Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

30 Charged Particle and Photon Interactions with Matter
dΩ in the direction Ω(θ,ϕ), measured from the polar axis taken along the direction of the incident
electron,
can be written as
I =
NI d E
d
n
n
0
0 0
( )
( , )
Ω
σ Ω
Ω
0
(3.1)
The subscript 0n indicates a transition from the ground state 0 to an excited or ionized state n. The
quantity dσ
0n
(E
0
,Ω)/dΩ is called the differential cross section for the excitation 0 → n.
Theoretically, the DCS is expressed in terms of scattering amplitude f
0n
(E
0
, Ω), which is derived
from
the asymptotic behavior of the electron wave function, that is,
d E
d
=
k
k
f E
n n
n
σ Ω
Ω
Ω
0
0
( , )
,
0
0
0
( )
2
(3.2)
where
k
0
and k
n
are magnitudes of the electron momentum before and after collision, respectively.
160°
140°
120°
100°
80°
60°
40°
20°
10
4
10
3
10
2
Electron energy (eV)
10
1
10
–24
DDCS (cm
2
/eV sr molecule)
DDCS (cm
2
/eV sr molecule)
10
–23
10
–22
10
–21
10
–20
10
–19
10
–18
10
–24
10
–23
10
–22
10
–21
10
–20
10
–19
10
–18
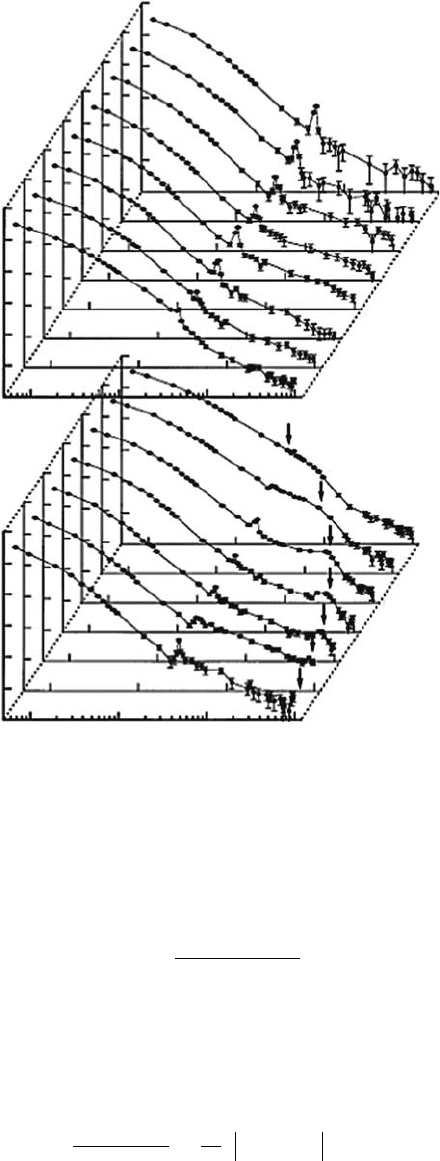
Figure 3.1 DDCS for 6.0MeV/u He
2+
impact on water vapor. The secondary electrons are measured at
7–10,000 eV and 20°–160°. Arrows indicate the binary-encounter peaks. (Reprinted from Ohsawa, D. etal.,
Nucl. Instrum. Methods B,
227, 431, 2005. With permission.)
Electron Collisions with Molecules in the Gas Phase 31
The integral of the DCS over all scattering angles, namely,
q E =
d E
d
d d
n
n
0 0
0
( )
( )
sin
0
σ Ω
Ω
θ θ φ
,
∫∫
(3.3)
is
called the (integral) cross section for the excitation 0 → n.
The
elastic-scattering cross section, q
0
(E
0
), is dened similarly, by replacing the nal state n with
the ground state 0 in Equations 3.1 through 3.3. The effect of elastic scattering on electron transport
phenomena
is normally explained by the momentum-transfer cross section dened by
q E =
d E
d
d d
0 0
0
M
( )
( )
(1 )sin
0
σ Ω
Ω
θ θ θ φ
,
cos−
∫∫
(3.4)
Thesumofthe cross section(3.3) over all possibleexcitation processes(includingthe elastic one), namely,
Q q
n
( ) ( ) ( )E = q E E
0 0 0 0 0
+
∑
(3.5)
is called the total scattering cross section (TCS). From this denition, it is clear that the TCS gives
an
upper bound of the cross section for any individual process.
If
the incident particle has a velocity distribution given by F(v), then the reaction rate constant for
a
process with a cross section q
0n
is calculated as
κ
0
( )
n
v vdv=
∫
q F
n0
(3.6)
0.001
0.01
0.1
1
10
2 4 6 8
10
2 4 6 8
100
2 4 6 8
1000
Energy of secondary electron (eV)
SDCS (10
18
cm
2
eV
–1
)
50 eV
E
0
=100 eV
300 eV
e+H
2
O ionization
200 eV
500 eV
1000 eV
2000 eV
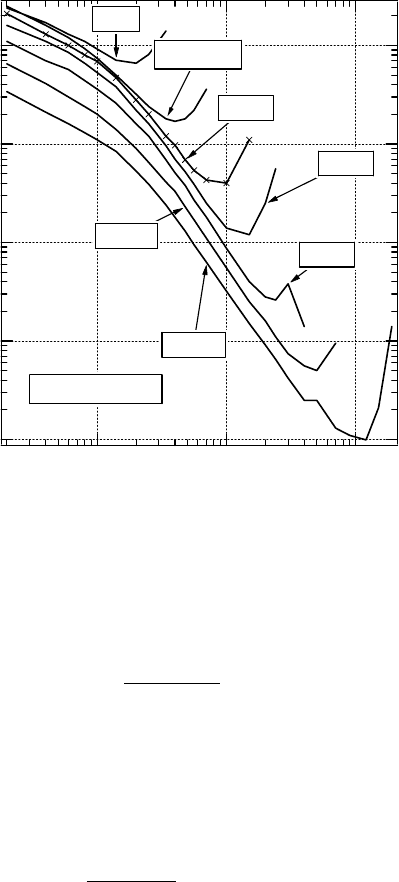
Figure 3.2 SDCS for electron collision with H
2
O measured by Bolorizadeh and Rudd (Bolorizadeh and
Rudd
1986). Incident electron energies (E
0
) are indicated.

32 Charged Particle and Photon Interactions with Matter
Finally, an average energy loss per unit path length of the incident electron is given as
−
=
∑
dE
dx E
S E i
( )
( ) ( ); N i
(3.7)
S E i dE q E
if if
( ); ( ) ( )=
∑
(3.8)
where
N(i)
is the number density of the target molecule in its ith state
S(E;
i)
is called the stopping cross section
The
summation on the right-hand side of (3.8) is taken over all possible transitions i → f with an
energy loss (dE)
if
. Strictly speaking, elastic scattering also has a contribution to the stopping cross
section.
The contribution is given by
S E
m
M
Eq E
e
M
elas
( ) ( )=
2
0
(3.9)
Here, m
e
and M are masses of the electron and the molecule, respectively, while
q
M
0
is the momen-
tum-transfer cross section dened by Equation 3.4. When the energy of the incident electron is
above the threshold of the rst electronic excited state, the elastic stopping cross section can be
ignored in comparison with inelastic ones. But for subexcitation electrons, the elastic contribution
should
be considered in the evaluation of the energy-loss rate in Equation 3.7.
3.2.3 theory and itS role
Electron collisions with molecules have been studied theoretically for many years. Several elaborate
methods have been developed to calculate collision cross sections (Huo and Gianturco, 1995). This
section does not address the details of these theoretical methods. Instead, general aspects of the
theory
as a tool to complement experimental results are presented in the following text.
One
of the main objectives of theory is to prepare a framework to interpret the results obtained
experimentally (Schneider, 1994). In other words, theory provides the means with which we can
gain insight into the physics underlying collision processes. Furthermore, it is only with theory that
we can predict the results of future experiments. One of the typical examples is the interpretation of
the resonant structure in the energy dependence of the cross section (see Section 3.3). This structure
is thought to be caused by a temporary capture of the incident electron to the molecule. This inter-
pretation is conrmed only through theory. Whenever any structure is found in the experimental
cross section, it tends to be assigned to a resonance. But the assignment should be tested against
theory. Another example is the asymptotic form of the cross section in the limit of high impact
energy. The Born–Bethe theory shows that the cross section for any dipole-allowed transition has
the asymptote (ln E)/E as a function of collision energy, E (Inokuti, 1971). This is a universal law
and often used as a check of the validity of experimental data.
Another important role of collision theory is to supplement the cross-section data obtained
experimentally. In many cases, experimental data do not satisfy the trinity requirement mentioned
in Section 3.1. Normally, relative measurements are much easier than absolute ones. In some cases,
the
experimental cross section on the relative scale is normalized to an absolute value theoretically
obtained at some point of energy. Most of the measurements of the cross section are performed at
a limited number of independent parameters (e.g., incident energies and scattering angles). In this
sense, most experimental data are not comprehensive but fragmentary. Theoretical models have
been
proposed to make these data more comprehensive.
As
is mentioned in Section 3.2.1, a close collision between an electron and a molecule can be approx-
imately described by a binary-encounter theory for collision of the incoming electron and the molecular
Electron Collisions with Molecules in the Gas Phase 33
one. Kim and his colleagues (Kim and Rudd, 1994; Hwang etal., 1996; Kim etal., 1997) derived an
ionization cross section from the classical two-body collision theory. To make the resulting formula
more physically reasonable, they combined this ionization cross section with an asymptotic form of
the quantum mechanical cross section in the high-energy limit (i.e., the Bethe formula). To take into
account the characteristics of the molecule, they incorporated into the resulting formula the binding and
kinetic energies of the molecular electrons. They called this the binary-encounter-dipole (BED) model.
The practical application of this model is shown in Section 3.3.4.1. Kim (2007) also proposed a simple
model to produce cross sections for the excitation of the electronic state. This (called the BEf-scaled
Born cross section) is given in Section 3.3.3.3 in relation to the excitation of H
2
O.
For their Monte Carlo study, Garcia and his group (Muñoz etal., 2007, 2008) obtained elastic cross
sections (particularly DCS) with a theoretical model. First, they prepared a model optical potential for
an electron–atom collision. With this potential, they approximately took into account the effects of
inelastic processes on elastic scattering. Then they adopted the independent atom model (IAM), that
is, the elastic cross section for an electron–molecule collision was obtained by an incoherent sum of
the elastic cross sections for electron scattering from the constituent atoms. In so doing, they approxi-
mately included screening effects to consider the molecular nature of the target. They showed that, at
least for the integral cross section, their model reproduces the experimental data available.
In principle, theory can produce cross sections on an absolute scale. When no experimental data are
available, theoretical cross sections are used for application. However, it is very difcult to evaluate the
accuracy of these cross sections. The reliability of the theory used can be sometimes judged, but it is
generally impossible to numerically estimate the possible error of the cross sections calculated. Great
care should be taken when any theoretical values are adopted in the database for application.
3.3 reCent advanCes in low-energy eleCtron
Collisions
with m
oleCules
3.3.1 overview of the croSS SectionS for electron colliSionS with MoleculeS
Two typical examples of the cross-section set are presented here: one for H
2
O (Figure 3.3) (Itikawa and
Mason, 2005) and the other for CH
4
(Figure 3.7) (Kurachi and Nakamura, 1990). The electron-impact
energy is covered from 0.1 to 1000eV for H
2
O, and from 0.01 to 100eV for CH
4
. As is described
in Section 3.2.2, collision is classied into two kinds, namely, elastic (0 → 0) and inelastic (0 → n)
processes. An elastic collision, in which internal energy of the molecule is not changed during the
collision, takes place at any energy. Strictly speaking, a small part, ΔE, of the kinetic energy of the
electron is transferred to the target molecule. The relative amount of energy transfer is given by ΔE/E ∼
m
e
/M ∼ 10
−4
, where m
e
is the electron mass and M is the mass of the molecule. In an inelastic collision,
internal energy is changed according to the processes, such as rotational, vibrational, and electronic
excitations; dissociation; ionization; and electron attachment. The relative amounts of energy transfer
to rotational, vibrational, and electronic degrees of freedom are roughly of the order of (m
e
/M)
1/2
:
(m
e
/M)
1/4
: 1. From this, it is clear that the adiabatic approximation holds for rotational and vibrational
motions. In the following text, some details of the cross-section set for H
2
O (Figure 3.3) are given.
Cross sections for CH
4
are discussed in Section 3.3.2.2 in relation to the swarm experiment.
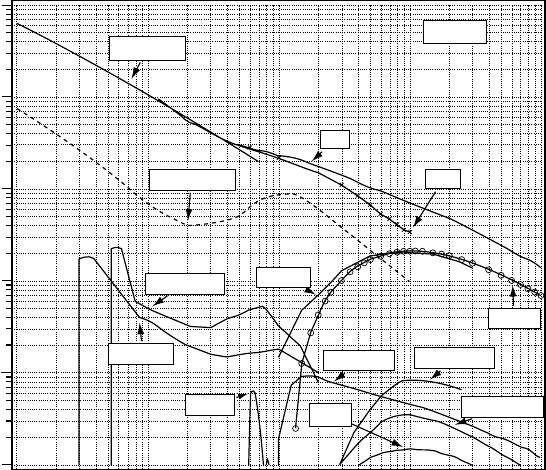
Figure 3.3 shows the recommended values of the cross sections for total scattering, elastic scat-
tering, momentum transfer, rotational transition, vibrational excitations of bending and stretching
modes, and total ionization (Itikawa and Mason, 2005). The gure also shows cross sections for
several different processes of molecular dissociation. They are dissociative attachment to produce
H
−
, dissociative emissions to produce Ly alpha and Balmer alpha lines of H, and A-X emission of
OH, and nally the production of OH in its ground (X) state and O in
1
S state. Each inelastic cross
section
has a threshold and a specic shape of energy dependence.
To
determine a cross section of a certain process over a wide range of energies, several differ-
ent experimental methods described below (i.e., an attenuation method, a swarm technique, and a
34 Charged Particle and Photon Interactions with Matter
crossed-beam method) must be used in combination. To obtain a reliable (though not necessarily
complete) set of the cross sections, there is no standard way to employ. As one can see in an exten-
sive data compilation, such as is shown in Figure 3.3, it takes efforts of many workers in many
different institutions to produce cross sections for one molecule. In spite of the efforts, some of the
data sets may be fragmentary. Furthermore, results from different laboratories are often discordant.
It is therefore necessary to collect as many sets of data as possible from the literature, to assess their
reliability and to determine the most trustworthy set of data for use in applications. Efforts toward
such data compilations and analysis are being made by various groups, as described in Section 3.4.
As for H
2
O, the cross-section set recommended by Itikawa and Mason (Figure 3.3) does not nec-
essarily satisfy every user. The most serious problem is the excitation of the electronic state. At the
time of compilation, no reliable experimental data were available for the process so that nothing was
recommended for the excitation of the electronic state, as is seen in Figure 3.3. However, the situa-
tion is now improved (see Section 3.3.3.3). Total and elastic-scattering cross sections and the ioniza-
tion process have been well studied. No experimental data are available for rotational excitation,
and values plotted in Figure 3.3 are the result of a theoretical calculation. Some of the other cross
sections are now being revised, which are discussed in the relevant sections below. Particularly for
dissociation
processes, a recent review by McConkey etal. (2008) is very informative and useful.
Once
the cross-section data set is determined, useful physical quantities like Equations 3.6 and 3.8
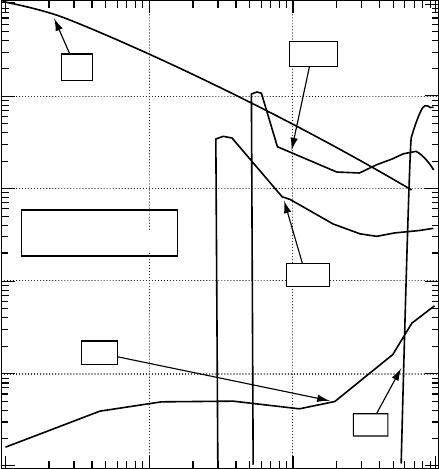
are evaluated. For instance, the stopping cross sections thus calculated are shown for H
2
O in Figure 3.4
(Itikawa, 2007). In thisgure, the stoppingcrosssections are presented only overthe energy region below
10eV. In this region, rotational transition is the most dominant process for stopping the electron. The two
curves for vibrational excitation correspond to the bending (vib2) and stretching (vib13) modes, respec-
tively. The stopping cross section for the excitation of the electronic state (designated as exc.) is based on
a theoretical cross section so that its quantitative accuracy is uncertain. For electron energies above 10eV,
ionization and electronic excitation dominate. We need more detailed information about these processes
(particularly about the excitation of electronic states) to evaluate the stopping cross section.
10
–13
10
–14
10
–15
10
–16
10
–17
10
–18
0.1 1 10
attach
OH A-X
H Lyman α
H Balmer α
ion tot
O(
1
S)
vib bend
vib stretch
tot
mom transf
rot J = 0–1
elas
e+H
2
O
OH(X)
Electron energy (eV)
Cross section (cm
2
)
100 1000
Figure 3.3 Summary of the recommended data on electron collision cross sections for H
2
O. (Reprinted
from
Itikawa, Y. and Mason, N.J., J. Phys. Chem. Ref. Data, 34, 1, 2005. With permission.)
Electron Collisions with Molecules in the Gas Phase 35
3.3.2 MeaSureMentS by electron-beaM attenuation and tranSport MethodS
3.3.2.1 total scattering Cross section—an upper bound of Cross section
The TCS, Q, dened by Equation 3.5 is obviously an upper bound of any of the individual cross
sections. It is normally determined with a beam attenuation method. With this method, however,
no information is available for each q
0n
(E
0
) component on the right-hand side of Equation 3.5.
Each individual cross section has to be determined by the measurement of the crossed-beam type
described
in Section 3.3.3.
To
determine Q, one may use the Lambert–Beer law, commonly used in photoabsorption mea-
surements. Suppose that one sends an electron beam (having uniform velocity) of intensity I
0
per
unit area into a gas consisting of N molecules (of a single species) per unit volume. If one determines
the number I of these electrons passing through unit area at a distance L in the gas, then one may
write I/I
0
= exp(−NQL). This relation is valid under a condition of single collision during the pas-
sage of the electron in the gas cell. Such a measurement is feasible for electrons of kinetic energies
between
0.1 and 1000
eV
or higher.
The
apparatus for the attenuation experiment appears to be simple in principle, but a great deal of
ingenuity and care is required to achieve a precision of a few percent or better in the results. In this
experiment, the electrons are assumed to be lost from the beam once they collide with molecules.
Some of the electrons, however, move in the forward direction even after collision. The intensity,I,
should not include these forward-scattering electrons. The reliability of the measured value of Q
critically depends on the care taken about this point. Sometimes, a uniform magnetic eld is applied
in parallel to the electron beam so as to limit the spatial divergence of the electron beam. When a
magnetic eld is applied, its inuence on a transmitted beam must be compensated by a calculation.
10
–15
10
–16
10
–17
rot
elas
exc
10
–18
10
–19
10
–20
0.01
2 24 46 68 8 2 4 6 8
0.1 1 10
Electron energy (eV)
Stopping cross section (eV cm
2
)
vib 2
vib 13
H
2
O
stopping cross section
Figure 3.4 Stopping cross sections for electron collisions with H
2
O. Only those for low-energy electrons
are shown. (Reprinted from Itikawa, Y., Molecular Processes in Plasmas: Collisions of Charged Particles
with Molecules,
Springer, Berlin, Germany, 2007. With permission.)

36 Charged Particle and Photon Interactions with Matter
Care is needed also in the correction for the effective cell length, because some gas leaks from both
the
entrance and the exit orice of the cell.
Assessing
the total scattering cross-section data available in the literature, Itikawa and Mason
determined the recommended values for H
2
O, as shown in Figure 3.3, which is reproduced in
Figure 3.5. Due to the permanent dipole moment of H
2
O, the TCS increases steeply with decreasing
electron energy. For a comparison, the TCS for CH
4
(collected from a data compilation by Karwasz
etal., 2003) is also plotted in Figure 3.5. In the energy region below about 10 eV, the two sets of
cross sections differ very much from each other. The minimum in the Q of CH
4
at around 0.5eV
corresponds
to the Ramsauer–Townsend minimum, discussed in Section 3.3.2.2.
The
beam attenuation method has been recently extended to extremely-low-energy electrons
(Field etal., 2001). By using photoelectrons, instead of electrons from hot laments, electron beams
of ∼10meV of energy are successfully produced with a very high resolution of a few meV. A beam
of monochromatized light of 786.5 Å from a synchrotron radiation source is used to excite Ar to an
autoionizing state, Ar
+
(
2
P
3/2
), and emit electrons of 5 meV to 4.0eV, with a resolution of about 5meV.
However,
the beam intensity is as weak as about
10
−10
A. Great care is necessary to account for the
contact potential of the analyzer material, and to prevent the leakage of the electric eld outside the
electrode region. Until now, only one laboratory has produced the TCSs in this way, and it has been
suggested
to conrm their result with other experiments.
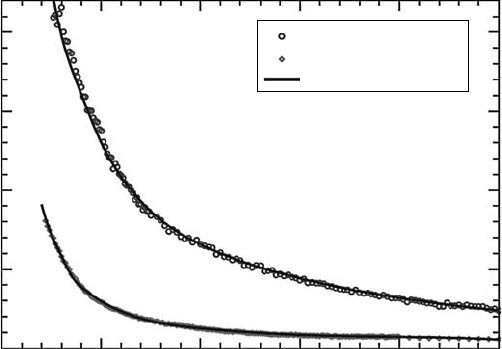
Figure
3.6 shows the TCS measured by Field and his group using their very-low-energy electron
beams (C
ˇ
urík etal., 2006). It should be noted that the electron energy goes down to about 0.02eV,
where the cross section has a value as large as 10
−13
cm
2
. One also should note that the measured
cross section is not the real value of the TCS. Actually, the measured value corresponds to the cross
section excluding a certain part of the forward-scattering electrons. In this sense, the values shown
Total scattering cross section
5
4
3
2
10
–14
10
–15
10
–16
2
3
4
5
6
2
3
4
5
6
6
5
0.1 1 10
Electron energy (eV)
Cross section (cm
2
)
100 1000
H
2
O
CH
4
Figure 3.5 TCSs for electron collisions with H
2
O and CH
4
. The values for H
2
O are the same as those
shown
in Figure 3.3.
Electron Collisions with Molecules in the Gas Phase 37
in Figure 3.6 do not exactly correspond to the ones in Figure 3.5. Further experimentation is needed
for
the TCS of H
2
O at very low energies.
3.3.2.2 the
s
warm
m
ethod
to d
etermine
a Cross-
section
s
et
When electrons are emitted from a source and ow through a gas under an applied uniform elec-
tric eld, they undergo many collisions with gaseous molecules. Macroscopic properties of this
electron swarm can be measured. These properties are the drift velocity, the diffusion coefcient,
and other transport coefcients, as well as the rate constants for excitations, ionization, and elec-
tron attachment. These are functions of the ratio of the electric-eld strength to the gas density,
and determined by the electron energy distribution function (EEDF). Thus, the measured values
of the transport properties can be related to electron–molecule collision cross sections through
the EEDF, which is obtained by solving the Boltzmann equation. The swarm method provides a
set of cross sections, especially at low electron energies (down to 0.01eV), where the electron-
beam method is difcult to apply.
As is easily understood, electron transport involves various kinds of collision processes. It is
difcult to derive cross sections without any ambiguity. Consequently, an analysis of swarm data
usually takes into account some information from other measurements and determines a full set of
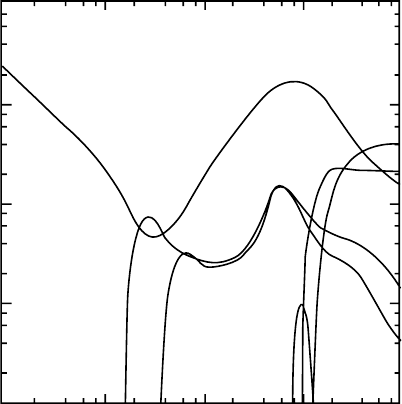
cross sections in such a way that the set is consistent with all the available data. Figure 3.7 shows a
set of cross sections thus determined for CH
4
(Kurachi and Nakamura, 1990). Methane is a proto-
type of the single-bond hydrocarbon molecules and is used in many applications, such as radiation
counters. The momentum-transfer cross section,
q
M
0
, dened by Equation 3.4 dominates over all the
electron
energies
shown. In the case of CH
4
,
q
M
0
has a minimum at around 0.34eV. This means that
at the energy around the minimum, electrons pass through the gas almost freely. This minimum
arises from the Ramsauer–Townsend effects, observed also for heavier rare gases (Ar, Kr, and Xe).
To be specic, the phase shift of the s wave (l = 0) of the scattered electron is an integral multiple
of π at this energy, whereas higher partial waves have negligible contributions at such a low energy.
Cross sections for inelastic processes are also obtained, as is shown in Figure 3.7. They are excita-
tions
of vibrational modes, dissociative electron attachment (DEA), dissociation to produce neutral
fragments,
and ionization. Details of vibrational excitation are discussed in Section 3.3.3.3.
2000
1500
1000
Integral cross section
Backward cross section
Fit of the data
500
0
0 50 100
Collision energy (meV)
Cross section (10
–20
m
2
)
150 200 250
Figure 3.6 Results of attenuation experiment with very-low-energy electrons in H
2
O. The upper curve
is the TCS, but with a part of forward scattering excluded (see Section 3.3.2.1). The lower curve is a cross
section for total backward scattering. (Reprinted from C
ˇ
urík, R. etal., Phys. Rev. Lett., 97, 123202-1, 2006.
With permission.)
38 Charged Particle and Photon Interactions with Matter
3.3.3 croSSed-beaM MethodS for the MeaSureMent of croSS Section
3.3.3.1 Crossed-beam methods and the measurement of dCs
In a crossed-beam method, one sends a well-collimated beam of electrons of a xed kinetic
energy, E
0
, into a molecular target (normally in a beam), and analyses the kinetic energy (i.e.,
the so-called residual energy, E
r
) of the scattered electrons. This method provides much more
detailed information than that provided by the attenuation method and certainly reects the
dynamics of the collision process. The electron energy analyzers commonly used are the 127°
electrostatic cylinder and the 180° electrostatic hemisphere (Celotta and Huebner, 1979). These
are also used as monochromators of the energy of the incident electron beams. A hot lament is
commonly used as a source of electrons, with an energy spread of 0.3–0.5 eV. After energy selec-
tion with a monochromator, a resolution of about 30meV and a beam of intensity of about 10
−9
A
(∼17 meV and ∼10
−10
A at best) are usually obtained. In order to maintain the single-collision
condition, the pressure of a gas target must be kept at about 10
−3
torr. This limits the scattering
intensity available. This situation is in sharp contrast with the electron spectroscopy of a solid
surface, which has a much higher atomic density, and hence readily accomplishes an energy
resolution of several meV.
The electron spectrometer can be operated in several different ways. One of the standard ways of
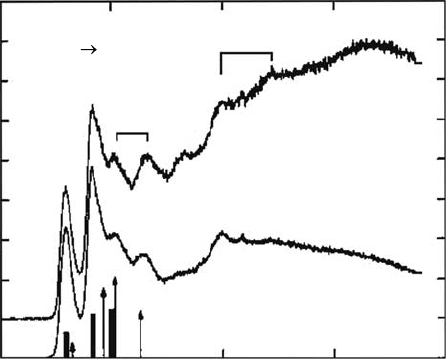
measurement (called the energy-loss mode, EL) is to derive an energy-loss spectrum. In this spectrum,
one plots the intensity of electrons scattered into a xed angle as a function of the energy loss, ΔE
(i.e., ΔE = E
0
− E
r
), for a xed incident electron energy. The location of the energy-loss peak indicates
the excited state of the target molecule, and the height of the peak is proportional to the correspond-
ing cross section for the excitation of the state. Figure 3.8 (Dillon etal., 1989) shows energy-loss
spectra of one of the DNA bases, adenine. Other ways to operate the spectrometer are as follows:
(1) Detecting only the electrons with a specied energy loss with the analyzer, and then sweeping
the incident energy, to observe the energy dependence of a specic excitation process (the excitation
10
–14
10
–15
10
–16
0.01
q
v24
q
m
q
v13
q
v13
q
v24
q
dn
q
i
100q
a
0.1 1 10 100
Electron energy (eV)
Cross section (cm
2
)
10
–17
10
–18
Figure 3.7 A set of electron collision cross sections for CH
4
, determined with a swarm method. (Reprinted
from Kurachi, M. and Nakamura, Y., Electron collision cross sections for SiH
4
, CH
4
, and CF
4
molecules, in
Proceedings of the 13th Symposium on Ion Sources and Ion-Assisted Technology (ISAT’90), Tokyo, Japan,
pp.205–208,
1990. With permission.)
Electron Collisions with Molecules in the Gas Phase 39
function mode, EF, see Figure 3.15). The resulting intensity distribution can reveal the structure due
to resonance. (2) Keeping the residual energy of the scattered electron constant and changing both
the incident energy and the energy loss, to detect the excitation function (the constant residual energy
mode, CRE). With this method, each energy-loss feature is recorded at the same energy above thresh-
old. Thus, it can avoid the effect of different efciencies, if possible, of the detector for different-energy
electrons. (3) Optimizing the spectrometer to detect scattered electrons with near-zero kinetic energy
and sweeping the incident energy (the threshold electron spectroscopy mode, TES [see Figure 3.17]).
Electron scattering from molecules of biological signicance, or biomolecules, is an area that
has been actively studied in line with the recent trends in science and technology. Crossed-beam
methods have been employed for these studies, because these biomolecules are now available com-
mercially and have sublimation characteristics that make a molecular beam easily producible. These
molecules are, however, often in a condition different from the circumstances of real biomolecules.
For example, the target molecules in the experiment are not hydrated and are at a high temperature.
Therefore, there have been serious discussions among atomic physicists, radiation scientists, and
biologists as to how such elementary collision processes can be effective in understanding the radia-
tion effect. As frequently repeated in this book, it is obvious that the OH radical dissociated from
H
2
O is an essential reactant for the radiation damages. But, in this chapter, an electron scattering
from biomolecules is descried as an example of the general study of electron–molecule collisions,
although
it may contribute little to the actual radiation damage.
Typical
examples of the DCS measurement are presented in Figures 3.8 (Dillon etal., 1989) and
3.9 (Vizcaino etal., 2008). The rst measurement of an electron collision with a gas-phase DNA-
base molecule was performed for adenine by Dillon etal. (1989). The electron energy-loss spectrum
was recorded over a range of excitation energy of 3–22eV for scattering angles of 3° and 6°. In
addition to observing accurate positions of the energy-loss peaks, the data solved the long-held con-
tention that the group of peaks in the 6–10eV range belong to transitions originating from valence
πorbitals. In the vapor-phase spectrum, a Rydberg transition corresponding to an n → 3s excitation
is readily observed with a term value of 3.45eV relative to the rst lone-pair ionization potential.
Since early 1990, the bond-breaking process in the molecular constituents of DNA has been
extensively studied by the dissociative attachment method, as described in Section 3.3.4.3.
2 7 12
θ=3°
θ=6°
σ σ
*
π π
*
?
π π
*
π π
*
n 3s
W (eV)
I (rel)
17 22
Figure 3.8 Electron energy-loss spectra for adenine. The incident electron energy is 200eV. Data for two
scattering angles (3° and 6°) are shown. See Dillon etal. (1989) for details. (Reprinted from Dillon, M.A. etal.,
Radiat. Res.,
117, 1, 1989. With permission.)
