Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

50 Charged Particle and Photon Interactions with Matter
produced in their excited states and emit radiation, they are easily detected. But, when the fragments
are in the ground or metastable states, they cannot be easily detected. Two examples of the detection
of
neutral fragments are given as follows:
3.3.4.2.1
Neutral Radical Measurement for CH
4
All electronically excited states of CH
4
are repulsive states, leading to dissociation. This implies that
in any plasma containing CH
4
, chemically active neutral molecules (or radicals) are formed with
high efciency. For example, we expect to have CH, CH
2
, and CH
3
in large quantities. However, it
is very difcult to measure these neutral fragments. Tanaka and his group have developed a new
technique to measure an absolute cross section for the production of CH
3
through electron-impact
dissociation of CH
4
. This method is based on the combination of the crossed-beam method and the
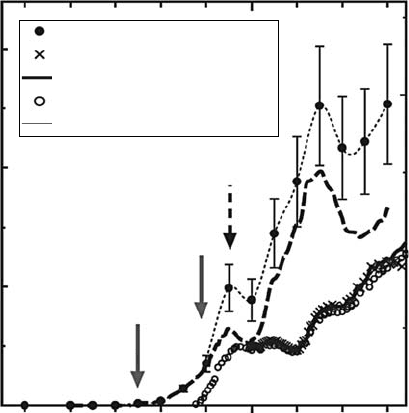
threshold ionization technique (for details, see the paper Makochekanwa etal., 2006). Figure 3.23
(Makochekanwa etal., 2006) presents the cross section measured. In this gure, the threshold for
the neutral CH
3
formation is observed at 7.5 ± 0.3eV. Comparing with photoabsorption data, the
authors conclude that all the excitations of CH
4
below 12.5eV predominantly result in dissociation
via
the production of CH
3
.
3.3.4.2.2
Production of OH Radicals in H
2
O
In radiation interaction with matter, OH radicals are of particular importance. It is a fundamental
question, therefore, that how many OH molecules are produced when electrons collide with water
molecules. Harb etal. (2001) measured the cross section for the production of OH in its ground state
through electron-impact dissociation of H
2
O. They applied the laser-induced uorescence technique
to detect neutral OH. To obtain an absolute cross section, they compared the channel of produc-
tion H + OH to that of H
−
+ OH. The absolute cross section of the latter process is available in the
literature. The resulting cross section is shown in Figure 3.3. It is noted that, compared with other
processes,
the OH(X) production has a large cross section.
1.5
1
0.5
3
T
2
1
T
2
E
thr
: Present work
: CH
4
neutral diss., Kameta et al.
: CH
4
photoab., Kameta et al.
: CH
4
photoab., Au et al.
: Electron–photon
0
6 8 10
Impact energy(eV)
Cross sections (10
–16
cm
2
)
Cross sections (10
–16
cm
2
)
12
0
0.5
1
Figure 3.23 Absolute cross sections for the production of CH
3
radicals upon electron collisions with
CH
4
. Also included are the photon impact results from the literature. For more details, see Makochekanwa
etal. (2006).
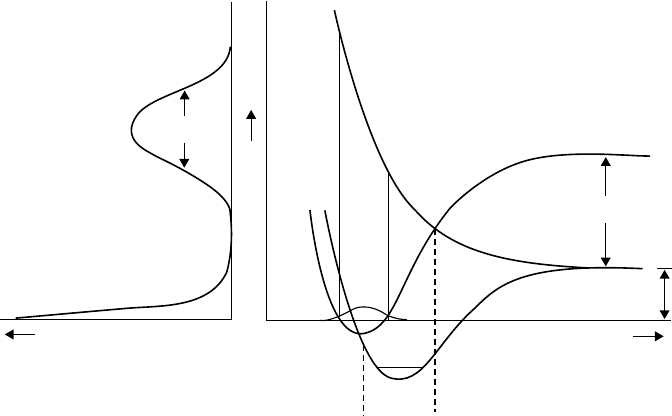
Electron Collisions with Molecules in the Gas Phase 51
3.3.4.3 negative-ion production
Electron interactions with biomolecules have been the subject of considerable interest, since it was
discovered that low-energy electrons can cause signicant DNA strand break (Boudaiffa etal.,
2000). Electrons with a specic energy readily attach to the molecular constituents of DNA to form
transient negative ions, which then dissociate to produce fragment negative ions and neutrals. This
bond-breaking process, called dissociative electron attachment, has been demonstrated to occur in
various
molecules, such as water, DNA bases, amino acids, and uorocarbons.
Dissociative
attachment is a kind of resonance process. It proceeds through a (doubly) excited
state
of the negative molecular ion in a way as follows:
e AB AB A+ B+ → →
− −
( )
**
The intermediate negative-ion state is unstable and called the “resonance state.”
Figure 3.24 shows one representative scheme of the DEA. In this case, the negative-ion state is
repulsive and crosses to the ground state of the neutral molecule at the internuclear distance R = R
c
.
When R is smaller than R
c
, the negative ion is unstable against the autodetachment of the electron
(i.e., AB
−
→ AB + e). Once R exceeds R
c
, dissociation to A + B
−
takes place automatically. When
theneutral molecule AB is initially in its vibrationally ground state, the attachment occurs only in
the Franck–Condon region. Depending on the steepness of the repulsive curve, dissociative attach-
ment has a nite value of the cross section only in a very narrow range of electron energy. When the
negative-ion state has an attractive potential, dissociative attachment can occur through the excita-
tion to the vibrational continuum of the negative ion.
Electron attachment to a molecule is studied by crossing an electron beam with a molecular
beam. The negative ions formed are recorded mass spectrometrically as a function of the electron
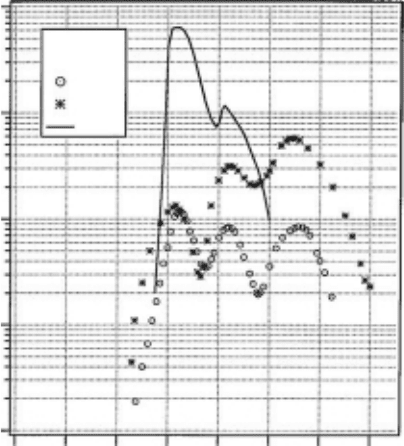
energy. Figure 3.25 (Itikawa and Mason, 2005) shows the cross sections of the dissociative attach-
ment to H
2
O to produce three different ions (H
−
, O
−
, and OH
−
). This is cited from the review article
by Itikawa and Mason (2005). After the publication of this article, a new measurement has been
reported by Rawat etal. (2007). Their results are a little, but not much, different from the values
shown
in Figure 3.25.
Franck–Condon
region
B
–
AB
–
A+B
–
A+B + e
–
ε
aff
ε
diss
R
e
R
c
Capture cross section
Nuclear separation
Energy
Γ
d
Figure 3.24 Potential diagram of a diatomic molecule for the mechanism of dissociative attachment.
52 Charged Particle and Photon Interactions with Matter
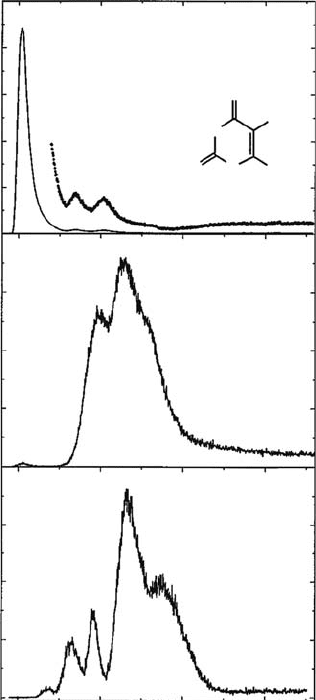
One example of the DEA study of biomolecules is given here. Figure 3.26 shows the electron
energy dependence of the yield of a variety of negative-ion fragments, induced by a resonant attach-
ment of subionization electrons to thymine (Huels etal., 1998). To produce an effusive molecular
beam, high-purity (>99.5%) thymine is sublimated at 120°C–180°C, i.e., well below its decomposi-
tion temperature of about 320°C. The molecular beam is crossed at right angles with an electron
beam generated by a trochoidal electron monochromator, and the negative ions produced are mass-
analyzed by a quadrupole mass spectrometer. Table 3.1 (Huels etal., 1998) summarizes the peak
positions observed, and Figure 3.27 (Huels etal., 1998) shows the channels for the production of
each ion from gaseous thymine. This subject has been extended to the studies of fragmentation of
elementary compounds from condensed H
2
O, hydrated DNA, sugar analogues, oligonucleotides,
and
so on. For further information about these DEA fragmentations, see Sanche (2005).
3.3.5 colliSion proceSSeS involving vibrationally excited MoleculeS
In most of the experiments of electron–molecule collision, target molecules are in their (vibra-
tionally and electronically) ground states. In the nature, there are many molecules in their (par-
ticularly, vibrationally) excited states. Sometimes, the interaction between electrons and excited
molecules is signicantly different from that of the ground-state molecules, leading to a new subject
of study in atomic and molecular physics (Christophorou and Olthoff, 2001). One typical example
is the DEA. The DEA process is very sensitive to the internal degrees of freedom of the initial
molecule (Christophorou and Olthoff, 2001). The distribution of the rotational–vibrational states of
molecules depends on the temperature of the molecular gas. Hence, the cross section of the DEA
depends sensitively on the temperature of the target molecular gas (Christophorou et al., 1984;
Ruf etal., 2007). For other collision processes, little has been known about the target-temperature
dependence of the cross section. It is usually difcult to perform any experiment of electron colli-
sions with vibrationally excited molecules. This is mainly due to the difculty in producing excited
10
–17
10
–18
OH
–
e+H
2
O
diss. attach.
O
–
H
–
10
–19
10
–20
10
–21
0 2 4 6
Electron energy (eV)
Cross section (cm
2
)
8 10 12 14
Figure 3.25 Dissociative attachment cross sections for H
2
O. Partial cross sections for the production of
H
−
, O
−
, and OH
−
are shown. (Reprinted from Itikawa, Y. and Mason, N.J., J. Phys. Chem. Ref. Data, 34, 1,
2005.
With permission.)
Electron Collisions with Molecules in the Gas Phase 53
species in a sufcient number density. Vibrationally excited molecules can be produced by heating,
laser
photoabsorption, or electron bombardment of the molecule.
The
rst quantitative experiment to study low-energy electron scattering from vibrationally
excited CO
2
was performed by Buckman etal. (1987). They measured the TCSs as a function of
temperature using a linear attenuation TOF spectrometer. They observed a substantial increase in
the TCS at electron energies below 2eV, which they attributed to the enhanced scattering by a bent
CO
2
. No signicant change in the TCS was observed at higher energies. Ferch etal. (1989) repeated
the experiment of Buckman etal., also using a TOF spectrometer, and conrmed the earlier result.
They also found a signicant change in the resonance structure of the TCS at around 3–5eV, due to
vibrational
excitation of the target molecule.
A
DCS measurement for an electron collision with hot CO
2
was performed by Johnstone etal.
(1993), but only at one energy point (4eV). Johnstone etal. (1999) repeated the experiment at 3.8eV.
Recently, a similar but more extensive experiment was performed by Kato etal. (2008b). They cov-
ered the collision energies in the range of 1–9eV. This includes the resonance region around 3–5eV.
100
80
60
40
20
0
1.2
0.8
0.4
0.0
0.6
0.4
0.2
0.0
0 5 10
O
–
from
thymine
CN
–
from
thymine
[T]
–
from thymine
(×10)
HN
T
O
O
N
H
H
(a)
(b)
(c)
CH
3
Incident electron energy (eV)
Mass-selected negative ion yield (10
3
counts)
15
Figure 3.26 Yields of representative negative ions produced in the electron irradiation of gas-phase
thymine, as a function of incident electron energy. Panel (a) shows the yield of the negative ion of the
parent molecule (thymine), panel (b) is the CN-yield, and panel (c) is the O-yield. (Reprinted from Huels,
M.A. etal., J. Chem. Phys., 108, 1309, 1998. With permission.)
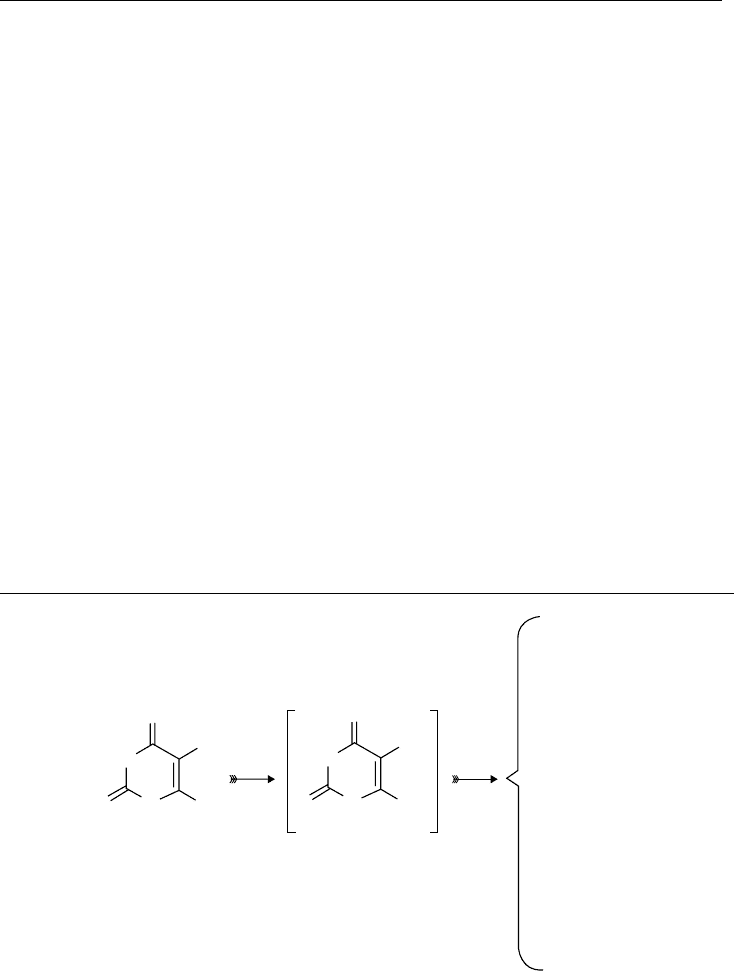
54 Charged Particle and Photon Interactions with Matter
They obtained absolute cross sections for the inelastic (i.e., vibrational excitation) and the super-
elastic (i.e., vibrational de-excitation) transitions of CO
2
in its rst excited state of the bending mode
of vibration (as shown in Figure 3.28). The resonance structure of these cross sections has been
revealed for the rst time. This may be helpful in theoretically understanding the resonance in the
electron–molecule collision. Further extension of this kind of study is desirable also from the point
of view of application. It is well known that vibrationally excited molecules are abundant in the
upper atmosphere of the Earth and other planets and discharge plasmas.
table 3.1
peak
p
ositions
(in e
v,
with u
ncertainty
of ±0.15
ev)
of the y
ield
of n
egative
Fragment
i
ons
from electron Collisions with t
hymine
and Cytosine
thymine (t)—C
5
h
6
n
2
o
2
–126 amu
t
−
oCn
−
Cn
−
oCnh
−
o
−
h
−
oCnh
2
−
Ch
2
−
0.18 0.20* 0.28* 1.8
3.4 2.8* 2.8* 3.2
4.6 4.8 4.6 4.5 4.0 4.6
5.2 5.8* 6.0* 5.2
6.4 6.6 6.6 6.6
7.7 7.8 8.0 8.7 8.0*
Cytosine
(C)—C
4
h
5
n
3
o–111 amu
C
−
oCn
−
h
−
C
4
h
3
n
3
o
−
Cn
−
o
−
or nh
2
−
C
4
h
5
n
3
−
or C
4
h
3
n
2
o
−
C
3
h
3
n
2
−
(0.1*)1.4 1.0 (0.1)1.4 1.5 1.2 2.3*
3.8 4.5
5.3 5.0 5.6 5.3* 5.1 5.2 5.2
6.9* 7.4 7.3 7.3*
7.7 7.8 8.2*
9.5 9.2
Source: Reprinted
from Huels, M.A. etal., J. Chem. Phys., 108, 1309, 1998.
With
permission.
Note:
The
asterisks indicate a faint peak or a shoulder.
O
O
HN
7 reaction pathways with
7 anion and associated
neutral fragments
N
H
H
T
CH
3
OCN
–
+ {C
4
H
5
NO + H}
CN
–
+ {C
4
H
5
NO + O + H}
OCNH
–
+ C
4
H
5
NO
OCNH
2
–
+ C
4
H
4
NO
O
–
+ C
5
H
6
N
2
O
H
–
+ C
5
H
5
N
2
O
2
CH
2
–
+ {C
4
H
3
N
2
O
2
+ H}
+ {C
4
H
4
NO
2
+ H + H}
*
–
O
O
HN
N
H
H
T
CH
3
e
–
+
Figure 3.27 Pathways of producing negative fragment ions from electron collisions with thymine.
(Reprinted
from Huels, M.A. etal., J. Chem. Phys., 108, 1309, 1998. With permission.)
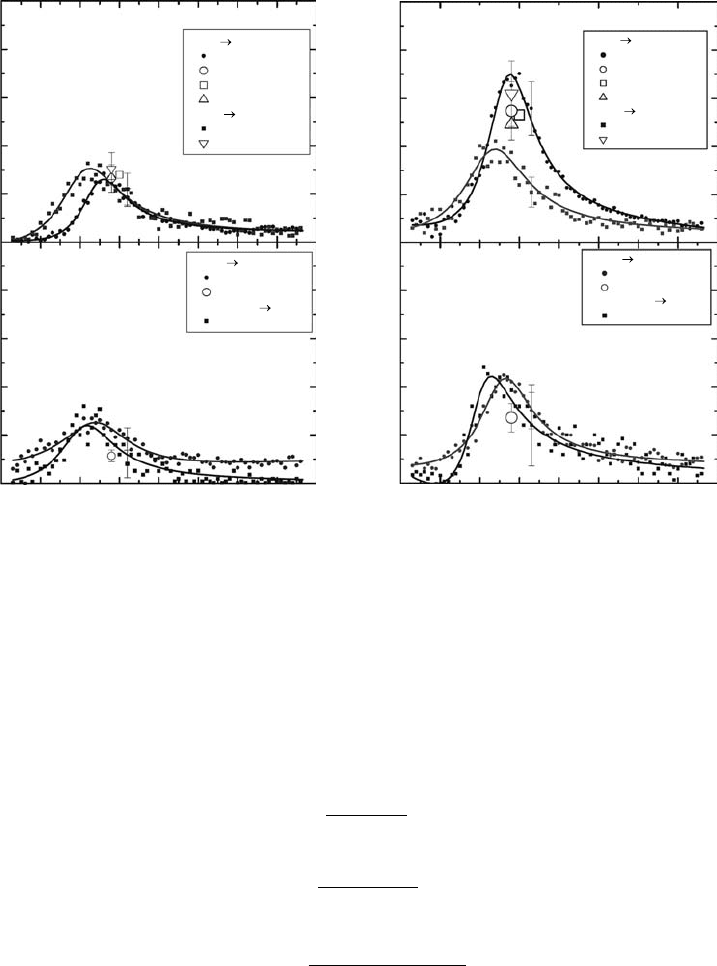
Electron Collisions with Molecules in the Gas Phase 55
3.3.6 coMplete colliSion dynaMicS in (e, 2e) experiMentS for h
2
o
As described in Section 3.3.4.1, partial and total ionization cross sections are of great importance
for practical applications, but these provide only limited insight into the ionization process itself.
Conversely, a great deal of information may be obtained from the study of DCSs (Märk, 1984), that is,
SDCS :
,d T W
dW
σ
( )
DDCS :
( , , )d T W
dW d
2
σ θ
Ω
Triple DCS :
( , , , , )d T W
dW d d
3
1 2 2
1 2
σ θ θ ϕ
Ω Ω
where
W
is the energy
θ
and φ are the polar angles of detection of the electrons after collision (see Figure 3.29; Märk, 1984)
In addition to this electron–electron spectroscopy, an ion kinetic energy spectroscopy and the mea-
surement of angular distributions of fragment ions are necessary for a full picture of the ionization
event. It is outside the scope of this chapter to give a detailed description on the subject, but a brief
comment
about the recent advances is given here.
0.25
0.20
0.15
Angle=30°
(a) (b)
0.10
0.05
0.00
0.20
0.15
0.10
0.05
0.00
0.25
0.20
0.15
0.10
0.05
0.00
0.20
0.15
0.10
0.05
0.00
1 2 3 4 5 6
Impact energy(eV)Impact energy(eV)
DCS (10
–16
cm
2
/sr)
DCS (10
–16
cm
2
/sr)
7 8 9 1 2 3 4 5 6 7 8 9
Present work
Present work
Johnstone et al.
Johnstone et al.
Register et al.
Antoni et al.
Present work
Present work
Johnstone et al.
(000)
(010)*
(010)
(010)*
(000)
(020)(100)
(020)*
(100)* (010)*
Present work
Johnstone et al.
Present work
(010)*
(000)
(020)*
(100)* (010)*
Angle=60°
Present work
Johnstone et al.
Register et al.
Antoni et al.
Present work
Johnstone et al.
(000) (010)
(010)*
(020)(100)
Figure 3.28 Vibrational excitation functions for the inelastic (000)→ (010) and (010) → (020) (100) and
the superelastic (010) → (000) and (020) (100) → (010) transitions of CO
2
at impact energies of 1–9 eV and at
scattering angles of (a) 30° and (b) 60°. See Kato etal. (2008b) for further details. (Reprinted from Kato, H.
etal.,
Chem. Phys. Lett., 465, 31, 2008b. With permission.)
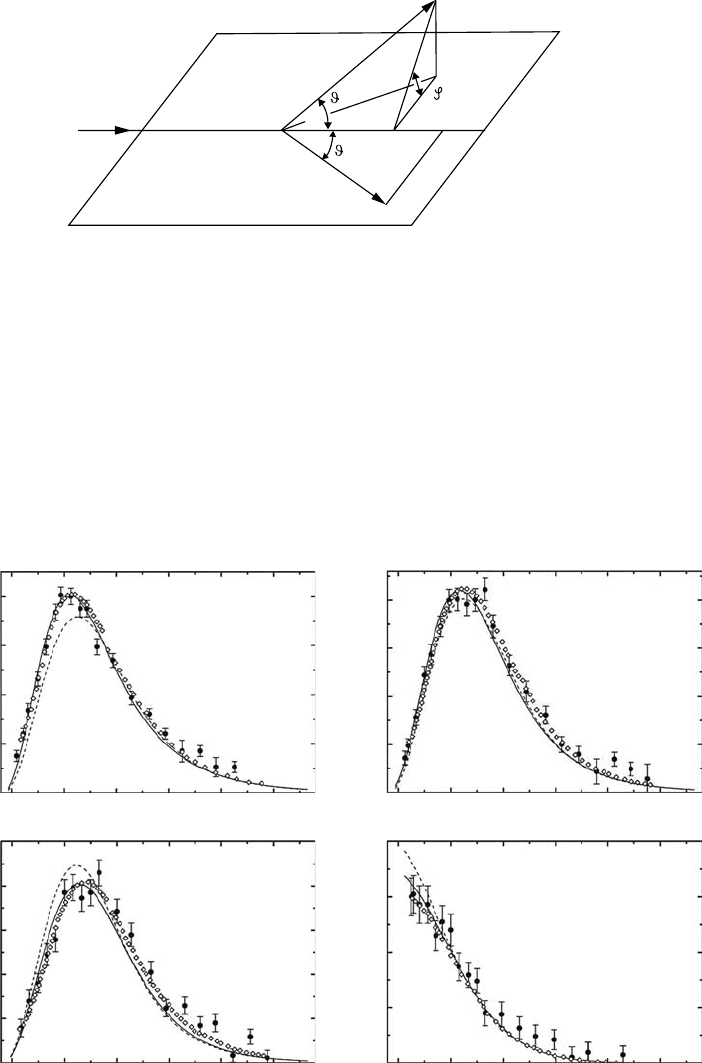
56 Charged Particle and Photon Interactions with Matter
Electron momentum spectroscopy (EMS), also known as (e, 2e) spectroscopy, has made a sub-
stantial contribution to the study of the electronic structure of matter. The basic principle of EMS is
a complete measurement of the momenta of the two electrons emerging after collision. It is a kine-
matically complete study of electron-impact ionization events. Figure 3.30 shows typical examples
of the results of the (e, 2e) experiment for gaseous water (Haed etal., 2007). This experiment was
performed in a symmetric noncoplanar geometry. A high-energy incident electron (E
0
= 1200 eV)
knocks out one electron from H
2
O, and the two outgoing electrons are detected in coincidence at the
k
2
,W
2
k
1
,W
1
1
2
2
k
0
,T
0
e
1
e
2
e
0
Figure 3.29 Schematic view of the kinematics of an electron-impact ionization: incident electron e
0
with
kinetic energy T
0
and momentum k
0
; and scattered and ejected electrons e
1
and e
2
with kinetic energies W
1
and W
2
and momenta k
1
and k
2
, respectively. (Reprinted from Märk, T.D., Ionization of molecules by elec-
tron impact, in Christophorou, L.G. (ed.), Electron-Molecule Interactions and Their Applications, Vol. 1,
Academic
Press, New York, 1984, 251–334. With permission.)
8×10
–4
6×10
–4
4×10
–4
2×10
–4
1×10
–3
8×10
–4
6×10
–4
4×10
–4
2×10
–4
0
4×10
–3
3×10
–3
2×10
–3
1×10
–3
0
0
8×10
–4
6×10
–4
4×10
–4
2×10
–4
0
0.0 0.5 1.0 1.5 2.0 2.5
0.0 0.5 1.0 1.5 2.0
Momentum(a.u.)
TDCS (a.u.) TDCS (a.u.)
Momentum(a.u.)
2.5 0.0 0.5 1.0 1.5 2.0 2.5
0.0 0.5 1.0 1.5 2.0 2.5
1b
1
1b
2
2a
1
3a
1
Figure 3.30 EMS momentum proles for the four valence orbitals of gas-phase H
2
O. Full dots with error
bars are the experimental data taken from Bawagan etal. (1987). Lines are the results of calculations using
different levels of wavefunctions. (Reprinted from Haed, H. etal., Chem. Phys. Lett., 439, 55, 2007. With
permission.)
Electron Collisions with Molecules in the Gas Phase 57
same high energies (E
1
= E
2
≃ 600eV) and the same polar angles (θ
1
= θ
2
= 45°). The experimental
result (Bawagan etal., 1987) is compared with several quantum chemical calculations. All the theo-
retical proles in Figure 3.30 have been folded with the instrumental momentum resolution. The
results of these studies have been applied practically to the charged-particle track structure analysis
in
both vapor and liquid waters.
The
(e, 2e) experiments, however, only give the result averaged out over molecular directions.
Of late, Takahashi and his colleagues (Takahashi etal., 2005) have started a new experiment that
provides the information of a molecule xed in space. They measure the momentum of a fragment
ion in a dissociative ionization, in coincidence with the two outgoing electrons (i.e., a triple coinci-
dence experiment). In Figure 3.31, the scheme of the measurement is given. For further details, see
Takahashi etal. (2005).
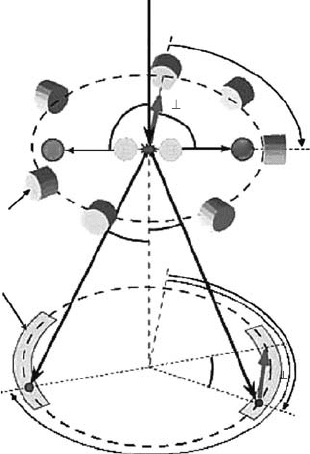
3.4 appliCations oF Cross-seCtion data and databases
3.4.1 energy depoSition Model baSed on colliSion croSS SectionS
The aim of the study of electron–molecule collisions is not only to learn the physics of collision
dynamics but also to provide a set of recommended data of the cross section for various practical
applications. As an example of applications of the cross-section data, a model of energy deposition
of radiation in water vapor is shown here. As is mentioned earlier, it is understood now that even
sub-ionizing electrons can produce damages (in terms of strand breaks and molecular dissociation).
We need to consider collision processes over a very wide range (from meV to MeV) of electron
energies.
Garcia and his colleagues have developed a new simulation program based on the Monte
Carlo method (Muñoz etal., 2008). For this program, they prepared an extensive set of cross-
section data for elastic-scattering and inelastic (ionization and excitation) processes. They also
e
0
–
(E
0
, p
0
)
e
2
–
(E
2
, p
2
)
e
1
–
(E
1
, p
1
)
φ=0°
φ
DMD
φ
M
Δφ
φ
2
φ
1
θ
M
CEMs
EAs
θ
2
θ
1
H H
+
q
q
Figure 3.31 Schematic diagram of an (e, 2e + M) experiment for a symmetric noncoplanar geometry.
This shows seven channel electron multipliers (CEMs), and a pair of entrance apertures (EAs) of a spherical
analyzer with a pair of position-sensitive detectors. (Reprinted from Takahashi, M. etal., Phys. Rev. Lett., 94,
213202-1,
2005. With permission.)
58 Charged Particle and Photon Interactions with Matter
used the TCS to estimate the mean free path of incoming electrons. When no comprehensive data
were available experimentally, they resorted to theoretical calculations. For instance, the angular
dependence (i.e., DCS) of the elastic cross section was derived from a model potential calculation.
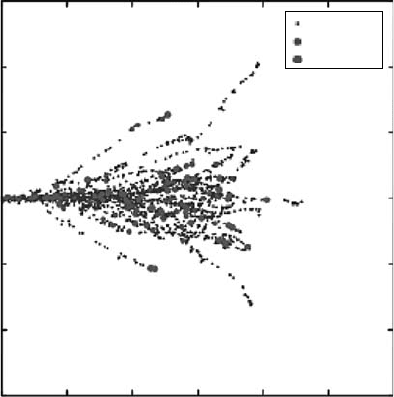
Figure 3.32 shows one sample result of their simulation of 5 keV electron tracks in 1 atm water
vapor. This shows one aspect of the track structure: local distribution of energy deposition events.
They can identify each event with a specic collision process. They plan to extend this model to
water in the liquid phase.
3.4.2 electron tranSport in high-preSSure xe doped with ch
4
High-pressure Xe has been studied extensively in relation to the development of a new-generation
gamma-ray camera for applications in planetary science (Pushkin etal., 2006), as well as of com-
pact and faster versions of radionuclide imaging in nuclear medicine (Barr etal., 2002). As a more
familiar example, the Xe gas has been commonly used in the plasma display panel (PDP) (Sobel,
1998) for producing, efciently and steadily, near-UV light (at a wavelength of 147nm or photon
energy of 8.43eV). The light is emitted as a result of a transition from the resonant state to the
ground state (5P
5
[
2
P
3/2
] 6s→5P
6
[
1
S
0
]). For lowering the discharge voltage, a mixture of Xe and a
few
percent of He or Ne is used to produce metastable states of He
*
and Ne
*
.
In
developing the next-generation gamma-ray imager (Pushkin etal., 2006), it was found that the
electron diffusion and scintillation properties in a high-pressure Xe gas (Hunter and Christophorou,
1984) are greatly inuenced by adding molecules such as CH
4
and H
2
. Scintillation in xenon, initi-
ated by a Compton electron, is used to generate a start signal, which enables the point of interaction
of the γ-ray quantum with the detector material to be reconstructed by the drift time of the ejected
secondary electrons. Xenon is characterized by a low drift velocity of “hot” electrons, which limits
the diffusion of the electron cloud drifting toward the electrode (the anode). An addition of methane
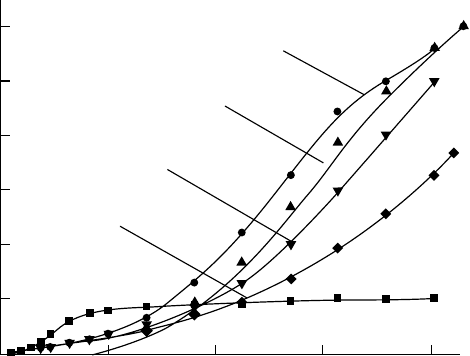
(∼0.2%) to xenon signicantly increases the drift velocities of electrons, as shown in Figure 3.33.
1.5
1
0.5
0
–0.5
y (mm)
–1
<50 eV
50–100 eV
>100 eV
–1.5
0.50 1 1.5
x (mm)
2 2.5 3
Figure 3.32 Simulation of 5keV electron tracks in 1atm of water vapor. Three different amounts of energy
deposition are separately indicated. (Reprinted from Muñoz, A. etal., J. Phys.: Conf. Ser., 133, 012002-1,
2008.
With permission.)
Electron Collisions with Molecules in the Gas Phase 59
Thus, a doping of methane helps in improving the time and coordinate characteristics of the Xe time
projection
chamber counter.
3.4.3 databaSeS
Electron–molecule collisions have been extensively studied for a long time. Cross sections have
been reported in a wide range of publications. Sometimes it takes time to survey the literature to
nd suitable data. It is therefore useful to compile cross-section data and publish them. A list of data
compilations of electron–molecule collision cross sections is given in the book by Itikawa (2007).
This
book also summarizes the methods to nd the cross-section data.
One
of the most comprehensive compilations of cross-section data for electron–molecule colli-
sions has been published as a volume of the Landolt–Börnstein series of data books (Itikawa, 2003).
This includes the cross sections for ionization, electron attachment, total scattering, elastic scatter-
ing, momentum transfer, and excitations of rotational, vibrational, and electronic states of more than
70 molecular species. For individual species of a molecule, Itikawa and his colleagues published a
series of data compilations in the Journal of Physical and Chemical Reference Data (Itikawa, 2002,
2006,
2009; Itikawa and Mason, 2005; Yoon etal., 2008).
Almost
all the compilations of cross-section data published so far are the collection of an integral
cross section (i.e., the cross section integrated over scattering angles). As is emphasized earlier, the
Monte Carlo simulation of radiation tracks needs the information of the scattering angle distribution
of electrons (i.e., the DCS). Normally, the numerical data on DCS are too detailed to be presented
in a compact form. Tanaka and his group recently published a collection of DCS for elastic scatter-
ing of electrons from polyatomic molecules, which they experimentally obtained (Hoshino etal.,
2008). This paper provides at least a part of the information needed in the Monte Carlo simulation.
Those who use the databases should notice one thing: any recommended values of cross-section
data can be changed due to the development of experimental techniques and theoretical methods.
One
should be careful to use the most recent version of the databases.
6
Drift velocity of electrons, W, 10
5
cm/s
Xe+ CH
4
(0.2%)
Xe+ CH
4
(0.4%)
Xe+ CH
4
(0.6%)
Xe+ CH
4
(1.0%)
Pure Xe
5
4
3
2
1
0 0.1 0.2 0.3
E/N, 10
–17
V cm
2
0.4
Figure 3.33 Drift velocities of electrons in pure gaseous xenon and xenon–methane mixtures with differ-
ent methane concentrations (0.2%–1%), as a function of reduced electric eld E/N. (Reprinted from Pushkin,
K.N.
etal., Instrum. Exp. Tech., 49, 489, 2006. With permission.)
