pass filter (350 nm) was found to significantly reduce the UV artifact described
earlier. This combined with the apparently high water solubility of the contaminant
species serves to confirm earlier suggestions that HNO
3
contamination of the cell walls
plays a role in the artifact signal. The cell pressure is maintained at 250 torr (typically
flown on a mid-altitude P3 aircraft) for a cell residence time of 0.8 s and 1/e response
time of 0.65 s. The conversion factor of NO
2
to NO is 0.7. Achieving such high conversion
at relatively short residence times has enabled high resolution aircraft measurements to
be made in atmospheric features with small spatial scales, such as plumes from power
plants and petrochemical facilities (Ryerson et al., 2003).
7.4.4 The measurement of total reactive nitrogen (NO
y
)
The lack of converter specificity for NO
2
or any other particular species is turned to
an advantage in the measurement of total reactive nitrogen, or NO
y
, which includes
NO, NO
2
,NO
3
,N
2
O
5
, HONO, HNO
3
,HO
2
NO
2
, ClONO
2
, PANs, and other organic
nitrates. For an NO
y
measurement it is desired that all reactive nitrogen species be
converted to NO, but not other nitrogen-containing species (e.g. NH
3
,N
2
O, HCN). As a
practical matter, it is required that near-unity conversion be achieved for all NO
y
species
contributing significantly to the total NO
y
amount, and that there be only minimal
conversion of potential interferents, as determined by the product of the conversion
efficiency and abundance for a particular species.
Several early studies used a variety of converters that were originally intended for use
as NO
2
-to-NO converters, but which suffered interferences from the conversion of other
nitrogen-containing species as well. So, these converters were pursued as means to measure
HNO
3
and other nitrogen species. Joseph & Spicer (1978) used a dual channel instrument,
each channel having a molybdenum converter in line, and placed a nylon filter in line for one
of them to scrub HNO
3
, so that the difference signal would yield the HNO
3
mixing ratio.
Kelly and Stedman (1979) used the same principle to measure HNO
3
, but instead with a
converter consisting of a quartz tube at 350
C packed with Pyrex beads. Helas & Warneck

Chemical Methods 329
(1981) used the difference in signals from molybdenum and ferrous sulfate converters to
infer a measure of what they termed ‘excess NO
x
’, or non-NO
x
NO
y
compounds, which they
recognized might include N
2
O
5
,HO
2
NO
2
,CH
3
O
2
NO
2
, and PAN, although the conversion
efficiencies for these species were not demonstrated. The molybdenum converter found
application in commercial instruments, as flown by Dickerson (1984) to measure total
reactive nitrogen in the free troposphere, where convection was demonstrated to have a
significant impact on reactive nitrogen mixing ratios. One difficulty observed with the
molybdenum converter is a ‘memory effect’ in which, after exposure to elevated levels of
reactive nitrogen, the background signal remains high, introducing uncertainty into the
measurement. Controlled, stepped heating has been used to thermally dissociate PANs,
organic nitrates, and HNO
3
to NO
2
for detection by LIF (Chapter 4).
Bollinger et al. (1983) and Fahey et al. (1985) introduced the use of gold catalysts
for the measurement of NO
y
via the reduction of NO
y
species to NO by CO. Bollinger
et al. (1983) employed a gold-coated quartz tube and tested the conversion efficiency
of NO
2
, HNO
3
, and n-propyl nitrate (NPN), selected as being representative of organic
and inorganic reactive nitrogen compounds. Conversion was measured as a function of
converter temperature and CO mixing ratio. Under appropriate conditions, complete
conversion was found for all three species. Fahey et al. (1985) extended this work by
testing the conversion of a thin-walled solid gold tube, which was found to be more
reliable than the coated quartz tube used earlier. Nickel and stainless steel converters were
also tested, but were not as good. NO
y
species chosen for tests of conversion efficiency
and linearity were NO
2
, HNO
3
,N
2
O
5
, and PAN. For a converter at 300
C, the conversion
efficiencies of all species exceeded 90%, and the conversion was linear for mixing ratios
in the range 0.1–50 ppbv. Temperatures higher than 300
C were not used in order to
minimize potential interferences, such as from N
2
O.
In addition, the non-NO
y
compounds NH
3
, HCN, N
2
O, CH
4
, and various chlorine
and sulfur compounds were tested as interferents. NH
3
and HCN were found to be the
principal interferents, at least in dry, synthetic air. Conversions of 2–8% and 2–20% were
found for NH
3
and HCN, respectively. The addition of 0.5–2.5% H
2
O was found to
reduce these conversion efficiencies to about 2%. Using laboratory air at 20% relative
humidity resulted in conversion efficiencies of less than 1%. In contrast with this,
later work by Kliner et al. (1997) found near-complete conversion of HCN. On the
other hand, Weinheimer et al. (1998) measured HCN conversion efficiencies for three
different converters sampling ambient air from a DC-8 aircraft, with results for two of
the converters being consistent with the results of Fahey et al. (1985), and one, not
consistent. Conversion in humidified ambient air for two converters was small, at about
5%. However, the third converter showed 30% conversion. Taken all together, the results
from the various studies show that outwardly identical converters can experience different
interferences from HCN, perhaps due to their own particular histories (air sampled,
cleaning history, temperature history). However, these effects are minimized by ambient
humidity, and irrespective of humidity, conversion is smaller in ambient air than in
synthetic air. This result points to the value of testing each converter for its own specific
interference from HCN in order to verify that ambient levels of NH
3
and HCN are
generally small enough that interferences will usually be negligible in field measurements.
This also points to the general value in testing converters for the conversion efficiency of
several component species, as well as interferents. Fahey et al. (1986) used the then new

330 Analytical Techniques for Atmospheric Measurement
gold-converter technique in conjunction with measurements of many of the component
NO
y
species at a ground site in the Colorado mountains to show that PAN was often the
dominant NO
y
species. They also found that correlations of NO
y
with O
3
gave evidence
for the production of O
3
in polluted air masses and showed that some NO
y
species are
subject to surface loss.
The molybdenum and gold converters have been compared in side-by-side tests, with
mixed results. Fehsenfeld et al. (1987) found that the two gave similar estimates of NO
y
in
ambient air over a wide range of mixing ratios, ranging from 0.4 ppbv in clean continental
air to about 100 ppbv in very polluted conditions. In this test, the molybdenum converter
did not suffer from memory effects noted above. However, it did suffer significant
degradation in conversion efficiency after extended operation in polluted conditions. In a
second ground-based test, Williams et al. (1998) report generally more favorable results.
Seven converters were compared, all followed by chemiluminescence detection of NO,
but some with gold catalysts using either CO or H
2
as the reducing agent and some
using molybdenum converters. No significant difference between gold and molybdenum
converters was found for polluted conditions experienced, where NO
y
was in the range
2–100 ppbv. This limits these favorable conclusions to these high mixing ratio conditions.
The authors note that differences could become significant at lower mixing ratios, and
especially for conditions when NO
x
is a smaller fraction of NO
y
.
Another important condition for measurement reliability is the transmission of HNO
3
through inlets and sample lines, as HNO
3
can be a dominant component of NO
y
, and
it is also very sticky. That is, it is prone to adsorb onto surfaces, either reversibly or
irreversibly. Neuman et al. (1999) used a sensitive, fast-response HNO
3
CIMS instrument
to test various surfaces for their ability to pass HNO
3
. They found that teflon above
10
C is very good for this purpose, but that stainless steel, glass, fused silica, aluminum,
nylon, silica-steel, and silane-coated glass all take up a large fraction of the HNO
3
. HNO
3
is reversibly taken up by teflon at low temperatures, but if it is kept moderately warm
>10
C it is a suitable material for inlets and sample lines. Inlets for NO
y
are typically
composed of teflon and are often heated to somewhat higher temperatures of 30–70
C
to insure good transmission (e.g. Ridley et al., 1994).
NO
y
measurements from the NASA ER-2 aircraft have proven to be a valuable
component of extensive field missions aimed at understanding polar ozone loss processes.
Fahey et al. (1990) found that correlation of NO
y
and N
2
O, which derives from N
2
O
being the source of stratospheric NO
y
, could be used to infer the degree of denitrification
in the winter polar stratosphere, a process critical to ozone chemistry, as the removal of
reactive nitrogen from an air mass allows chlorine species to catalytically destroy ozone.
This nitrogen loss process was later observed by Fahey et al. (2001) in the form of
large HNO
3
-containing particles that were precipitating in the winter arctic stratosphere,
causing denitrification and hence making ozone loss more likely (Figure 7.9).
A new and interesting use of an NO
y
instrument is unattended measurements aboard
civil in-service commercial aircraft. The MOZAIC (Measurements of Ozone and Water
Vapour aboard Airbus in-service Aircraft) project includes a small NO
y
instrument
installed on long-haul aircraft to provide regular surveys of the poorly sampled tropopause
region. These are difficult constraints under which to operate a research-grade instrument,
and especially with the long inlet line required, but with the large number of flights taken,
and with proper attention to quality control, a large quantity of valuable data are being
generated (Volz-Thomas et al., 2004).
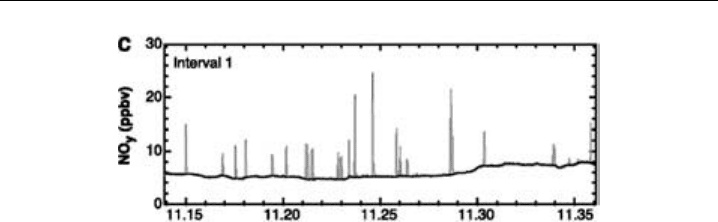
Chemical Methods 331
Figure 7.9 Simultaneous measurements from two Au-converter/chemiluminescence NO
y
channels
aboard the NASA ER-2 in the Arctic stratosphere. The darker trace is for air sampled through an aft-facing
inlet and so is primarily gas-phase NO
y
(mostly HNO
3
in this case) as particles are inertially separated.
The lighter trace is for air and particles sampled through a forward-facing inlet, and the two traces closely
overlap, except at the “spikes” which are due to the presence of HNO
3
-containing particles, likely nitric
acid trihydrate, which evaporate and release a burst of HNO
3
. By means of sedimentation, these particles
irreversibly remove HNO
3
from the air mass and this denitrification allows chlorine to remain active for a
longer time in destroying ozone. (Figure 2c of Fahey et al., 2001, reprinted with permission from AAAS.)
7.4.5 Routine NO
x
monitoring
As NO
x
is a major urban pollutant, central to photochemical ozone production, its routine
monitoring has become an important aspect of pollution control in urban environments.
In the UK, a network of over 200 automated sites employs chemiluminescence NO
x
instruments, most with molybdenum converters, to allow an assessment of ambient NO
x
levels for the purpose of determining whether NO
2
concentration objectives, imposed by
government agencies, have been met (AQEG, 2004). These objectives are not to exceed
200 gm
−3
over a one-hour average, nor to exceed 40 gm
−3
as an annual average (divide
these values by 2 to get the rough equivalent in ppbv for standard temperature and
pressure). These data also allow the observation of patterns, cycles, trends, and correlations
with measurements of other species that can be tested against our understanding of the
basic processes of pollution chemistry as embodied in numerical models. In particular, it
enables the connection of emission rates to ambient concentrations, so that predictions
of future concentrations may be inferred from projections of future emissions.
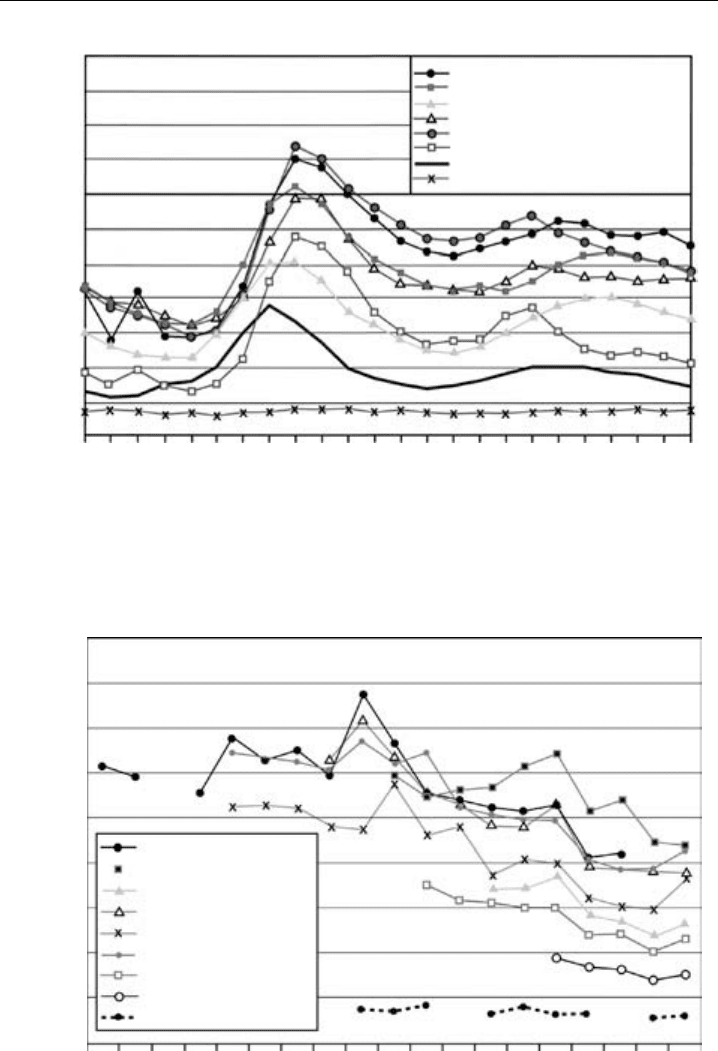
Figure 7.10a shows the diurnal variation in NO
x
at various sites in the monitoring
network (AQEG, 2004). That road traffic is the largest source is reflected in the diurnal
cycle with a maximum during the morning rush hour and secondary maximum in the
evening, except for the most remote site, which shows little in the way of a diurnal cycle.
The monitors are also valuable for the determination of longer-term, multi-year trends.
Emissions in the UK have declined by 37% from 1990 to 2000, and this is reflected in
the decadal trend in NO
x
mixing ratios shown in Figure 7.10b (AQEG, 2004). Emissions
are expected to decrease another 25% by 2010 due to tighter vehicle emission standards,
so it is important to continue to monitor ambient levels to see whether their decline is
commensurate with this. The monitoring network may also be used to construct the large-
scale spatial distribution of NO
x
and correlate the measured concentrations to emission
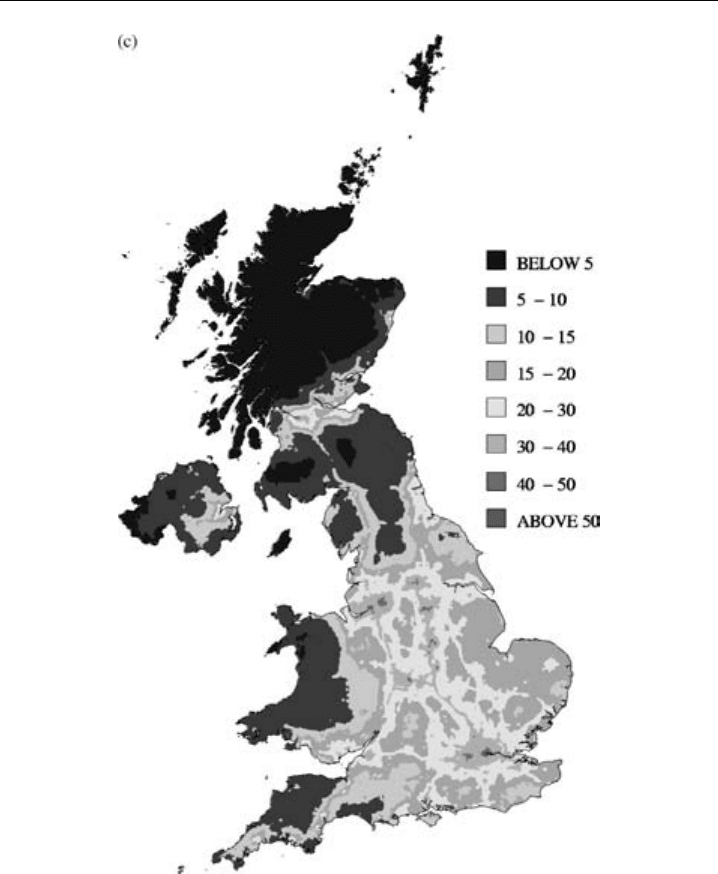
estimates. Figure 7.10c shows the distribution within the UK of annual-average NO
x
concentrations derived from the network dataset interpreted through use of an empirical
model (Stedman et al., 2002). As the density of sites throughout the monitoring network
332 Analytical Techniques for Atmospheric Measurement
(a)
200
180
160
140
120
100
NO
x
(μg m
–3
, as NO
2
)
London Bloomsbury (μg m
–3
, as NO
2
)
London Bexley (μg m
–3
, as NO
2
)
West London (μg m
–3
, as NO
2
)
Manchester Town Hall (μg m
–3
, as NO
2
)
Glasgow City Chambers (μg m
–3
, as NO
2
)
Belfast Centre (μg m
–3
, as NO
2
)
Port Talbot (μg m
–3
, as NO
2
)
Lullington Heath (μg m
–3
, as NO
2
)
80
60
40
20
0
01234567891011
Hour of Day
12 13 14 15 16 17 18 19 20 21 22 23
Figure 7.10a The diurnal cycle of NO
x
at eight sites in the UK, showing the morning rush hour maximum
due to vehicle traffic and a secondary maximum in the evening. (Figure 6.14 of AQEG, “Nitrogen Dioxide
in the United Kingdom”, 2004, printed with permission from Crown.) (Reproduced in colour as Plate 3a
after page 264.)
225
(b)
200
175
150
125
100
Bridge Place
Annual mean NO
x
(μg m
–3
, as NO
2
)
London Bloomsbury
London Bexley
West London
Manchester Town Hall
Glasgow City Chambers
Belfast Centre
Port Talbot
Lullington Heath
75
50
25
0
1982
1983
1986
1987
1988
1989
1990
1991
1992
1993
1994
1995
1996
1997
1998
1999
2000
2001
Figure 7.10b The decadal trend in NO
x
at nine sites in the UK, showing the gradual decline due to
the tightening of vehicle emission standards. (Figure 6.5 of AQEG, “Nitrogen Dioxide in the United
Kingdom”, 2004, printed with permission from Crown.) (Reproduced in colour as Plate 3b after page 264.)
Chemical Methods 333
Figure 7.10c The geographical distribution of NO
2
in the UK, derived from monitoring-network data
interpreted with an empirical model. (Figure 3.1 of Stedman et al., 2002, printed with permission from
AEA Technology.) (Reproduced in colour as Plate 3c after page 264.)
is not always high, and in order to elucidate the dependence of measured concentrations
on NO
x
sources, it is useful to interpolate the network measurements and to model
empirically the contributions of local and more distant sources to the NO
x
measured at
a particular site. In turn, the empirical model is validated by comparison of its results
with measurements at specific sites. Maps such as that shown in Figure 7.10c can be
used to formulate statistics on the regions in which concentration objectives for NO
x
are

334 Analytical Techniques for Atmospheric Measurement
exceeded, as well as the fraction of the population thus affected. Furthermore, it serves
to illustrate the impact of urban areas on more rural regions.
7.5 Ozone via homogeneous chemiluminescence
7.5.1 O
3
+ Ethene
The first gas-phase chemiluminescence instrument was for the measurement of O
3
and
was based on the chemiluminescent reaction of O
3
with ethene. Nederbragt et al. (1965)
mixed flows of ozone-containing air and ethene directly in front of a PMT. The detection
limit was 30 ppbv, so it was not useful for routine atmospheric monitoring but was
useful for monitoring the elevated levels (1000s of ppbv) that are produced by beams
of electrons near accelerators. Warren and Babcock (1970) made further improvements,
especially with regard to portability, but, as the application was still for accelerators, they
were satisfied with a detection limit too high for atmospheric applications.
Commercial versions based on this principle were produced and used for atmospheric
monitoring, and some were modified for use in research applications, including aircraft
deployment (Routhier et al., 1980; Gregory et al., 1983; Kondo et al., 1987). The emission
spectrum for the O
3
–ethene reaction peaks near 430 nm (Finlayson et al., 1972), and the
products include excited OH and HCHO. The instruments consist of inlet flow system,
an ethene flow system, and a reaction chamber viewed by a PMT. The modifications to
the commercial instruments for aircraft use generally include flow control and pressure
regulation, so that instrument sensitivity does not change with altitude or cabin pressure.
Gregory et al. (1983, 1984) report a detection limit of 2 ppbv, with a 90% response in
about 2 s, and an accuracy of 5% or 5 ppbv, whichever is larger.
7.5.2 O
3
+NO
Just as O
3
can be added in excess to the sample air stream for the measurement of
NO, so can NO be added in excess for the measurement of O
3
. Stedman et al. (1972)
built a prototype instrument based on this principle. A typical reaction vessel was a
cylindrical tube, 4
long and 2
in diameter, viewed from one end by a PMT through
a red filter (as for NO instruments). Conical reaction vessels were also tested. A typical
sample flow was 20 cc min
−1
, with a reagent flow of 3 cc min
−1
of pure NO. The response
time was less than 1 s, excluding inlet sampling. Calibration was achieved in multiple
ways using a photolytic O
3
source. One is via NO titration, which adds a measured flow
of a known concentration of NO to the sample stream before it enters the instrument
to allow reaction prior to that in the reaction vessel. As NO is increased the O
3
signal
decreases. An extrapolation to zero O
3
gives the known amount of NO corresponding
to the O
3
level being used. Another method used an infrared absorption measurement
of O
3
from the photolytic source. A linear response was found for the wide range of
50 ppbv–50 ppmv. Several species were tested for interference at the 100 ppmv level: SO
2
,
NO
2
,Cl
2
,H
2
O, C
2
H
2
,C
2
H
4
, and C
3
H
6
. None gave detectable chemiluminescence. The
instrument was tested in the field where it showed excellent response to atmospheric

Chemical Methods 335
variability. The chemiluminescence technique using NO is much more sensitive than that
using ethene due to the high rate of reaction of O
3
with NO.
The high sensitivity and fast response of the chemiluminescence detector using NO
has led to its use in making eddy-correlation flux measurements of O
3
to the ground, as
surface deposition is a major sink for O
3
. Pearson (1990) describes an instrument designed
to make such measurements from an aircraft, where frequency response requirements are
much more demanding than for an instrument fixed to the ground. A filtered frequency
response of 12 Hz was achieved. An earlier version of the instrument (Pearson & Stedman,
1980) was improved upon by doubling the sample flow to 37 L min
−1
and by increasing
the diameter of the photocathode of the red-sensitive PMT to 51 mm. The reagent flow
is 28cm
3
s
−1
of 5–10% NO. The cylindrical reaction vessel has an ID of 4.7 cm and a
length of 8 cm and is operated at 8–10 mb. The inlet flow is regulated by a stainless steel
frit in an aft-facing inlet to prevent cloud and rain water from entering. The reaction
vessel is maintained at 39
C in order to keep the sensitivity constant in what may be
expected to be a warm aircraft flying in the boundary layer. The PMT is operated in the
analog mode and is low-pass filtered at 10–12 Hz. An operational consideration when
flying the poisonous NO reagent gas is the safety requirement for a containment vessel
to house the compressed gas bottle with a line plumbed to the outside of the aircraft, so
that in the event of a leak, the NO is vented outside the aircraft.
Lenschow et al. (1981) used an earlier version of this instrument to analyze the relative
contributions of chemistry and transport to the ozone budget in the boundary layer over
range and crop land and to show that the time rate of change of the O
3
concentration
was several times larger than contributions from horizontal advection and the divergence
of the vertical eddy flux, thus demonstrating the dominance of local chemistry over
transport in determining the local time rate of change of the O
3
concentration.
Ridley et al. (1992) describe a high-sensitivity chemiluminescence instrument that is
designed to have small size and weight, suitable for use as part of a multi-instrument
package on a small aircraft and still with good frequency response. Photon counting
is employed to give a sensitivity of 2000 counts s
−1
per ppbv of O
3
and a detection
limit well below 0.1 ppbv for a 2 s integration. The overall measurement uncertainty at
higher mixing ratios is estimated to be 2 ppbv plus 5%. The reaction vessel is conically
shaped, with a volume of 17 cm
3
(eight times smaller than that of Pearson, 1990), and
is made of highly polished stainless steel coated with gold. It operates at a pressure of
about 10 torr, with a small sample flow of 180 sccm that is combined with a reagent flow
of 1.5 sccm of pure NO. The instrument is calibrated prior to flight using a standard
UV absorption instrument. Ridley et al. (1992) offer several interesting points about the
chemiluminescence measurement of O
3
compared to that for NO. First, tropospheric
O
3
is often several orders of magnitude larger than that of NO, so high signal-to-noise
ratio is expected. Second, the reagent NO is much easier to provide (from a bottle)
than is the reagent O
3
for the NO measurement (silent discharge). Third, since the
reagent NO is pure compared to 3–5% for reagent O
3
, smaller reaction vessel volumes
are possible as the reaction occurs more quickly and less residence time is required. And
fourth, the limiting background signal in an NO instrument due to reagent O
3
is not
present in the O
3
instrument. All these factors combine to make possible instruments
which are very fast and sensitive. The high sensitivity and low noise of this instrument
were put to test in low altitude ∼100 m aircraft measurements in the Arctic, where

336 Analytical Techniques for Atmospheric Measurement
ozone depletion events occurred in which O
3
mixing ratios were measured to be as
low as 0.03 ppbv due to processes not yet fully understood but likely involving bromine
chemistry.
7.6 NO
2
via luminol
Luminol has found application in the measurement of NO
2
via chemiluminescence
near 425 nm. As luminol was described earlier to be used for the chemiluminescence
measurement of O
3
, an obvious concern is the interference from O
3
to the NO
2
determi-
nation. Despite this potential limitation, the luminol technique initially offered some hope
of avoiding the significant interferences encountered in the NO
2
techniques described
earlier (Section 7.4.3) that coupled heated catalytic converters with NO chemilumines-
cence detectors. Although photolytic converters for NO
2
detection do not suffer serious
interference problems and are generally used instead of the catalytic converters, the
luminol technique still enjoys application in commercial NO
2
instruments, as well as in
some peroxy radical instruments (Section 7.9.1). It has the advantages of small size, low
power consumption, and high sensitivity.
Maeda et al. (1980) converted a gas-phase reaction vessel so that it could hold a pool
of liquid luminol solution with a constant surface area, and they varied the luminol
concentration to optimize the response to NO
2
. KOH was added to the solution, and its
concentration was optimized at 0.05 M. KOH was determined to facilitate the dissolution
of NO
2
in the luminol solution, and the OH
−
may participate in the chemiluminescent
reaction. Potential interferents were tested. No interference was found from NH
3
, organic
nitrate, organic nitrite, NO, and hydrocarbons. A negative interference was found with
CO
2
, but this was minimized by increasing the pH with additional KOH (1.0 M). O
3
is a very strong interferent with a chemiluminescence signal 30 times that for NO
2
,
but that is remedied by the addition of sodium sulfite to the luminol solution, which
also removes an SO
2
interference. The detection limit for NO
2
for this prototype was
50 pptv.
Wendel et al. (1983) improved on the earlier design. Rather than use a pool of luminol,
they used a wetted strip of cellulose fiber filter paper so that the detector would be less
sensitive to movement and orientation, and so that the PMT would not view the reservoir
of liquid. This also improved the time response by eliminating the signal that comes
from the liquid volume at some time lag after the NO
2
encounters the surface. Other
modifications were a cotton trap to remove O
3
in the inlet and the use of NaOH instead of
KOH. Also, the measurement of NO was achieved by its conversion (60–70%) to NO
2
by
passing the sample over CrO
3
adsorbed on silica gel (in contrast with the more common
conversion of NO
2
to NO followed by NO detection). Also, Na
2
SO
3
(0.01 M) was used to
reduce the sensitivity to O
3
and at the same time enhance the sensitivity to NO
2
. This also
reduces the induction period (the time period after changing the solution during which
the sensitivity to NO
2
increases) to one hour rather than days. It was also found that the
addition of methanol to the solution further increases the sensitivity and specificity for
NO
2
. No interference was found from 20 ppbv each of HNO
3
,NH
3
, and HCN. At best,

Chemical Methods 337
the NO
2
response is 30 times that for O
3
. PAN was found to respond like NO
2
(indeed
a gas chromatograph in conjunction with a luminol detector was later used to measure
PAN and NO
2
; Burkhardt et al., 1988). The detection limit for field measurements was
reported to be 30 pptv S/N =2, with a 2 Hz response to 20 ppb changes. However, Ray
et al. (1986) subsequently report for a later version of this instrument a detection limit
of 1 pptv and a 3 Hz response.
Schiff et al. (1986) describe a commercial instrument based on luminol (Luminox
LMA-3, Unisearch/Scintrex). In contrast to the complex solution described in the Wendel
et al. (1983) paper, comparatively little information is available in the open literature
about this commercial instrument. However, it was evaluated by Fehsenfeld et al. (1990)
in a comparison with a photolysis/chemiluminescence instrument (Section 7.4.3) and
a tunable diode laser absorption spectrometer (TDLAS, Chapter 2). For high levels of
NO
2
above 2 ppbv, similar results were found for all three instruments. On the other
hand, below 2 ppbv, interferences from O
3
and PAN were evident. However, they were
consistent enough that they could be corrected for down to 0.3 ppbv, provided there
are simultaneous measurements of O
3
and PAN. An O
3
scrubber placed on the inlet to
remove this interference worked to do that, but it did not remove PAN and it removed
about 50% of the NO
2
as well. Kelly et al. (1990) also assessed the Luminox instrument
for airborne measurements and determined several corrections that needed to be applied
for low mixing ratios. Schmidt et al. (1995) determined that recommended O
3
corrections
did not work, in contrast to the Fehsenfeld et al. (1990) study, but they, too, found that
the proprietary scrubber had the unacceptable trait of removing NO
2
as well. However,
they created a scrubber using natural rubber that removed most of the O
3
without
significantly affecting NO
2
.
In summary, the luminol detector is sensitive, lightweight, fast, and has low power
consumption. It suffers from strong interferences from O
3
and PAN, which, in the past,
have been corrected for with varying degrees of success. Also of concern are calibration
instabilities, nonlinearities, and zero offsets, but Kelly et al. (1990) found that with care,
errors as low as 15% could be attained.
7.7 Peroxides, HCHO, and HONO via liquid
techniques
7.7.1 H
2
O
2
via dissolution and chemiluminescence
Peroxides are products of atmospheric photochemistry that serve as reservoirs of odd-
hydrogen radicals and as indicators of prior photochemistry in an air mass. One of the
early techniques for the measurement of H
2
O
2
employed its collection from the gas phase
for detection in the liquid phase via the chemiluminescent reaction of H
2
O
2
with the
ubiquitous luminol reagent. Kok et al. (1978a) designed two methods for ‘scrubbing’
H
2
O
2
from the gas phase, one of them being a coil through which both the sample
air and distilled water flowed, extracting ambient H
2
O
2
into an aqueous solution. Once
in solution, it is mixed with a luminol solution and a solution of copper ions directly
in front of a blue-sensitive PMT to detect the 450 nm emission. The chemilumines-
cence mechanism is not fully known, but it involves the decomposition of H
2
O
2
by
