Heard D.E. (editor) Analytical Techniques for Atmospheric Measurement
Подождите немного. Документ загружается.


242 Analytical Techniques for Atmospheric Measurement
lenses. In a magnetic field-free linear separation system, an ion with mass m and total
charge q = ze, has a kinetic energy KE defined by Equation (5.13).
KE =
mv
2
2
= zeV (5.13)
Since t = v/d, we can rearrange this equation to give
t
2
=
m
z
×
d
2
2Ve
(5.14)
Equation 5.14 shows that ions with lower masses will therefore reach the detector first.
By accurately measuring the time t and keeping V and d constant m/z can be derived.
An advantage of this technique is that although the time differences are extremely small,
time can be very accurately measured. The principle is depicted in Figure 5.6.
Time of flight mass filters have very high transmission efficiencies, which leads to very
high sensitivities. All the ions formed are directed to the detector and so, in principle,
all ions are analysed. This is in contrast to the quadrupole mass filter, described in
Section 3.2.1, which measures masses sequentially. An important drawback of time of
flight instruments is difficultly in achieving good resolution. This stems from the physical
limits of creating ions, of the same energy, instantaneously, in the same place. These are
the following: the length of time in the ion formation pulse is finite, creating a time
distribution; there is a minimum size of the ion-generating volume creating a spatial
distribution; and the ions produced do in reality have a kinetic energy distribution. All
these factors contribute to uncertainty in the measurement of the flight time and hence
to lower resolution. To counter this, TOF-MS practitioners lengthen the flight tube and
hence the flight time, thus increasing the resolution. A long flight tube would be typically
1–2 m in length. This is a considerable restriction when operating this instrument on small
jet aircraft where space is a precious commodity. One major advantage of current TOF
systems is that they may be operated at high measurement frequencies. Commercially
available systems may be operated at 500 Hz (in contrast to typical quadrupole data acqui-
sition frequency of 10 Hz), making them ideally suited to comprehensive chromatography
applications, see Chapter 8.
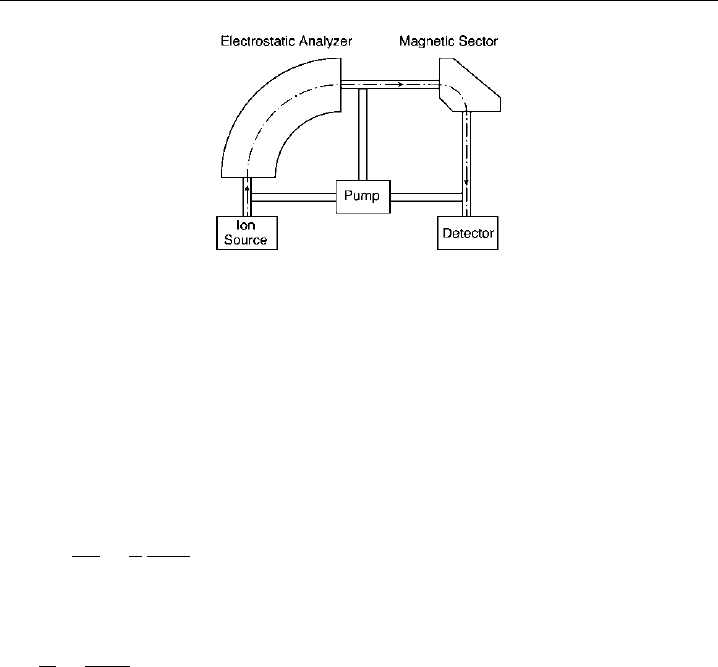
5.3.2.3 Magnetic sector
The mass separating effect of a magnetic field comes from the fact that ions of equal
energy but different m/z describe different radial trajectories when passing through a
Figure 5.6 A representation of the principle of time of flight mass spectrometry (Jochum, 1988 with
permission from Expert Verlag GmbH).
Mass Spectrometric Methods for Atmospheric Trace Gases 243
Figure 5.7 A schematic of the Nier-Johnson type magnetic sector mass spectrometer design.
magnetic field. A schematic diagram of a magnetic sector mass filter is given in Figure 5.7.
In a homogeneous magnetic field of strength B, ions with a velocity v experience a
force F , in a direction perpendicular to the field lines and to the direction of motion
of the ions. In order to traverse the circular path, the magnetic or Lorentz force FL
and the centripetal force Fc must be equal. The radius of the resulting trajectory can be
calculated by equating the Lorentz force FL =evB with the centripetal force Fc =mv
2
/r.
Substituting the relationship eU =mv
2
/2 for the ion energy (where U is the acceleration
potential), we obtain:
r =
mv
eB
=
1
B
2mU
e
(5.15)
Rearranging we can obtain an expression for m/z.
m
z
=
B
2
r
2
e
2V
(5.16)
From Equation (5.16) it can be seen that mass spectra can be obtained by varying one
of three variables (B V ,orr) while holding the other two constant. Classical magnets
were not well suited to the fast scanning necessary for coupling with GC due to magnetic
heating by Foucauld currents induced by rapidly changing the magnetic field. Most
modern mass spectrometers contain an electromagnet in which ions are sorted by holding
V and r constant while varying the current in the magnet and hence the field strength B.
In practice, most magnetic sector instruments are built to deflect ions through circular
paths of 60, 90 or 180
. When a collection of ions with the same m/z but with small
diverging directional distribution is acted upon by the magnetic field, a converging
directional distribution is produced as the ions leave the field (directional focussing).
For this reason the simplest magnetic sector mass filters, are called single focussing
spectrometers. It is important for mass spectrometry with magnetic sectors that the
incident ions have the same energy as far as possible. A large span of energies leads to a
low resolution. Therefore to obtain the high resolution required for isotope measurements
an electric and a magnetic field are combined so that the energy dispersing effect of the
magnet is compensated by the electric field. By this so-called double focussing method,
directional and energy focussing is achieved. Figure 5.7 shows a schematic diagram of
the so-called ‘Nier-Johnson geometry’ design.

244 Analytical Techniques for Atmospheric Measurement
A wide variety of double focussing mass spectrometers are available commercially.
Resolutions of up to 10
5
can be obtained and this is necessary when analysing isotopic
ratios in ambient air.
5.3.2.4 Ion trap
An ion trap is a device in which gaseous ions can be formed and confined for extended
periods by electric and/or magnetic fields. In a sense, the ion trap may be thought of
as a ‘three dimensional’ quadrupole (see Section 3.2.1.). The electric fields in a standard
quadrupole assembly constrain ions into stable two-dimensional motion between the
quadrupole rods. By constructing an arrangement of two end-cap electrodes, one on
either side of a third annular electrode and all of hyperbolic cross section, ions injected
into the volume between the three electrodes can be ‘trapped’ into a complex circular
motion by applying suitable DC and RF electric fields. Ions with an appropriate m/z
circulate in a stable orbit within the cavity. When the radio frequency is increased, the
orbits of the heavier ions become stabilised, while those of the lighter ions become
destabilised and eventually collide with the electrode. The ions repel one another in the
trap and as a result their trajectories expand as a function of time. To avoid ion losses
through this expansion, a small pressure of helium gas (typically 0.13 Pa) is maintained
in the trap. This removes excess energy from the ions by collision.
Like a quadrupole, the ion trap is usually a low resolution mass spectrometer, being
usually able to resolve unit mass (1 amu). A considerable advantage of the ion trap
mass filter is that it can be used to perform MS/MS experiments. This involves trapping
ions of a characteristic m/z as described above and then collisionally fragmenting them
further before detection. In this way two different species having the same m/z can be
differentiated provided they produce distinct fragments. Usually the precursor ions are
given more kinetic energy through the use of an extra RF voltage applied to the end caps
so that total collision energy can be increased and the selected ions induced to fragment.
This is of great help in atmospheric studies where many different species may lie on the
same mass (e.g. propanal, acetone, glyoxal all have mass 58 at unit mass resolution, see
Table 5.2) but when fragmented can be expected to be different.
5.3.3 Detectors
Following the successful application of the mass filters described above we obtain a
current of ions with a well-defined m/z. These ion currents lie typically between 10
−8
and 10
−16
A. To measure these currents a number of systems are available. The three
most commonly in use in atmospheric science are described below.
5.3.3.1 The Faraday cup
The Faraday cup collector is the simplest and most inexpensive of detectors available.
The cup is aligned so that ions exiting the mass filter strike the collector electrode. The
charge carried by the ions that hit the cup is earthed via a high ohmic resistance and the
potential difference across this resistance is measured with a high impedance amplifier.
Mass Spectrometric Methods for Atmospheric Trace Gases 245
The electrode is surrounded by a cage that prevents the escape of reflected ions and
ejected secondary electrons. The collector electrode is inclined with respect to the entering
ions so that particles striking or leaving the electrode are reflected away from the entrance
to the cup. The response of this detector is independent of the mass, the energy and the
chemical nature of the ion. The main disadvantage of the Faraday cup is the amplifier
required, since this limits the speed at which a spectrum can be obtained. Moreover,
as the cup detector uses no internal amplification (see Sections 5.3.3.2 and 5.3.3.3) it is
less sensitive. The Faraday cup is widely used in systems requiring high precision, as the
charge of the cup is not dependent on the mass and energy of the detected ion. It is
necessary to use such detectors for isotopic analyses, see Section 5.4.3.
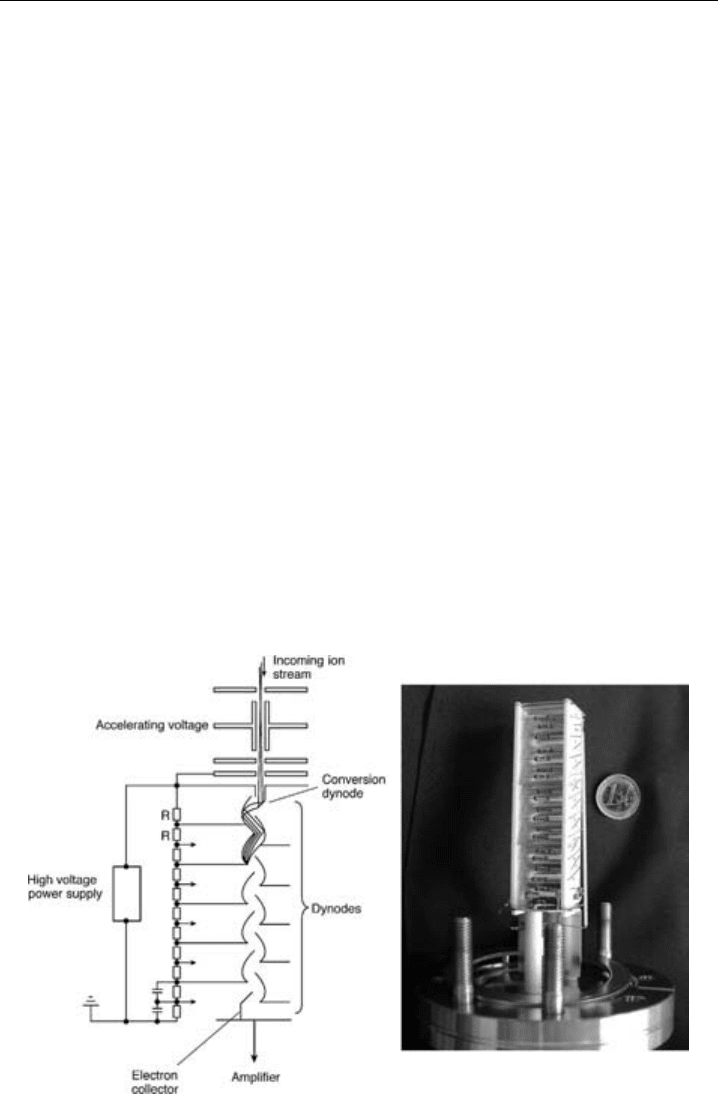
5.3.3.2 The Secondary electron multiplier (SEM)
Where extremely high sensitivity is required to detect ions, a secondary electron amplifier
is used. The secondary electron multiplier comprises a conversion dynode, a series of
further dynodes and a collector. Every mass-filtered ion that hits the conversion dynode
releases a burst of electrons, which are accelerated to the next dynode. In turn, each
electron hitting the second dynode release a similar burst of secondary electrons. With
each successive dynode the electron current is amplified with the result that the current
measured at the final cup is several orders of magnitude greater than the incident ion
current. A 20-stage SEM can produce a current gain of ca 10
7
. A combined schematic
diagram and picture of an SEM is shown in Figure 5.8. The SEM pictured is one used
in the proton transfer mass spectrometer described in Section 5.4.2. To better judge the
size of this detector a 1 Euro coin is included in the photograph.
Figure 5.8 Schematic diagram and photograph of a secondary electron multiplier.
246 Analytical Techniques for Atmospheric Measurement
(a) (b) (c)
–2
kV
–4
kV
+2
kV
–150
V
Ions Ions
e
–
Conversion
dynode
Conversion
dynode
Channel electron
multiplier
(Channeltron)
Signal Signal
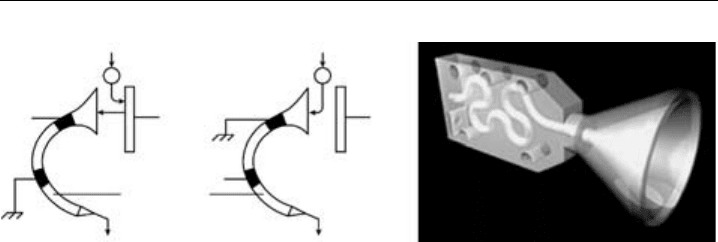
Figure 5.9 A schematic diagram of a channeletron when used for (a) negative and (b) positive ion
detection and (c) a cross section picture of a commercial version (Picture courtesy of Sjuts Optotechnik
GmbH).
In general, SEM detectors are robust and reliable, providing high current gains in
nanosecond response times. The detector response is, however, dependent on the mass,
the charge, the speed and the molecular structure. This necessitates empirical assessment
of the ion calibration factors for quantification work. For magnetic sector instruments,
the detector may be set directly behind the mass filter exit slit as ions reaching the detector
usually have enough energy to eject electrons from the first dynode. When used with
quadrupoles the ions must be accelerated prior to the first impact. The lifetime of these
detectors is 1–2 years because of surface contamination of the Cu/Be surface from the
incident ions. They are not as precise as Faraday cups but the higher sensitivity allows
for rapid scanning.
5.3.3.3 The Channeletron
The channeletron (or channel electron multiplier) is a variant of the secondary electron
multiplier described above, but it has a single continuous dynode rather than several in
series. It consists of a glass or ceramic tube with semiconducting inner surfaces. This
detector is used in the API-CIMS instrument described in Section 5.4.1. When used for
positive ions, see Figure 5.9a, a conversion dynode at high negative potential (3–4 kV)
is used to release electrons when the ion impacts. The resulting electrons are led into
the channeletron where they cause an electron avalanche, sufficient to be detected by a
preamplifier. In the case of negative ions, Figure 5.9b, the ions themselves are led into the
channeletron and detected. Current gains of 10
5
are typical for such detectors. A cross
section of a commercially available channeletron is shown in Figure 5.9c.
5.4 Mass spectrometry used in atmospheric chemistry
5.4.1 Atmospheric pressure ionisation-chemical ionisation
mass spectrometer (API-CIMS)
As the name suggests, in API-CIMS primary ions are produced in an ion source operating
under approximately atmospheric pressure. This instrument has also been termed as
Mass Spectrometric Methods for Atmospheric Trace Gases 247
ion molecule reaction mass spectrometer (IMRMS). In such an instrument, positive or
negative reagent ions interact with the trace gas molecules of interest and produce new
ions by chemical ionisation, as described in Section 5.3.2. For these reactions to be fast
and effective, the ionisation has to occur at high pressures, and for atmospheric scientists
it is practical to choose approximately atmospheric pressure.
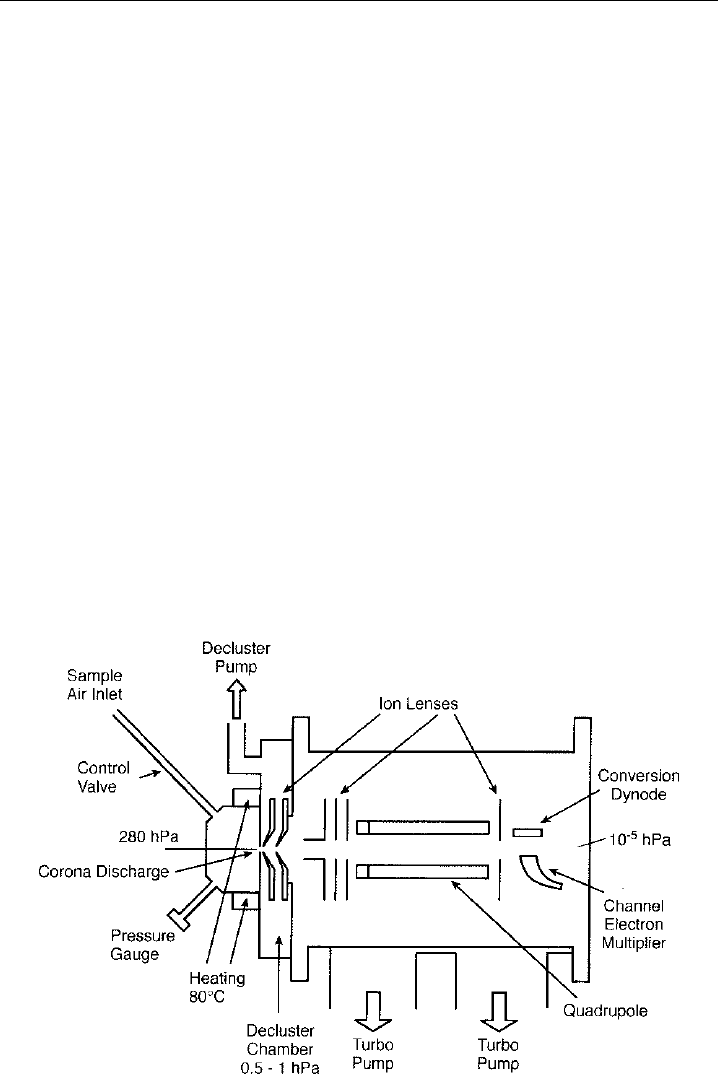
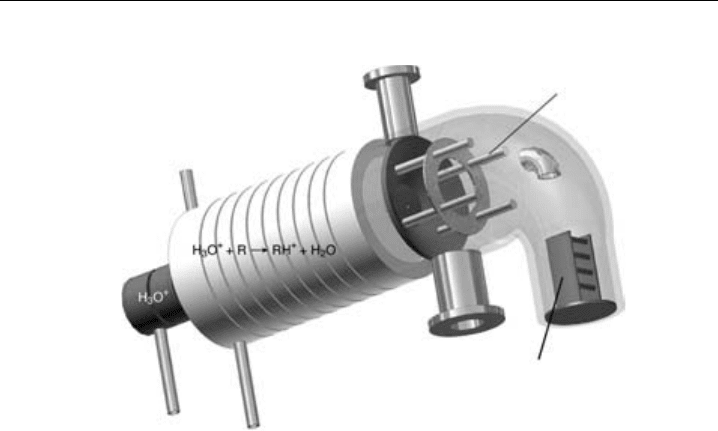
Figure 5.10 shows a schematic diagram of a mass spectrometer that has been used to
measure SO
2
(when in negative ion mode) and acetone (when in positive ion mode) on
various field campaigns on the ground and from on-board aircraft. Ions are produced by
corona discharge between a needle and the aperture lens housed within the ion source,
which is kept at constant temperature 80
C and pressure (280 hPa). The polarity of the
needle is chosen to have the same polarity as the ions to be detected since this improves
the transmission of ions through the system. Further details of such chemical ionisation
systems can be found in Huang et al. (1990). In this case the pressure in the ion source is
chosen to be slightly lower than the lowest pressure encountered in flight (at maximum
altitude of the aircraft, in this case 8.5 km–350 hPa).
The ion source is separated from the decluster chamber by a small 0.25 mm orifice.
As the decluster chamber is at lower pressure 0.5–1.0 hPa, a small flow (200 ml) of air is
drawn through the source region into the decluster chamber. When ionisation of ambient
air is done at these pressures, significant clustering of water molecules can occur on the
ions of interest. Since the number of water clusters can vary greatly, the ion signal can
be spread over many masses (e.g. X
+
H
2
O
n
, complicating the mass spectra in similar
fashion to fragmentation. In the decluster chamber the mean free path of the gas particles
is typically 100m, which is enough for them to be accelerated in the electric field present
in the decluster chamber. The energy thus imparted is sufficient to induce declustering
Figure 5.10 A schematic diagram of an atmospheric pressure chemical ion mass spectrometry. (Jost,
2002)
248 Analytical Techniques for Atmospheric Measurement
of the ions upon collision with another gas molecule. The desired ion-molecule reactions
can occur from when the ions are first produced until the ions are drawn into the high
vacuum of the spectrometer, where the pressure is too low for ion-molecule reactions to
be effective. A number of ion lenses are used to improve the transmission of the ions
from the decluster chamber to the channel electron detector. The ion lenses always have
the same polarity as the ions to be detected, and the lens potential must be optimised.
If it is too low the transmission will be poor, while too high causes the ions to enter the
quadrupole too fast and poorly resolved mass spectra result.
Let us now examine the underlying principle of operation. Having produced a positive
or negative reagent ion A
±
, we wish to react it with a neutral molecule of interest, X.
This can occur in a time dt, between ion source and high vacuum.
The rate of reaction can therefore be described,
dA
±
dt
=−kA
±
X (5.17)
where k is the rate coefficient and the terms in square brackets are concentrations.
Assuming that only a small fraction of the trace gas X reacts then it follows that [X] is
approximately constant. Therefore we may integrate to derive the expression,
X =
1
k
ln
A
±
t=0
A
±
t=T
(5.18)
By taking into account that the number of ions does not change in-ion molecule reactions
we may further transform Equation (5.18) to include B
±
, the product ions, as shown
below
X =
1
kT
ln
A
±
t=T
+B
±
t=T
A
±
t=T
=
1
kT
ln
1 +B
±
t=T
A
±
t=T
(5.19)
If the reaction time T is sufficiently short, the concentration of reactant ions A
±
remains
much higher than the concentration of the product ions B
±
, and the approximation
ln1 +x ≈x when x<<1 can be applied, simplifying this equation to
X =
1
kT
B
±
t=T
A
±
t=T
(5.20)
The concentration ratio of the reagent ion A
±
to the product ion B
±
can be determined
from the relative count rates of the mass spectrometer. Therefore the concentration of
gas X may be determined if we know the reaction time t, the rate coefficient k and
the count rate ratio of B
±
and A
±
. The same principle is used in the proton transfer
mass spectrometer described in Section 5.4.2. The reaction time T can be calculated or
measured although the measurement is complicated. The rate of an ion–molecule reaction
is very fast and the rate coefficient k typically has a value of 10
−9
cm
3
molecule
−1
s
−1
.
In the case of the atmospheric pressure ionisation mass spectrometer described here,
multiple reagent ions can be formed (e.g. in positive mode H
3
O
+
H
2
O
n
. The distribution
of these ions can change markedly with humidity. Each may react with X with different

Mass Spectrometric Methods for Atmospheric Trace Gases 249
rates, which are known only with ca 30% accuracy. Additionally, back reactions and
continuing reactions of the product ions can also be sources of error. Clearly, careful
calibration is essential to determine the proportionality coefficient between the X, the
molecule of interest and the product/reagent ion ratio under a variety of temperature and
humidity conditions. The measurement principle of API-CIMS is in many ways similar to
the PTR-MS technique. The accuracy, precision and errors of atmospheric measurements
made with these techniques are discussed in Section 5.4.2.
The API-CIMS instrument described here has been successfully deployed on several
ground and airborne campaigns. Over the west coast of Africa it has been used to track the
emission and subsequent photochemical production of acetone (using H
3
O
+
as a reagent
ion) and sulphur dioxide (using the CO
−
3
ion) from the large-scale biomass burning in
the dry season (Jost et al., 2003). Furthermore, the instrument was employed in the
laboratory to investigate the direct emission of compounds (e.g. acetonitrile, CH
3
CN)
when selected African biomass materials were burned under controlled conditions, see
Figure 5.11.
If the global distribution of biomass is known, then laboratory emission rates deter-
mined in this way may be used to derive global emission strengths. Furthermore the
emissions rates may be included in global models in order to estimate the atmospheric
effect of these global emissions on species such as ozone.
By adopting different reagent ions, other gases of atmospheric interest may be selectively
detected. The SF
−
6
reagent ion has been used in laboratory or field studies to detect
molecules of atmospheric interest such as HNO
3
,NO
2
,O
3
, HOCl, CF
2
O and SO
2
(Huey
et al., 1995) and in 2004 hydrochloric acid (HCl) was measured from aircraft in the
upper troposphere/lower stratosphere with a 5 pptv detection limit and an accuracy of
25% (Marcy et al., 2004).
5.4.2 The proton transfer reaction mass spectrometer
The proton transfer reaction mass spectrometer (PTR-MS) is a specific example of
the general chemical ionisation scheme described above, being developed by Lindinger
and co-workers at the University of Innsbruck (Lindinger et al., 1998). The underlying
principle is the same as described in Section 5.4.1 but in this case, the reagent ion is H
3
O
+
.
Complications resulting from water clusters present in API-CIMS are reduced by judicious
15
Mass 42 (CH
3
CN)
10
5
0
14:25 14:30 14:35 14:40 14:45
Time
0.0
0.5
1.0
1.5
2.0
CO and CO
2
CO
CH
3
CN
CO
2
Figure 5.11 Example of a laboratory application of API-CIMS – Biomass burning analysis.

250 Analytical Techniques for Atmospheric Measurement
Table 5.3 Proton affinities
Compound Formula Proton affinity kJ mol
−1
Nitrogen N
2
464.6
Oxygen, Ozone O
2
,O
3
396.3, 595.9
Noble gases Ar, Ne, He 346.3, 174.4, 148.5
Carbon mon/di oxide CO, CO
2
562.8, 515.8
Methane CH
4
544
Water H
2
O 660.0
Methanol CH
3
OH 725.5
Acetonitrile CH
3
CN 779.2
Acetone CH
3
COCH
3
782.1
Dimethylsulphide CH
3
SCH
3
801.2
Isoprene CH
2
CCH
3
CHCH
2
797.6
Source: Data from Hunter and Lias (1998).
choice of pressure and energy parameters, as detailed below. The key step is the transfer
of a proton from the reagent ion H
3
O
+
to the molecule of interest (X). Through proton
transfer reactions it is possible to measure all compounds with a proton affinity larger
than that of H
2
O. Fortunately for atmospheric scientists, it is ‘blind’ to the major air
constituents N
2
,O
2
, Ar, and CO
2
, as can be seen from Table 5.3. However, it can detect
many important atmospheric species such as acetone and isoprene. The ionisation occurs
at relatively low energies so that protonation usually does not cause the molecule to
fragment, or if it does, by ejecting only one H
2
O molecule. This considerably simplifies
the mass spectra. The proton affinity or gas-phase basicity is defined as the negative
molar Gibbs energy, −G, of the hypothetical protonation reaction,
H
+
+X → XH
+
(5.21)
A selection of these proton affinities is given here in Table 5.3. A comprehensive list can
be found at the NIST website – http://webbook.nist.gov/chemistry/. All the species in
Table 5.3 with proton affinities greater than water have been measured by PTR-MS.
A schematic diagram of the PTR-MS apparatus is shown in Figure 5.12. The ion source
consists of a hollow cathode and an earthed anode, through which water vapour flows at
about 8 cm
3
min
−1
. When held at a pressure of about 2 mbar (200 Pa), a cathode voltage
of 600 V is sufficient to cause an electric discharge between cathode and anode. The
result is an intense source of H
3
O
+
ions: count rates of typically 1×10
6
counts s
−1
being
detected by the mass spectrometer. An alternative source of H
3
O
+
ions has also been
developed using alpha particles emitted from a strip of Am
241
to ionise water vapour
(Hanson et al., 2003). Connected to the ion source is the reaction chamber or ‘drift tube’,
where the proton transfer reactions occur between H
3
O
+
and the compounds of interest
in ambient air. The reactor consists of a series of stainless steel rings separated by thin
isolating teflon rings. The steel rings are connected by resistors so that a voltage of up to
600 V can be applied over the entire set of rings to obtain a homogenous electric field.
Ambient air is pumped through the drift tube which is maintained at 2 mbar (200 Pa)
pressure. The residence time of air in the drift tube is about 1 second. During this time
Mass Spectrometric Methods for Atmospheric Trace Gases 251
Pump
Pump
Pump
Hollow
cathode
H
2
O vapor
inlet
Ambient
air inlet
Drift tube
600 V, 2 mbar
Secondary electron
multiplier
Quadrupole
mass
filter
Figure 5.12 A schematic diagram of the PTR-MS.
H
3
O
+
ions are accelerated through the drift tube containing ambient air and ionising
reactions may occur. As we have seen in Section 5.4.1, H
3
O
+
ions can form clusters with
water of the type H
3
O
+
H
2
O
n
. The distribution of the clusters is highly dependent on
the sample humidity. More humid conditions promote larger clusters. Their presence
complicates the mass spectra and for some species it can lead to a strong humidity
dependence in the calibration (see Section 5.4.1). This is because some trace gas species
react with H
3
O
+
but not with H
3
O
+
H
2
O
n
ions, an effect that has been shown to be
important for non-polar compounds such as benzene (Warneke et al., 2001). The drift
tube electric field is applied to increase the average kinetic energy of the ions to prevent
clustering so that in PTR-MS, H
3
O
+
is the predominant reagent ion. The disadvantage
of the electric field is that it effectively limits the reaction time and pressure in the
reaction chamber, making the PTR-MS technique less sensitive than API-CIMS. From
the drift tube the ions are directed to a quadrupole mass filter held at 10
−5
bar by a turbo
molecular pump.
Quantification is based on the difference in counts between ambient air and air that has
been scrubbed of organics by passing it through a heated trap containing a platinised wool
catalyst. The interpolated background signal before and after an ambient measurement
is then subtracted from the ambient signal to derive a mass signal corrected for any
background contamination originating from the mass spectrometer. As the effectiveness
of the mass filter and detector is mass dependent the ion counts must be corrected for this
transmission difference. The transmission as a function of mass can readily be determined
by sequentially injecting high concentrations of species covering the entire mass range.
At sufficiently high concentrations all the reagent ions (e.g. H
3
O
+
) are converted to
product ions (e.g. CH
3
COCH
+
3
. The ratio of the initial reagent ion counts per second
(typically 1 ×10
6
to the acetone counts indicates the relative transmission of the two
