Hawkes P.W., Spence J.C.H. (Eds.) Science of Microscopy. V.1 and 2
Подождите немного. Документ загружается.

Chapter 5 High-Speed Electron Microscopy 425
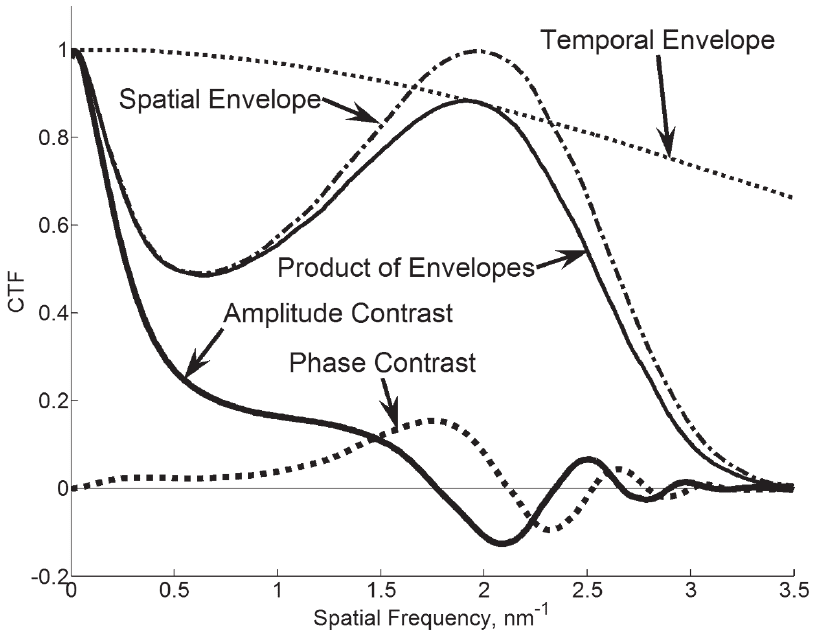
Figure 5–9. Phase and amplitude contrast transfer functions (CTF’s) as simulated by the commercial
software package Java EMS [68] for the following parameters: E = 100 keV, DE = 5 eV (the maximum
allowed by the software), CS = CC = 2 mm, convergence semi-angle 10 mrad, defocus 105 nm, no objec-
tive aperture. The cross-interaction between convergence angle and aberrations is primarily respon-
sible for the low contrast. Phase contrast will be very weak, however amplitude contrast mechanisms
should produce reasonable images with better than 1 nm resolution under the assumptions in this
model. Therefore the effects that limit DTEM resolution must be among those not included in this
simulation, which was designed for conventional TEM.
426 W.E. King et al.
very important to control the convergence angle to obtain a good
DTEM image. Reasonably high-quality selected area diffraction
patterns have been achieved in DTEM over areas of some tens of
micrometers,
69
indicating that the convergence angles can be kept to
reasonable levels.
In short, the estimated resolution limit accounting for all known
(and measured) effects in the gun, accelerator, condenser system, and
objective lens would appear to be ∼1 nm, dramatically better than is
achieved experimentally. This suggests that the limitation is in one of
the effects not included in conventional TEM simulations; this also
suggests that if the limiting factor can be identifi ed and eliminated, a
great improvement in resolution should be possible. The above argu-
ment indicates that the culprit should be either in the sample, in the
postsample lens systems, or (as indicated in Domar and Bostanjoglo
52
)
in the camera (including detection statistics). The balance of this section
will discuss some of these effects.
Consider the interaction of a very intense beam with a solid sample.
In conventional TEM, 1 µA would be an extremely high current at the
sample; this corresponds to roughly 1 electron per 160 fs, with an axial
spacing of ∼33 µm at 200 keV. Plasmon lifetimes are in the femtosecond
range, while TEM samples are usually less than 1 µm thick, so each
electron encounters a sample that has had time to relax since the last
electron passed through it (apart from heating and radiation damage).
DTEM currents may be four orders of magnitude larger. More than one
electron will be in the sample at a time, and at high fl uences each
electron may encounter multiple previously excited plasmons on its
way through the material. The theory of electron–sample interactions
in the electron microscope always makes the one-electron-at-a-time
approximation. This theory may have to be modifi ed for the DTEM.
Further, a great deal of energy will be deposited in a region highly
localized in time and space, which raises the possibility of new radia-
tion damage mechanisms. It is not clear at this time whether and how
these effects might worsen the image resolution.
The abnormally high currents in the DTEM may also affect the
behavior of the intermediate lens system, specifi cally through elec-
tron–electron interactions in the intense crossovers. We have performed
rough estimates of the various effects involved (space charge, Boersch,
and stochastic particle displacement), following the formalism of Kruit
and Jansen.
65
Space charge effects can change the effective focal lengths and aber-
ration coeffi cients of each of the lenses. Space charge defocusing would
merely require a readjustment of the lens currents and a possible reca-
libration of the microscope in pulsed mode. The experience at TU
Berlin suggests that this is not a major issue. Estimating the effects of
space-charge-induced spherical aberration is somewhat more diffi cult
and has not yet been performed in detail. Part of the problem is that
this effect vanishes to fi rst order for a circular beam of uniform inten-
sity,
65
so any associated distortions would have to be driven by nonsta-
tistical inhomogeneities in the beam intensity (including those due to
Chapter 5 High-Speed Electron Microscopy 427
the image contrast itself). Little more can be said without extensive
modeling, but fortunately the relevant models are already well devel-
oped in other contexts.
66
The Boersch effect acts to increase the energy spread of a charged
particle beam by coupling the lateral and longitudinal degrees of
freedom via statistical electron–electron interactions.
64–66
This process
will saturate when all degrees of freedom are, in some sense, in thermal
equilibrium. Saturation energy spreads depend on accelerating volt-
ages and convergence angles.
66
Rough estimates suggest that this effect
has adequate time to saturate in the early part of the column, and that
it may well be responsible for much of the energy spread measured by
Bostanjoglo et al.
28
[a very rough estimate of the saturation energy
spread based on assumed parameters yields ∼10 eV, compared to the
measured 8.7 eV, while the original authors used Loeffl er’s formalism
(validated to within ∼20%)
70
to estimate a value of 7.6 eV]. In any case
it would seem that the maximal effect of chromatic spread would
already have occurred in the objective lens (with a resolution limit
∼1 nm), so that it seems doubtful that chromatic aberration in the inter-
mediate lenses is to blame for the observed resolution limit of
∼200 nm.
This brings us to the trajectory displacement effect,
65,66
which slightly
and randomly defl ects electrons in and near a crossover. Suppose we
take an intermediate lens with focal length 24 mm forming an image
of radius 0.1 mm at a distance of 60 mm, with a crossover at 27 mm. The
divergence angle α
0
is therefore 3 mrad. These values might come up
between the fi rst and second intermediate lenses of a typical TEM in a
moderate-magnifi cation imaging mode, with an illuminated area of
1 µm at the sample. Suppose 10
8
electrons are in a pulse of duration 1 ns,
for a current of 16 mA at 200 keV. Using the formulas quoted by Kruit
and Jansen,
65
we fi nd that we are in the Gaussian regime, with an
average random trajectory displacement of 2.2 µm at the crossover and
an estimated 5 µm at the image plane. The result will be an image blur
much like what happens in projection electron lithography systems.
Referencing this displacement back to the sample plane yields a resolu-
tion limit of ∼50 nm due to this crossover alone, assuming that the rest
of the optical system faithfully transfers this image plane to the screen.
This is a very signifi cant limitation to the resolution (comparable to the
quoted resolution limit of ∼200 nm in current instruments
52
), yet it must
be taken as only a very rough estimate of the effect in a real TEM
column. This resolution limit depends strongly on all relevant param-
eters, including the positions, intensities, and convergence angles of all
crossovers, the positions and degrees of magnifi cation at every image
plane, and the beam current and voltage. This means that (1) much
more involved calculations and experiments are required if we are to
understand if this is truly the resolution limit, and (2) if it is the resolu-
tion limit, then it may be possible to dramatically reduce its effects by
changing the lens excitations in the intermediate/projector lens system.
The idea would be to avoid intense crossovers with small convergence
angles at high-leverage parts of the column. A simple way to test the
428 W.E. King et al.
hypothesis would be to take the same pulsed images with and without
a small selected-area aperture. If trajectory displacement is the resolu-
tion limit, then when the aperture is inserted the electrons from outside
the region of interest will no longer be present at the intermediate lens
crossovers, and the effect should be greatly reduced.
We should also remember that the sample may well be moving,
rapidly, during the exposure. A phase front or shock wave moving at
roughly the speed of sound in the material may move several microm-
eters during a few-nanosecond electron pulse. The sample may also be
distorting from local heating or moving vertically (out of focus) due to
the effects of the pump laser. These effects must be considered in the
design of experiments. However this does not appear to be the resolu-
tion limit in the experiments to date; roughly the same resolution is
obtained in test shots on a static sample.
In summary, the observed resolution limit in DTEM may arise from
a combination of intermediate crossover interactions, motion blurring,
and low signal levels, while the effects that limit resolution in conven-
tional TEM would suggest a pulsed image resolution ∼1 nm. Various
experiments are proposed that may identify the true culprits, while
some methods of mitigating their effects are suggested. This is the
present state of knowledge at the time of writing; the near-future evolu-
tion of the understanding of these effects is likely to be rapid.
3.3 Damage
Due to the inelastic scattering of electrons in TEM, a large amount of
energy (typically some 10s of electron-volts per primary electron) is
deposited in the sample, and in many cases damage due to energy
deposition is a limiting factor in obtaining high-resolution images. For
continuous electron exposure used in standard TEM, damage is lin-
early related to the total radiation dose,
71,72
but to fully understand the
impact of damage on image quality in DTEM, the effects of higher dose
rate and the time-dependent nature of radiation damage must also be
considered. At this point, we do not know enough about either of these
subjects to confi dently predict the damage-related resolution degrada-
tion in a DTEM image. Nevertheless, here we briefl y review what is
known as a baseline for further investigation.
The consequences of energy deposition for TEM images depend
strongly on the nature of the sample. Although a wide variety of inor-
ganic and metallic samples can be readily imaged, sometimes with
very high resolution and contrast,
73
damage is the most signifi cant
limiting factor for imaging less robust samples such as biomaterials.
Although damage in solid-state material due to direct atomic displace-
ment can be signifi cant at high beam currents,
74
typically this is appre-
ciable only for a large integrated number of primary electrons incident
on the sample and can be avoided (in standard TEM) through the use
of a primary electron energy that is less than a sample-dependent
threshold. For a current density of 10
5
A/cm
2
through a carbon fi lm
sample at between 100 and 200 keV, the sputtering rate is roughly
10 nm/s.
74
Although the instantaneous current density in DTEM could
Chapter 5 High-Speed Electron Microscopy 429
be considerably higher than this (as high as 10
7
A/cm
2
in future instru-
ments), this current density will be sustained only over nanoseconds.
Assuming the sputtering rate is linearly related to the current density
(which may not be the case for DTEM), sputtering would be insignifi -
cant for less than millions of shots.
Since the time scale of the electron pulse can be comparable to the
thermal diffusion time, sample heating is a more signifi cant issue for
DTEM than standard TEM, but this depends strongly on the sample
and beam properties. The deposited energy density can be very large.
For water irradiated by ∼200 keV electrons at a density of 10 electrons/
Å
2
(a typical density for cryoEM) in a single pulse, straightforward
estimates of the inelastic cross section
71
result in a deposited energy
density of about 300 eV/nm
3
, about 10 eV per water molecule! For a
Gaussian beam with 1/e radius of 100 nm and 1/e half width of 1 ns in
water, naively assuming only standard thermal diffusion using room
temperature constants, the deposited energy density will reach 90% of
the value it would have with no diffusion. The situation is signifi cantly
better with the lower heat capacity and larger thermal conductivity
found in metals. With the same beam properties, the energy density
in an aluminum sample will reach only 5% of the zero diffusion energy
density. These results depend strongly on the pulse duration. A pulse
of suffi ciently short duration (less than 10s of picoseconds for a 100-nm
radius beam in aluminum) will still overwhelm diffusion within this
model. At a suffi ciently short pulse duration and electron density,
sample heating may result in sample melting and lowering the atomic
displacement energy threshold. Nonetheless, questions remain as to
the actual state of the material at these early times and high tempera-
tures—it is unlikely that any standard assumptions about thermal
transport or material stability would still apply.
Biomaterials (such as proteins) are especially ill suited to examina-
tion via TEM due to their native aqueous environment, caustic species
created in the irradiation of water, low contrast, intrinsic radiolytic
susceptibility, and the relatively weakly bound tertiary and quaternary
structures of proteins. The “lifetime” of irradiated protein crystals (i.e.,
the amount of time a crystal may be irradiated until its diffraction
pattern fades) is a well-documented obstacle
75
to high-resolution protein
structural characterization. Yet, biology would benefi t immensely from
the ability to effi ciently image proteins at high resolution. Knowledge
of the detailed functioning of biomolecules and biomolecular com-
plexes is essential to our understanding of the functioning and evolu-
tion of life.
76
Here we focus on the imaging of biomaterials, since this
application has great scientifi c benefi t as well as being very challeng-
ing. Damage mechanisms would be simpler for just about any other
condensed-state sample.
Specifi cally, how much will damage degrade the resolution of a
DTEM image over the time scale of the electron pulse? More optimisti-
cally, is it possible to effectively mitigate the effects of damage with
respect to a high-resolution image by using a short electron pulse?
Such an idea has already been proposed for imaging with X-rays,
77
where single particle diffractive protein imaging is thought to be
430 W.E. King et al.
possible using a 10-fs duration X-ray pulse. Although pulsed imaging
ultimately results in the destruction of the sample, the idea is to
obtain the image before damage has signifi cantly distorted the
structure being imaged. An incidental, yet highly benefi cial product of
this scheme is the ability to time-resolve structural changes, enabling
the direct visualization of nanobiostructural dynamics—a molecular
movie.
9
An analogous imaging scheme using electron pulses presents both
advantages and challenges. Electrons deposit much less energy per
inelastic scattering event than X-rays: less than 50 eV per event for
electrons
71
versus approximately 10 keV for X-rays, and the ratio of
inelastic to elastic scattering events is roughly a factor of three smaller
for electrons.
9
This results in about 600–1000 times less energy depos-
ited in the sample per unit signal for electrons compared to X-rays.
Furthermore, electrons scatter with 10
4
greater probability than X-
rays, allowing imaging with a much lower electron density. Yet, elec-
trons are electrically charged fermions, which (as discussed in the
sections on photoelectron guns and resolution) limits the fl uence at
the sample and both the spatial and temporal resolution. The chal-
lenge of pulsed electron microscopy will be to obtain a pulse of suf-
fi cient quality and electron density to obtain a high-resolution image,
which is also short enough to avoid damage to the sample during the
pulse.
Hydrodynamic modeling of single particle diffractive imaging
with electrons (in analogy with the X-ray method above)
78
indicates
that 4 Å resolution is possible for a molecule of 10 nm radius with
100 keV electrons at a density of 10
7
electrons/(100 nm)
2
, with a pulse
width of 2 ps. Using the same model for the X-ray case and suffi cient
photon density to achieve the same resolution, an X-ray pulse of 2 fs
is required. These results are reasonably consistent with expectations,
giving a difference in the maximum acceptable pulse length of 1000,
exactly the ratio of deposited energy per unit signal between the X-
ray and electron cases. These single particle models consider “naked”
molecules only where the damage mechanism is Coulomb explosion.
For the X-ray case, damage by this mechanism is the result of
ionized electrons exiting the sample and leaving a net positive charge,
resulting in rapid sample distortion due to electrostatic repulsion.
Charging is much less severe for an electron probe due to the much
lower kinetic energy of ionized secondary electrons. Also, it is likely
that damage will occur on a slower time scale in solution, where the
dispersion of molecules will be impeded by the presence of solution
and secondary electron transit will be signifi cantly reduced, resulting
in less charging. In this case, the primary damage mechanism may
be radical chemistry and diffusion, rather than Coulomb expansion.
Although a 2-ps electron pulse width is probably beyond the capabil-
ity of DTEM using standard electron guns, sub-100-ps pulse widths
are not inconceivable at the required electron density. Attaining the
necessary spatial and temporal coherence for diffractive imaging
using such a pulse will probably be very diffi cult but perhaps within
the reach of foreseeable improvements in electron gun technology.
Chapter 5 High-Speed Electron Microscopy 431
Although atomic resolution may be challenging, electron gun modi-
fi cations to shorten the pulses into the 10s of picoseconds may well
allow very effi cient imaging of protein complexes at suffi cient resolu-
tion to signifi cantly contribute to our understanding of their struc-
ture and dynamics. Furthermore, the RF accelerator community is
aware of the potential for ultrashort, high brightness electron guns
in electron microscopy.
30
Such sources do not currently exist, but if
these were developed, they could very signifi cantly improve our
ability to rapidly image biomolecules.
Currently, there is very little information directly concerning biosa-
mple radiolysis damage at the very high current densities that will be
found in DTEM. Although simulations such as those described above
can provide us with some insight into the damage process, they do not
consider the effect of an aqueous solution surrounding the sample. It
is likely that a surrounding solution will signifi cantly alter the damage
process and may impede the physical diffusion of a damaged biosam-
ple long enough to obtain a high-resolution image with an electron
pulse that is longer than 2 ps. Given the available information, it is
diffi cult to speculate. Nonetheless, here we attempt to approach the
problem based on what is known.
First, we consider standard TEM. In some cases, the diffi culty in
imaging biomaterials may be overcome with standard TEM via any
of three strategies, which all depend on the mitigation of damage:
increasing sample contrast (via, for example, staining), increasing
the sample damage threshold (as in cryoelectron microscopy
75
), or
reducing the dose per unit sample (as in diffraction and cryoEM)
with signal averaging over many samples. Of these techniques,
cryoEM most closely approximates imaging of biosamples in a native
environment, since this method does not require contrast enhance-
ment. In cryoEM, a sample is fl ash frozen such that the embedding
ice is vitreous rather than crystalline.
79
This minimally distorts the
sample and reduces diffractive background signal in the image. In
addition, cooling of the sample to cryogenic temperatures increases
the radiation tolerance by factors of thousands
75
with respect to
image-manifested damage. Although irradiation may have caused
the conditions under which sample damage would occur, the physi-
cal diffusion that would make this apparent in an image does not
occur when the sample is frozen in place.
74
This result indicates that
molecular diffusion, and temperature and phase-dependent chemi-
cal reactions play a role in damage,
74,75
and that damage resulting
from direct atomic displacement is insignifi cant at the doses (1–10
electrons/Å
2
) used in cryoEM.
To further establish the relevance of diffusion in damage, an early
time diffusional dependence is indicated by electron energy loss exper-
iments that demonstrate an orders of magnitude increase in the damage
threshold with reduced excitation volume at equivalent or greater irra-
diation density. Varlot et al.
80
observed a change in damage threshold
of 10
4
between a 100 nm and 0.7 nm radius cylindrical excitation volume
irradiating polymers. Their analysis did not attribute the change in
damage threshold to temperature effects and they speculated that dif-
432 W.E. King et al.
fusion of damaged molecules (e.g., free radicals) might explain the
effect. Siangchaew et al.
81
also observed a substantial increase in the
damage threshold of polyethylene using a small electron probe. They
speculate that the increase in damage threshold is due to fast second-
ary electrons (of energy >50 eV) exiting the 100-nm-diameter probe
region. These electrons create damage collateral to the probe region,
but leave the probe region relatively pristine. Egerton et al.
74
disagree
with this interpretation, asserting that (for polyethylene) the number,
range, and energy of fast secondary electrons (FSEs) should result in
75% of the FSE energy being deposited within 2 nm of the probe beam.
Slow electrons have longer mean free paths and may exit a small probe
region,
82
but it is not known how damaging these low-velocity elec-
trons would be to organic samples.
Diffusion also plays a primary role in radiolysis damage. In an
aqueous environment, radiolysis damage is complex and involves
many disparate processes. The physical excitation of secondary elec-
trons through inelastic scattering is followed by electron thermaliza-
tion and diffusional transport, and trapping occurring over a time
scale on the order of 1 ps, along with the initial generation of radicals.
This is followed by equilibrium with nuclei, which occurs in a few
picoseconds. At longer time scales, chemistry begins to occur. Ioniza-
tion and energy deposition in the fi rst stage result in a transient dis-
tribution of radicals and other species (including H
+
, OH, e
aqu
, and H
2
O
2
in water), which then diffuse and react with solutes and each other,
reaching a homogeneous chemistry limit within micro- to millisec-
onds.
83,84
With respect to solute interactions, pulse radiolysis studies
indicate that the primary damage mechanism in DNA is attack by
radiation-created hydroxyl radicals
85
and based on simulations, the
time scale of this process is thought to be hundreds of femtoseconds,
86
assuming low deposited energy density.
These data seem to indicate that the time scale for image manifested
damage of a biomolecule in aqueous solution would be no faster than
a few picoseconds, possibly much longer. Nonetheless, these data do
not directly address the question. Although pulse radiolysis studies
have provided a signifi cant amount of information,
87
this information
generally concerns the time domain behavior of irradiated water (cf.
time domain spectroscopy) and stoichiometric chemistry. Such data
and corroborating simulations
83,84
can be used to aid in the understand-
ing of the diffusion of energy and radiolytic species in water, but ulti-
mately they do not tell us about structural damage in solutes,
particularly within a few picosecond time scale. More specifi c experi-
mental studies of biomolecules such as DNA address damage, but
again, this typically provides information regarding the chemistry of
damage (typically via radiation-induced radicals) and the functional
viability of irradiated molecules without reference to detailed
structure.
85,88
Furthermore, the energy deposition used in pulse radi-
olysis studies is much lower (∼10 Gr)
89
than what is used in TEM
(>10
7
Gr),
75
and the excitation volume in TEM is correspondingly much
smaller. As a result, transport and relaxation of secondary electrons
71
and high-dose rate effects
90
will probably play a signifi cant role in the
Chapter 5 High-Speed Electron Microscopy 433
damage process in DTEM. These issues are not as signifi cant for con-
tinuous irradiation in conventional TEM since thermal diffusion occurs
on a faster time scale than the average time between electrons tran-
siting the sample. The instantaneous energy density in the DTEM will
be much higher, and these data do not provide information in this
regime.
Finally, we note that there is actually some empirical evidence that
damage will be signifi cantly reduced by the use of pulsed electron
exposure. Fryer
91
observed damage thresholds of 90–100 e/Å
2
using
10- to 100-ms pulses to image monolayer fi lms of aromatic hydrocar-
bons. Using continuous exposure, the damage threshold for these
samples was 3–4 e/Å
2
, comparable to the damage threshold for proteins
in cryoEM. This 22–25% improvement was observed for much longer
pulses than we will use in the DTEM and points in the direction of
trying even shorter pulses.
Unfortunately, no currently available data directly address the ques-
tion of resolution smearing in DTEM due to damage from short pulses
of electrons. As far as damage is concerned, currently available simula-
tions and empirical results do not unambiguously exclude the possibil-
ity of molecular bioimaging with short electron pulses, but they do not
clearly indicate that it is possible, either. As with many aspects of
DTEM, the development and use of the instrument will ultimately
provide these answers.
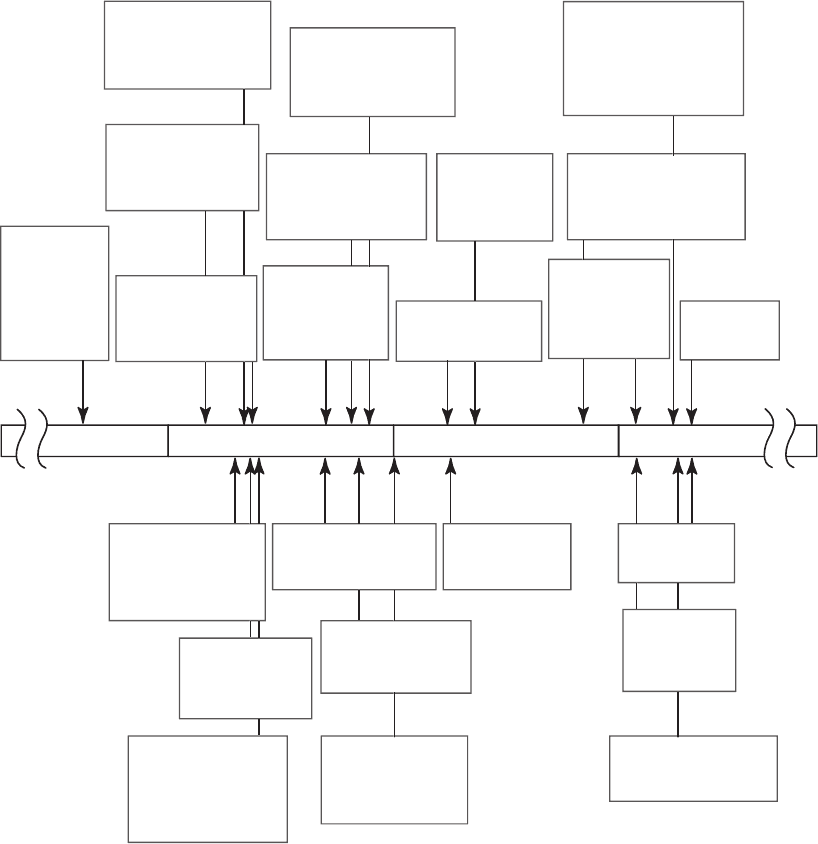
4 DTEM Applications
We include a brief historical review that attempts to put electron
imaging in the context of work on electron diffraction. For the
purpose of this discussion, we limit the review to time resolutions of
micro seconds or less. Figure 5–10 provides a summary. In the mid-
1970s, Bostanjoglo et al.
16,92
developed a technique to produce strobo-
scopic images in TEM using beam blanking. In these pump-probe
studies, ultrasonics were used to pump samples that were studied
stroboscopically. This led to a number of papers studying fast
transitions.
93–101
Stroboscopic diffraction studies were carried out in the early
1980s.
102–104
Real-time gas-diffraction experiments were reported in
1984.
105
The fi rst picosecond time-resolved structural studies in the
solid state occurred in the early 1980s with a streak camera used as the
basis of a diffraction instrument.
1,3
This led to the fi rst picosecond time-
resolution observation of a phase transition in Al.
2
In the late 1980s,
pulsed photocathode,
106
multiframe movies,
107
and dynamic imaging
were demonstrated at TU Berlin.
108
In the 1990s there was work in gas-
phase energy transfer,
109
superheating of lead, and structural dynam-
ics.
110
The techniques were applied to complex transient phenomena
including dynamics of cyclohexadiene,
6
photo-dissociation,
111
direct
observation of ultrafast thermal lattice expansion (Ag) on picosecond
time scales,
112
melting of amorphous Si,
7
phase explosion in metal
fi lms,
113
and melting of Al.
114
434 W.E. King et al.
TEM with high time resolution began at TU Berlin in the 1970s with
stroboscopic imaging of periodic processes using a periodically
defl ected electron beam.
16,115
In this confi guration, time resolutions of
200 ps were achieved for periodic processes up to 100 MHz.
116
This
technique was applied to ultrasonically driven disruption of crystals
and magnetoelastic effects
92,115
and magnetic-fi eld-induced oscillations
of the domain magnetization, of domain walls, and of their substruc-
tures.
98,116,117
After 1980, the instrument was modifi ed to study nonpe-
riodic processes. That instrument operated in three modes:
1980s
1970s
1990s
2000s
Melting of
Si (few ps)
7
Stroboscopic
electron
microscope
(200 ps)
16
Superheating
of Pb (200 ps)
140
Multiframe
"movie" (<10 ns
with 25 ns frames)
107
Photoreaction in gas
phase (few ps)
139
Photo-
dissociation
(few ps)
111
Time-resolved
RHEED (200 ps)
141
Stroboscopic
time-resolved
LEED (10 ns)
103
Stroboscopic
time-resolved
diffraction (100 s)
104
First time-resolved
pump-probe
diffraction (20 ps)
2
Pulsed cathode
TEM (20 ns)
15
Reflection
microscope (20 ns)
14
Gas phase energy
transfer (15ns)
109
First dynamic
TEM images (20 ns)
15
Melting of Al
(600 fs)
114
Dynamics of
cyclohexadiene
(few ps)
6
Electron diffraction
of Al (100ps)
1
"Real-time"gas
diffraction
(16 ms)
105
Stroboscopic
time-resolved
electron diffraction
(100 ns)
102
Direct observation of
lattice heating and
diffusion (Ag) on ps
time scales (<400 fs)
112
Structural
dynamics
(1.4 ps)
110
Phase change in
clusters (1 s)
142
Phase explosion in
metal films (3 ns)
10
Figure 5–10. Timeline showing the parallel development of ultrafast electron diffraction and DTEM
technologies.
