Heard D.E. (editor) Analytical Techniques for Atmospheric Measurement
Подождите немного. Документ загружается.

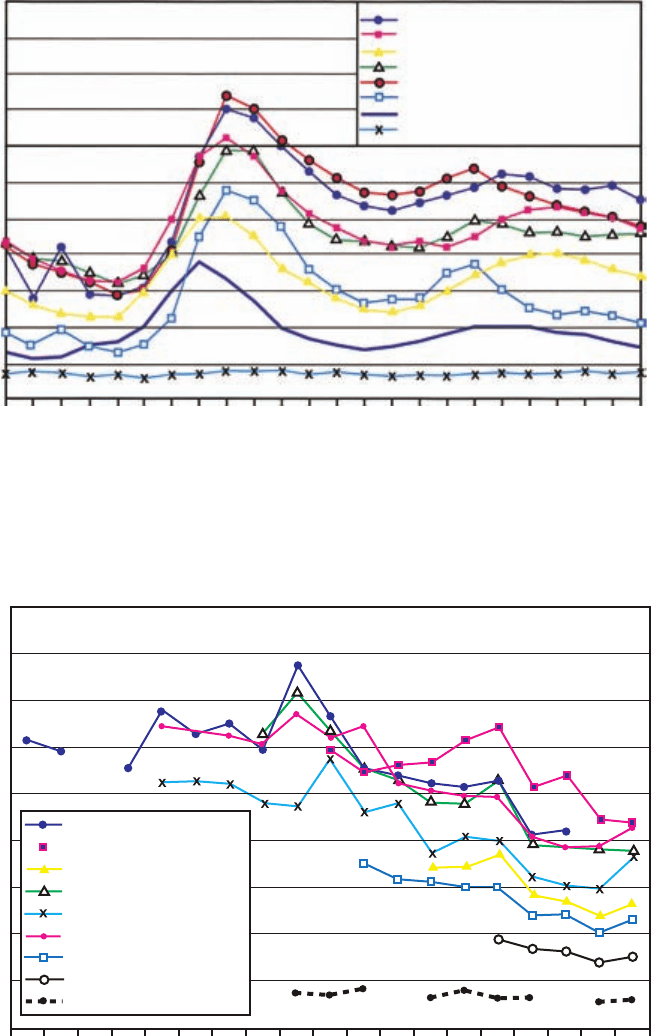
Plate 3a The diurnal cycle of NO
x
at eight sites in the UK, showing the morning rush hour maximum
due to vehicle traffic and a secondary maximum in the evening. (Figure 6.14 of AQEG, “Nitrogen Dioxide
in the United Kingdom”, 2004, printed with permission from Crown.) (See Figure 7.10a.)
Plate 3b The decadal trend in NO
x
at nine sites in the UK, showing the gradual decline due to the tight-
ening of vehicle emission standards. (Figure 6.5 of AQEG, “Nitrogen Dioxide in the United Kingdom”,
2004, printed with permission from Crown.) (See Figure 7.10b.)
(a)
200
180
160
140
120
100
NO
x
(µg m
–3
, as NO
2
)
London Bloomsbury (µg m
–3
, as NO
2
)
London Bexley (µg m
–3
, as NO
2
)
West London (µg m
–3
, as NO
2
)
Manchester Town Hall (µg m
–3
, as NO
2
)
Glasgow City Chambers (µg m
–3
, as NO
2
)
Belfast Centre (µg m
–3
, as NO
2
)
Port Talbot (µg m
–3
, as NO
2
)
Lullington Heath (µg m
–3
, as NO
2
)
80
60
40
20
0
01234567891011
Hour of Day
12 13 14 15 16 17 18 19 20 21 22 23
225
(b)
200
175
150
125
100
Bridge Place
Annual mean NO
x
(mg m
–3
, as NO
2
)
London Bloomsbury
London Bexley
West London
Manchester Town Hall
Glasgow City Chambers
Belfast Centre
Port Talbot
Lullington Heath
75
50
25
0
1982
1983
1986
1987
1988
1989
1990
1991
1992
1993
1994
1995
1996
1997
1998
1999
2000
2001
ALT-Colorplate 1/19/06 12:53 PM Page 2
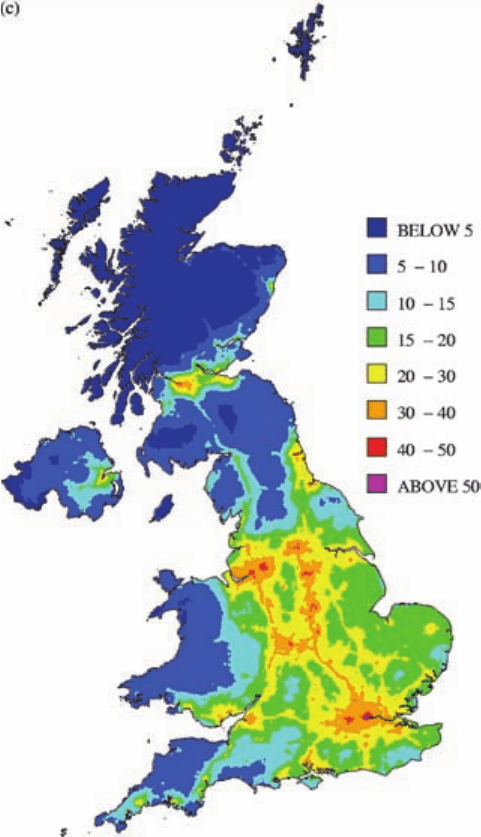
Plate 3c The geographical distribution of NO
2
in the UK, derived from monitoring-network data inter-
preted with an empirical model. (Figure 3.1 of Stedman et al., 2002, printed with permission from AEA
Technology.) (See Figure 7.10c.)
ALT-Colorplate 1/19/06 12:54 PM Page 3
A
A
B
B
C
C
D
D
E
F
G
H
1
2
3
4
5
3
3
4
5
5
6
7
8
8
9
3
4
5
7
8
9
9
11
50 55 61 66
66
72 77 82
75 84
400
300
200
100
0
0 10 20 30 40 50 60 70 80 90 100
51015202530354045505560 7065 75 80
Retention time (min)
1st retention time (min)
2nd column RT / secs
2nd column RT / secs
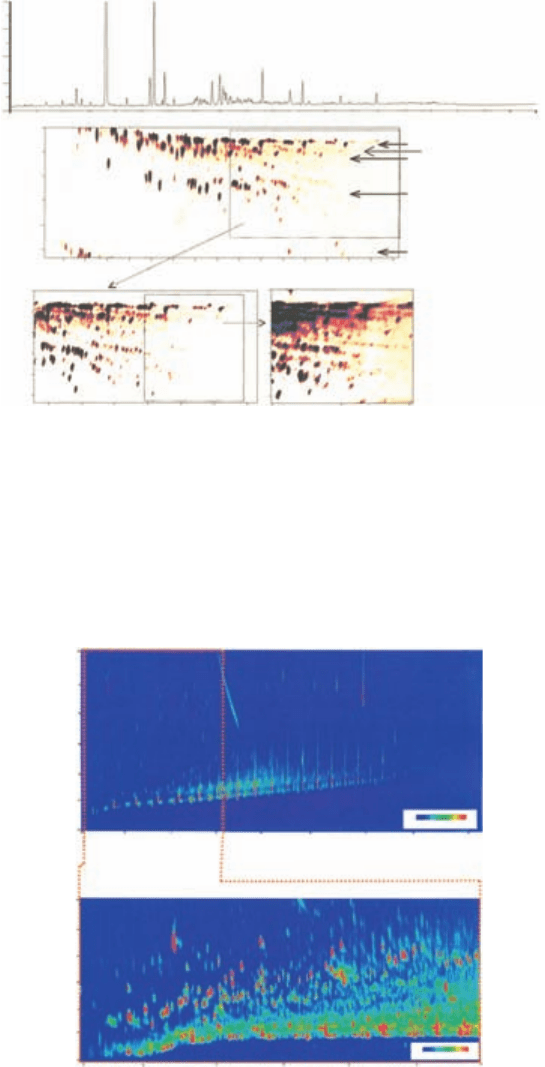
Plate 4 Comparison of single column (upper) and GC × GC separations (lower) of a Leeds urban air sam-
ple. Areas of the full chromatogram are successively extracted at higher gain to illustrate increasing isomeric
complexity at higher boiling points. GC × GC chromatograms are annotated with start of individual C
x
isomer band (running right to left) where A = C
2
, B = C
3
, C = C
4
, D = C
5
, E = C
6
, F = C
7
, G = C
8
,
H = naphthalene. Chemical banding assignments, 1; aliphatics, 2; olefinics, 3; oxygenated, 4; mono
aromatics, 5; polyaromatics. (From Hamilton & Lewis, 2003, reproduced with permission from Elsevier.)
(See Figure 8.10.)
Plate 5 Two-dimensional GC × GC-TOFMS total ion current (tic)-plot of an aerosol sample in 2D-contour
plot: (A) showing the full chromatogram of the analysed aerosol with (B) the extraction of the selected section
for data analysis. (From Welthagen et al., 2003, reproduced with permission from Elsevier.) (See Figure 8.11.)
GC × GC plot of Aerosol sample
(A)
(B)
4.0
3.5
3.0
2.5
2.0
1.5
1.0
4.0
3.5
3.0
2.5
2.0
1.5
1.0
Polarity 2nd dimension (seconds)Polarity 2nd dimension (seconds)
1000 1500 2000
2500 3000 3500 4000
600 1250 2500 3750 5000 6250 7500 8750 10
000
Volatility 1st dimension (seconds)
Volatility 1st dimension (seconds)
0
20
000
40
000
0
20
000 40 000
ALT-Colorplate 1/19/06 12:54 PM Page 4

Mass Spectrometric Methods for Aerosol Composition Measurements 271
dislodged after impaction and become re-entrained in the air flow, meaning they may
be collected on the wrong stage or not at all. To mitigate this problem, the substrates
are often pre-treated or coated before sample collection to promote adhesion. However,
these methods are only permitted when the treatment or coating will not perturb
the aspect of particle chemistry of interest or interfere with the analytical procedure
being used.
While these bulk sampling methods can employ many powerful and well-established
analytical techniques to determine the chemical nature of the particles, they carry many
intrinsic limitations. First, a measurable amount of particulate material has to be collected,
and the detection limits of laboratory chemical analysis instrumentation usually require
sampling times from several hours to days, so temporal resolution is generally poor.
Secondly, because of the intervening time between sample collection and analysis, volatile
components of the aerosol may evaporate and be lost or chemically unstable compounds
may react in the interim (Chow, 1995; Zhang & McMurry, 1987, 1992). Semi-volatile
chemicals may also interchange with the gas phase during sampling or before analysis
and there is the potential for contamination during handling. Thirdly, while cascade
impactors provide some size-resolved information, the resolution is generally poor and
particle bounce may result in erroneous size information. Finally, because the aerosol is
handled in bulk, no information on the extent of internal or external chemical mixing is
retained.
A common remedy for the problem of gas-phase interchanges during bulk sampling
is to place a suitable denuder (tubing lined with a reagent) upstream of the collector to
absorb any gas phase chemicals that would otherwise contaminate the substrate. A second
denuder or chemically treated filter can also be placed downstream and subsequently
analysed to measure the amount of particle-phase material that has evaporated from the
substrate during sampling (e.g. Mader et al., 2003).
6.3.2 Online bulk sampling and analysis methods
Some of the problems associated with bulk sampling such as gas-phase partitioning after
collection and contamination during handling can be avoided by performing the analysis
in situ by an automated instrument, for example the Thermo Electron Series 8400N
Ambient Particulate Nitrate Monitor. This collects a sample over time through impaction
and then, through heating, converts the nitrate fraction of a sample to NO
x
, which it
then measures to calculate the particulate nitrate mass concentration (Stolzenburg &
Hering, 2000). While this offers an increased time resolution (minutes are possible), it
is limited to one aspect of particle chemistry, although instruments employing variations
of this principle can be used in parallel to study other chemical species (e.g. sulphate and
OC/EC).
The particle into liquid sampler (PILS) (Orsini et al., 2003; Weber et al., 2001) and
the steam jet aerosol collector (SJAC) (Khlystov et al., 1995) are examples of automated,
in situ methods that use a more generalised approach. Particles are grown to large sizes
by introducing them into a supersaturated water environment, and then impacted on a
surface, over which water is flowed continuously. The sample water is collected period-
ically and analysed, frequently using IC, to quantify the amounts of various chemicals

272 Analytical Techniques for Atmospheric Measurement
present in the aerosol. This allows high time resolution data (minutes) on many ionic
components of the aerosol to be collected simultaneously. The collection technique is
also not limited to IC; with a suitable detector, it can be used to quantify fractions such
as water-soluble organic carbon as well.
Another novel in situ method is the thermal desorption aerosol GC-MS/FID (TAG)
(Williams et al., 2004). This collects particles in bulk on a cooled surface through
impaction over a period of time. After collection, the surface is isolated and then heated,
vaporising the components. The organic fraction is then transferred to a gas chromato-
graph for analysis, using either a flame ionisation detector for total mass measurements
or a mass spectrometer for compound identification. This method is similar to the online
methods involving thermal decomposition, discussed later. The analysis produced by this
instrument is in many ways analogous to the results obtained by offline GC analysis;
while many specific compounds can be positively identified and quantified, the majority
of the organic matter in particles remains unresolved. These methods are similar to those
employed for the gas-phase speciation of organic compounds, as discussed in Chapter 8.
These instruments are automated and therefore less labour-intensive and so are ideally
suited for continuous monitoring operations. However, while these online instruments
are very powerful and reliable, they have inherent limitations in that size-resolved data
are not delivered, and as is common to all forms of bulk sampling instrumentation,
cannot provide any information regarding mixing states.
6.4 Online aerosol mass spectrometry
The field of online aerosol mass spectrometry has emerged to provide a real-time method
for the chemical analysis of aerosols. These techniques overcome the problems associated
with bulk collection and offline analysis by analysing the composition in situ on a particle-
by-particle basis. The basic principle of an aerosol mass spectrometer is to introduce
airborne particles into the instrument, vaporise and ionise the material and then analyse
the ions produced using mass spectrometry. There have been many different designs of
aerosol mass spectrometer over the years, most developed in-house at research institu-
tions around the world and tailored to specific tasks, usually related to either ambient
sampling or specific laboratory studies. However, one of the more useful features of these
instruments is their versatility; in most cases, the individual instruments have proved
useful in multiple applications.
At the time of writing, two models of aerosol mass spectrometer are available commer-
cially: the TSI model 3800 Aerosol Time Of Flight Mass Spectrometer (ATOFMS), an
instrument based on the pioneering work of Prather et al. (1994) and developed further
by Gard et al. (1997), and the Aerodyne Research Inc. Aerosol Mass Spectrometer (AMS;
Billerica, MA, USA), a design introduced by Jayne et al. (2000). Both of these instruments
are designed to be portable and suitable for both field and laboratory studies, but the data
produced are fundamentally different. The ATOFMS, being a laser desorption/ionisation
and time of flight-based instrument, delivers quantitative size and largely qualitative
composition information on individual particles, while the AMS, being based around
thermal desorption, electron impact ionisation and quadrupole mass spectrometry, gives

Mass Spectrometric Methods for Aerosol Composition Measurements 273
quantitative data on both the size and composition of the entire aerosol ensemble, but
gives only limited data on specific particles and cannot study refractory components.
The technical aspects of these and many other examples of similar instruments will
be discussed in this section and the specific advantages of the various approaches
explored. Instruments developed at the beginning of twenty-first century that will be
referred to by name include the Aerosol Composition Mass Spectrometer (ACMS),
Atmospheric Pressure Chemical Ionisation Mass Spectrometer (APCI-MS), the Chapel Hill
instrument, the Laser Mass Analyzer for Particles in the Airborne State (LAMPAS), the
Particle Analysis by Laser Mass Spectrometry (PALMS), the Particle Blaster, the Rapid
Single particle Mass Spectrometry (RSMS), the Surface Ionisation Particle Beam Mass
Spectrometer (SI-PBMS), the Thermal Desorption Chemical Ionisation Mass Spectrometer
(TD-CIMS) and the Thermal Desorption Particle Beam Mass Spectrometer (TDPBMS).
Table 6.1 lists these instruments, their measurement technologies and selected publica-
tions. The list is not exhaustive, but represents a wide spectrum of the novel techniques
used. Examples of further implementations of these techniques can be found elsewhere
in the literature. (e.g. Hearn & Smith, 2004; Hunt & Petrucci, 2002; Öktem et al., 2004).
6.4.1 Mass spectrometer types
The principle of a mass spectrometer is to separate and count ions according to their
mass-to-charge ratios (m/z). A detailed discussion is given in Chapter 5, and so the
operation of a mass spectrometer is considered only briefly here. Quantitatively, m is
taken as the mass of the ion relative to the standard atomic mass (defined as one twelfth
of the rest mass of a
12
C atom, or 16606×10
−27
kg) and z is the charge relative to e, the
elementary charge 1602 ×10
−19
C. The charge of most ions detected is normally ±1e,
although multiple charging is possible, depending upon the composition of the ion and
the ionisation technique used. In this chapter, m/z is treated as being dimensionless,
although atomic mass units (amu or u), Daltons (Da) and Thompsons (Th) are used as
units for the same quantity elsewhere in the literature.
The most basic design of a mass spectrometer is the magnetic sector mass spectrometer,
which accelerates and focuses ions using electric fields and then bends their paths with trans-
verse magnetic fields. As the ions are accelerated and deflected by electric and magnetic fields
of specific strengths, their velocities and deflected trajectories are therefore dependent on
their m/z. Therefore, by using a fixed detector (such as an electrometer), the ions are filtered
according to their m/z prior to counting. This type of mass spectrometer can be scanned by
varying the electric or magnetic field strengths. While this method is generally not favoured
in current designs of aerosol mass spectrometers due to its bulk, it is capable of very high
resolutions, so is often used in other laboratory applications where mass measurements
of fractions of amu are needed, such as when identifying specific elements. An exception
is the RCMS, which has used the compact design introduced by Nier & Schlutter (1985).
Quadrupole mass spectrometers work by again accelerating the ions using electric fields
but this time, the ions are selected by passing them between four parallel rods. A voltage is
applied between the two sets of opposing rods, which consists of AC and DC components.
The ions adopt oscillating trajectories as they travel the length of the rods (see for example
Chapter 5, Figure 5.4), the magnitude of the oscillations dependent on their m/z, the AC

274 Analytical Techniques for Atmospheric Measurement
Table 6.1 Instruments to measure aerosol composition using mass spectrometry
Instrument Vaporisation
and ionisation
Mass
spectrometer
Sizing Selected literature
ACMS Thermal-EI Quadrupole or
magnetic sector
None Schreiner et al.,
2002
AMS Thermal-EI Quadrupole Polydisperse
aerodynamic
Jayne et al., 2000;
Jimenez et al.,
2003a
APCI-MS Thermal-CI Ion Trap None Hoffmann et al.,
2002
ATOFMS
(TSI 3800)
Single laser
∗
Bipolar reflectron
(originally
monopolar
linear) TOF
Polydisperse
opto-aerodynamic
Gard et al., 1997;
Prather et al.,
1994
Chapel Hill
Instrument
Dual laser or
thermal-laser
Linear TOF Polydisperse
opto-aerodynamic
Sykes et al., 2002;
Woods et al.,
2001
LAMPAS/
LAMPAS 2
Single laser Bipolar
(originally
monopolar)
linear TOF
Polydisperse
(originally
monodisperse)
opto-aerodynamic
Hinz et al., 1994;
Trimborn et al.,
2000
PALMS/
WB-57F
PALMS
Single laser Reflectron
(originally PSPF)
TOF
Polydisperse
optical, later with
opto-aerodynamic
Murphy and
Thomson, 1995;
Thomson et al.,
2000
Particle
Blaster
Single laser Reflectron TOF None Reents et al.,
1995; Reents and
Ge, 2000
RSMS/RSMS
II/RSMS III
Single laser Linear (originally
reflectron) TOF,
later bipolar
Monodisperse
aerodynamic
(originally none)
Carson et al.,
1995; 1997a;
Lake et al., 2003
SI-PBMS Thermal Quadrupole None Svane et al., 2004
TD-CIMS Thermal-CI Triple quadrupole None Voisin et al., 2003
TDPBMS Programmable
thermal-EI
Quadrupole None Tobias and
Ziemann, 1999;
Tobias et al., 2000
∗
Two-step laser desorption and ionisation was first demonstrated on an instrument of this type (Morrical et al., 1998),
although it has not been widely used since.
and DC voltages applied and the frequency of the AC voltage. Normally, the frequency is
kept fixed during operation, as it is easier to vary the magnitudes of the voltages. The AC
voltage acts as a high-pass m/z filter, as ions with too low an m/z adopt trajectories with
oscillations larger than the spacing of the rods, causing them to strike the rods, become
neutralised and not be detected. In a similar manner, the DC voltage acts as a low-pass

Mass Spectrometric Methods for Aerosol Composition Measurements 275
m/z filter by further perturbing the oscillations of high m/z ions. Therefore, if the two
voltages are selected correctly, a quadrupole can act as a filter for all ions but those of
the desired m/z.
After the rods, the ions are detected using devices such as electron multipliers or
conversion dynodes, sometimes after extraction using additional electric fields. While
essentially performing the same filtering function as a magnetic sector mass spectrometer,
quadrupoles are much more compact, robust and generally easier to implement, making
them ideal for field instrumentation. Their accuracy is also not affected by the initial
velocities of the ions prior to selection. However, a disadvantage of selecting the ions this
way is that the resolving power of quadrupoles is limited by the amount of time the ions
spend in the rod region, which is typically less than a microsecond. Because of this, the
m/z resolution of quadrupole mass spectrometers is normally around unity.
A third common type of mass spectrometry is the time-of-flight (TOF) method. The
basic principle is to again accelerate the ions over a specific electric potential, but the
time taken to travel a set distance to the detector (e.g. microchannel or microsphere
plates) is measured, from which the velocity and therefore the m/z can be calculated (see
Figure 5.6 in Chapter 5). This technique requires accurate timing electronics, rapid data
collection and the precise gating of the start of the time of flight, meaning the ions must
be delivered in discrete pulses. It does have a major advantage in that ions of many m/z
can be detected simultaneously, which is not possible with the previous two methods,
and does not have a maximum m/z imposed upon it. The TOF mass spectrometer
is capable of sub-unity m/z resolution, but is limited by several factors, including the
duration of the ion pulse, the spatial distribution of the ions before extraction and the
distribution of velocities of the ions prior to acceleration. There are ways of mitigating
these losses in resolution, post source pulse focusing (PSPF) being one such method
(Kinsel & Johnston, 1989). This involves applying an additional voltage pulse to part of
the drift section after the ionisation event, which causes any lagging ions to catch up with
those of the same m/z. While this produces a satisfactory improvement in the resolution,
it is intrinsically limited to a particular portion of the mass spectrum. The wider the
section it is applied to, the less pronounced the improvement. Many implementations
make use of a reflectron instead, as shown in Figure 6.1, which specifically reduces the
broadening due to uncertainties in the ion velocities in the drift region. Electric fields are
used to reverse the direction of travel of the ions halfway through their flight. As ions with
a velocity higher than intended for their m/z penetrate deeper into the reflectron, the
reflection time is increased, so the otherwise shortening of their time of flight is largely
Ion source
Reflectron
Detector
Figure 6.1 The basic principle of a reflectron time-of-flight mass spectrometer. Trajectories of ions with
higher and lower velocities than desired are shown with dashed lines.

276 Analytical Techniques for Atmospheric Measurement
cancelled out, providing the electric fields are tuned correctly (Weickhardt et al., 1996).
An advantage of this over PSPF is that providing it is correctly tuned, the increase in
resolution benefits the ions of all m/z.
Ion trap mass spectrometers use a system of two end-cap electrodes either side of a
ring electrode, which is effectively the rod geometry of a quadrupole wrapped around
on itself. By applying the AC and DC voltages between the caps and the ring in the
same manner as in a quadrupole, ions of a range of m/z’s are indefinitely held in
stable three-dimensional orbits between the electrodes. The voltages can be initially set
to capture and hold all ions and then subsequently ramped to selectively release ions
according to their m/z for detection. Like the TOF mass spectrometer, this is capable
of delivering a complete mass spectrum from a single ionisation event, but is not reliant
upon having a pulsed ion source. Also, it is capable of higher resolutions than quadrupole
mass spectrometers due to the increased resolving time. However, because it must be
scanned, it takes much longer than a TOF mass spectrometer to deliver a complete mass
spectrum, so is unsuitable for rapid data collection.
A method sometimes used to further investigate the origin of specific peaks within a
mass spectrum is tandem mass spectrometry or MS-MS. This can be performed using a
triple quadrupole mass spectrometer, which is in effect three quadrupoles in series. The
first quadrupole selects an m/z of interest, while the second does not act as a filter but as
a region where the ions are further fragmented by techniques such as collision induced
dissociation (CID). The third quadrupole then performs a scan to give a spectrum of
the secondary fragments, which is of particular use in identifying organic chemicals.
By disabling the initial selection and secondary fragmentation, this configuration is also
capable of performing the same task as a standard quadrupole, so the peaks of interest in
a sample can be identified. An MS-MS is also possible with ion trap mass spectrometers;
the basic method is to release all ions except for those of the m/z of interest from the
trap before returning it to a state where it will hold all ions. The remaining ions are then
fragmented using CID before the mass spectrometer is scanned as normal.
6.4.2 Inlet technologies
Mass spectrometers must be operated at high or ultra-high vacuum, because random
collisions with gas molecules can prevent ions from being detected. Therefore, a key
feature of aerosol mass spectrometry involves removing as much of the gas-phase material
from the aerosol sample as possible while retaining the particulate fraction for analysis.
The exceptions are the thermal desorption-chemical ionisation methods (e.g. APCI-
MS, TD-CIMS) because while the mass spectrometer is operated at high vacuum, the
collection, desorption and ionisation can take place at atmospheric or near atmospheric
pressure. The most common method of removing gas from the sample is to form a
collimated particle beam from the aerosol at the inlet and skim off the majority of the
gas-phase material using a series of apertures and differential pumping. In most early
designs, the particle beam was formed by accelerating the particles through a capillary
tube or nozzle into the vacuum of the instrument. Due to their inertia, the particulate
fraction of the aerosols forms a less divergent jet than the gas fraction at the point of
expansion. The particles pass through a series of channels or apertures, allowing the
Mass Spectrometric Methods for Aerosol Composition Measurements 277
excess gas to be removed by the pumping system. There is still some divergence in the
particle beams, with the amount of divergence dependent on the aerodynamic size of the
particles. Particles must hit a physical target or pass through laser focal points in order to
be detected and so there is a tolerance in the solid angle in which particles can travel and
be detected. This in turn leads not only to a reduction in sensitivity, but also to a bias
towards particles of a given aerodynamic size. These can both be mitigated by reducing
the distance between the nozzle and the detection point.
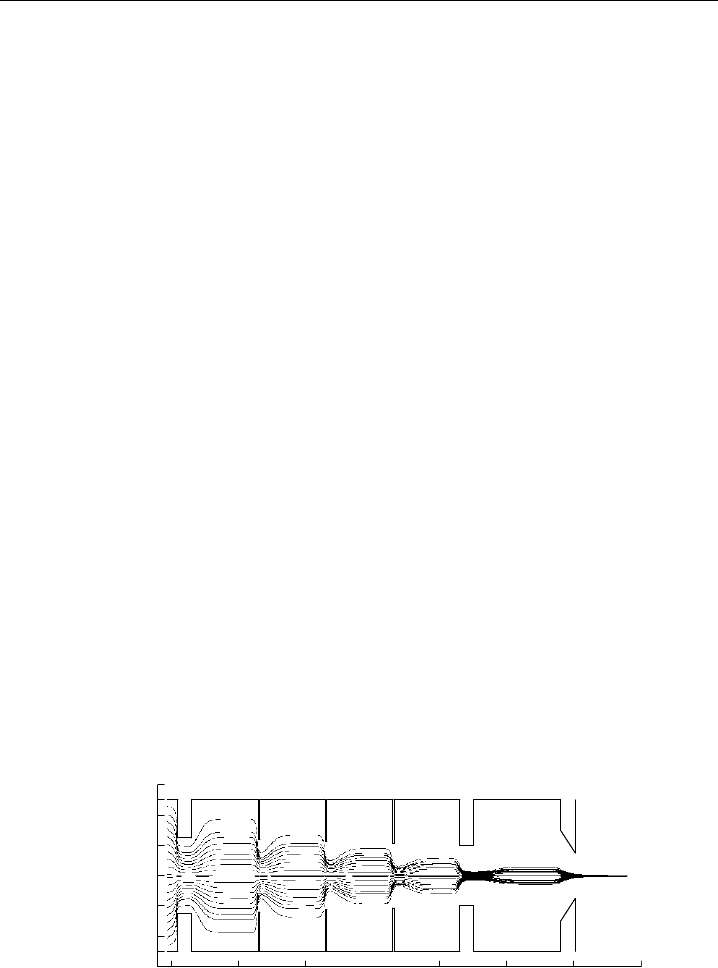
Since mid-1990s, the implementation of aerodynamic lenses prior to a nozzle expansion
has sought to address these problems (Liu et al., 1995a,b; Petrucci et al., 2000; Zhang
et al., 2002, 2004), as shown in Figure 6.2. Particles are drawn through a series of
concentric apertures with successively decreasing diameters, which causes the gas stream-
lines to rapidly compress and expand as the aerosol flows through the lens system. These
successive compressions cause the particle streamlines to converge on the axis of the
lens, so that when they are accelerated through the nozzle, the radial components of
their velocities are small and the streamlines take the form of a tightly collimated beam.
As the lens must be operated at a low pressure to reduce defocusing due to Brownian
motion, the aerosol must normally pass through a critical orifice before entering the lens.
The main advantage of aerodynamic lenses is that particles over a wide range of sizes
are focused into a narrow and very parallel beam, thereby helping to eliminate any size-
dependent detection biases an instrument may have over a given size range and improving
quantitative capabilities. The tighter beam also increases the number of particles detected,
which in turn improves the instrument sensitivity and overall data quality, particularly
for the smaller particles (Kane et al., 2001; Su et al., 2004). The TDPBMS, ACMS, AMS,
SI-PBMS and Chapel Hill instrument (see Table 6.1) are among the instruments that use
this technology and TSI have manufactured an aerodynamic lens (model 3801-030 AFL)
as an optional accessory for the ATOFMS. In the case of the ACMS, the lens design was
modified to allow sampling at high altitudes (Schreiner et al., 1998).
Normally, a particular lens implementation can effectively focus a specific range of
particle sizes; for instance, the 3801-030 is specified for particles between 30 and 300 nm
in aerodynamic diameter. Smaller particles do not posses enough inertia to be focused
Radial coordinate (m)
Axial coordinate (m)
0.00
–0.005
0.005
0.000
0.10 0.20 0.30
Figure 6.2 Computed 100 nm particle trajectories within an aerodynamic lens system. Several aerosol
trajectories enter from the left and become focused down the axes of the lenses during the successive gas
expansions and contractions. The right side is where the aerosol enters the first vacuum chamber of the
instrument. (Jayne et al., 2000, reprinted with permission from 2000 American Association for Aerosol
Research, published by Taylor and Francis.)
