Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.


312 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
point of about 1500°C is quite high. So, not trusting the computer fitting
of a large quantity of data, the value of ∆G
mot
was obtained by direct
observation of the evolution of the largest grain size as a function of time
and temperature. The activation energy thus obtained was the same: about
4 eV.
[96]
In a pure material, the activation energy for the motion of the
C49C54 boundary should be defined, as in recrystallization or grain
growth, by the grain boundary diffusion of the slow element; here, the
slow element was Ti. In a recent paper,
[97]
a maximum value of about 3 eV
was estimated for such a process. The key to this discrepancy between
anticipated and experimentally derived activation energies would be that
the material is not pure.
Some investigators
[98]
observing the motion of the C49C54 bound-
aries in an electron microscope equipped with a residual gas analyzer dis-
covered a quite strong hydrogen signal occurring simultaneously with the
growth. It too had an apparent activation energy of about 4 eV, the same
as the other(s). This cannot be the activation energy for the evolution of
hydrogen from the grain boundaries of TiSi
2
, which is most probably of
the order of 1 eV, half of which would be for diffusion and the other half
for desorption (perhaps 1.5 eV if we assume hydrogen traps with a trap-
ping energy of 1 eV). It is hard to guess, but for diffusion of hydrogen in
Ni (with a melting point close to that of TiSi
2
), the activation energy is
0.4 eV.
[99]
That value is for lattice diffusion, but it seems that the grain
boundary diffusion of hydrogen is hardly any larger than that in the
lattice.
[100]
The analysis of boundary motion during recrystallization
[101]
of
impure materials (with impurity contents in the range of parts per million)
depends on the impurity drag effect.
[102]
At low temperatures, the segrega-
tion of impurities to the boundaries and the attraction between these impu-
rities and a moving boundary cause the activation energy to have a higher
value, equal to that for lattice diffusion plus the energy of segregation
(or rather desegregation) of the impurities. At high temperatures, where
entropy dictates that the impurities should “evaporate” from the bound-
aries, the activation energy equals the value for grain boundary diffusion.
In an intermediate temperature region between “low and high,” the appar-
ent activation energy assumes meaninglessly very high values that cannot
be defined analytically.
[103]
In the present case of the C49 to C54 transfor-
mation of TiSi
2
in thin films, available data are unfortunately limited to a
very narrow range of temperatures (30 K in the most extensive study
[92]
),
so that we do not have any knowledge of its behavior in low, high, or
intermediate temperatures. Thus, the apparent activation energy value of
4 eV could be meaningless (intermediate range) or correspond to the sum
of the activation energy for the lattice diffusion of Ti (not measured, but
probably about 3 eV) plus those for the desorption and diffusion of hydro-
gen. The effect of hydrogen has been discussed as an example of the
Ch_06.qxd 11/12/04 4:06 PM Page 312

REACTIVE PHASE FORMATION, D’HEURLE ET AL. 313
impurity drag effect;
[102]
yet it may also result from the hindering effect of
impurities on grain boundary diffusion,
[71]
usually thought of as occurring
in fixed grain boundaries, although the two effects should not be consid-
ered to be exclusive of one another. Support for such a view is found in
the important retardation of electromigration failure in Al thin-film con-
ductors in the presence of hydrogen.
[104]
Furthermore, it was observed that
the formation of purple plague (Au
2
Al) at Al-Au contacts was almost
completely suppressed in samples annealed in hydrogen,
[103]
and similar
results were obtained in the reactions of Cu, Ag, and Ni with Sn.
[105]
This
impurity effect on grain boundary diffusion is also illustrated in the self-
aligned technology, where the goal is to obtain a Ti disilicide over source,
drain, and gate, but not over the short spaces that separate source and
drain from the gate. With a continuous Ti film annealed in a vacuum or an
inert atmosphere, a most unwanted bridging occurs via the diffusion of Si
either in Ti itself or in one of the precursor phases to C49 TiSi
2
. That phe-
nomenon is prevented
[106]
by annealing in nitrogen, seemingly because the
small interstitial nitrogen atoms diffuse rapidly in the grain boundaries
where they successfully block any subsequent diffusion of Si atoms.
With very thin films (10 nm) in current devices, the question of the
roughness of the layers used becomes important, which led to the system-
atic study of that roughness by optical means,
[107]
used in situ during the
heating of samples at constant ramp rates. Such a study
[108]
reveals a
strong roughening at about 600°C (see Fig. 6.8), corresponding to the for-
mation of the C49 phase of TiSi
2
, and no roughening at the temperature of
the C49 to C54 transition. The latter should not be surprising. (The small
light-scattering peak for distances of 5 mm at about 800°C for that trans-
formation is undoubtedly due to the difference in refractive indices for the
two phases, not to topological irregularities.) The paramorphic transfor-
mation implies small changes in volume and therefore small changes in
the surface aspect of the film. The signal obtained at 600°C for lateral dis-
tances of the order of 5 mm reveals a nucleation-controlled reaction, with
bumps on the samples for each nucleation center. (A study as a function
of scattering angles or laser wavelengths would indicate the average dis-
tance between such sites.) As the film transforms completely, the bumps
disappear, and so does the scattering signal. With the new phase, C49
TiSi
2
grows in “cauliflower” fashion from the nucleation centers.
Scattering corresponding to short distances (0.5 mm) reveals the local
roughness at the surface of the cauliflowers, which does not change sig-
nificantly with the completion of the process.
Before we attempt to analyze a possible nucleation process in the ini-
tial formation of TiSi
2
, we should consider what happens before that stage
in Fig. 6.8. In the diffraction pattern, at low temperatures, the line at about
45 degrees is the (101) line of Ti and at 44 degrees is the (002) line. The
Ch_06.qxd 11/12/04 4:06 PM Page 313
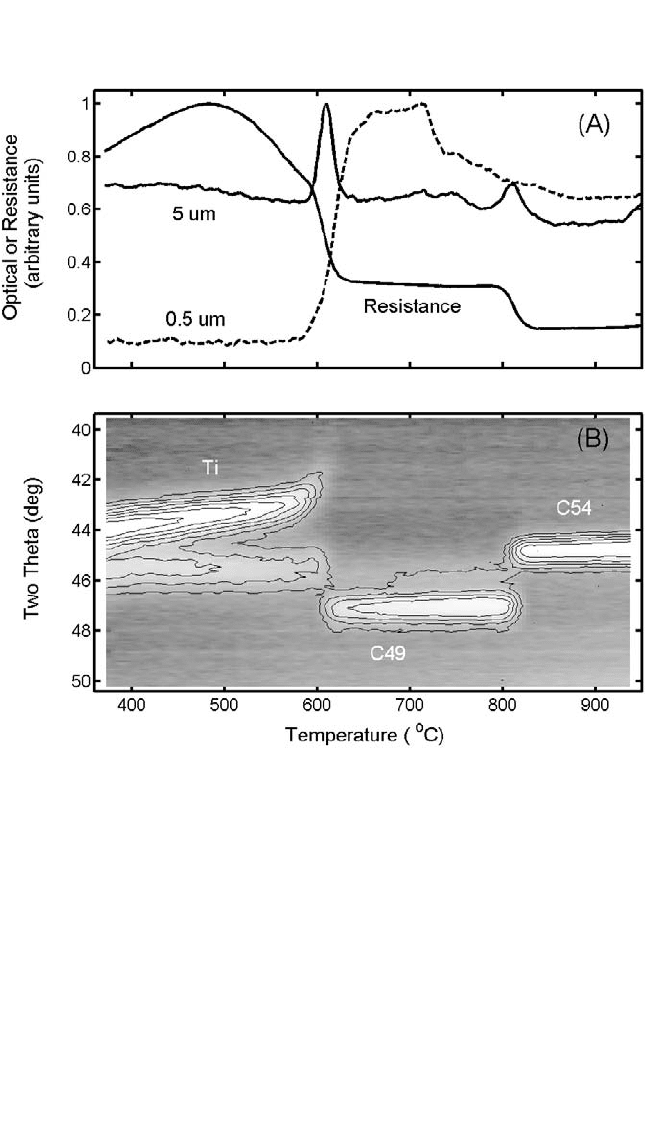
314 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
latter reveals what may be assumed to be an abnormally high expansion
coefficient, but that would be incorrect. The somewhat surprising behav-
ior is due to the dissolution of oxygen, which increases the c axis of
hexagonal Ti without significantly affecting the other axis, a. Values
obtained by Villars and Calvert
[109]
are a 0.2951 nm and c 0.4685 nm
for Ti, a 0.296 nm and c 0.483 nm for Ti
2
O, and intermediate values
for lower oxygen content. The oxygen intake causes the corresponding
increase in resistivity (Fig. 6.8), an increase that does not occur if the film
is protected against possible oxidation.
[110]
Figure 6.8. (A) Simultaneous measurements of resistance and roughness at two
different lateral scales during heating at 3 K/sec of a Ti film (20 nm thick) on poly-
crystalline Si. (B) Partial x-ray diffraction diagrams showing distinctly the formation
of the C49 and C54 phases of TiSi
2
. Note the correspondence between the fea-
tures in the two sets of pictures.
Ch_06.qxd 11/12/04 4:06 PM Page 314

REACTIVE PHASE FORMATION, D’HEURLE ET AL. 315
It had long been known
[26]
that the so-called ill-behaved silicides,
including TiSi
2
, became rough in appearance (to the naked eye) during
preparation, and that this roughness implies some nucleation-controlled
reaction. However, while in the case of NiSi
2
, for example, the preceding
phase NiSi was clearly identified, and the small ∆G driving the reaction
was thereby identified also, the same has not been true of TiSi
2
, or MoSi
2
,
and so forth. It was thus difficult to know at what stage of the reaction the
samples became rough. With the help of Fig. 6.8, we now know that
roughness occurs simultaneously with the nucleation of the C49 phase. In
the continuous absence of firm information about what precedes the C49
phase, we hazard here some educated guesses. It is probable that the pre-
ceding phase or phases have not been clearly identified for a number of
reasons: Amorphous phases are not clearly seen in x-ray diffraction. In the
case of Ti-Si reactions, a number of phases exist (TiSi, Ti
3
Si
4
, and Ti
5
Si
3
)
that are hard to identify because they have somewhat similar x-ray dif-
fraction patterns, often very poorly displayed from thin-film samples.
Finally, while the rough appearance of the C49 phase is constantly annoy-
ing in all samples, it probably varies with the sample preparation such as
the mode of film deposition and the doping of the substrates. A possible
reaction would be:
TiSi Si TiSi
2
(C49). (23)
(Because the phase considered here is C49, we must take into account
the small enthalpy difference between C49 and C54.) According to
Hodaj and Dumas,
[28]
the standard enthalpy change for this reaction
would be 3.5 ± 36 kJ/mol, which is extremely small. Although crys-
talline TiSi does not seem to form during the reaction of Ti with Si, there
are reports of the formation of an amorphous phase with a composition
close to TiSi.
[111, 112]
With TiSi in an amorphous state, the enthalpy
change for Eq. (23) would be increased (in absolute value) by an amount
corresponding to the crystallization of that phase; that may not be
enough for the formation of the TiSi
2
to cease to be nucleation-controlled.
Since Ti
5
Si
3
has been observed in some experiments,
[113, 114]
we can
consider:
15 Ti
5
Si
3
75 Si TiSi
2
(C49). (24)
The standard enthalpy change
[28]
for this would be 13.5 ± 36 kJ/mol.
This seems bigger than the preceding value (as anticipated, considering
the greater change in composition), but could still be small enough to
cause the reaction to be nucleation-controlled. [Compare this with
Eq. (21).] According to a recent publication, the phases Si, Ti
5
Si
3
, and the
Ch_06.qxd 11/12/04 4:06 PM Page 315

316 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
two forms of TiSi
2
can coexist, which implies some sort of equilibrium
among them.
[115]
Moreover, the phase Ti
5
Si
3
, like other such transition
metal silicides with the same formula, should be stabilized by interstitial
impurities: oxygen, carbon, and nitrogen.
[116]
A considerable amount of
research
[117–119]
has been devoted to this subject. The impurity atoms,
including hydrogen, occupy the center of trigonal antiprisms, with binding
energies that can be high enough to decrease the overall lattice parame-
ters. At high temperatures, oxygen impurities in Zr
5
Si
3
completely desta-
bilize adjacent phases such as Zr
2
Si, implying a considerable decrease in
the free energy of the 53 phase. Yet quantitative data for the low-
temperature reaction of interest here could not be found. In practice, with
unavoidable contamination, the ∆H and (∆G) for Eq. (24) could be con-
siderably smaller than evaluated here. Thus, whether considering Eq. (23)
or (24), we can find some theoretical justification for the nucleation-
controlled formation of the C49 phase of TiSi
2
. Nothing has been said
about interface energy and stress effects, although we know that these fac-
tors are very important physically. Experimentally, more effort should be
spent to identify the precursor phase in TiSi
2
formation, as well as for
other such phases, for example, MoSi
2
. We should note that in the reac-
tion of Ti with crystalline Ge, the reaction:
Ti
6
Ge
5
Ge → TiGe
2
(C54) (25)
is clearly nucleation-controlled.
[61]
When considering small driving forces, as in any form of nucle-
ation-controlled reactions or transformations, remember that the ∆G’s
and the ∆H’s cease to be comparable. Moreover, we should not use the
table values for the stoichiometric compounds, but should operate from
the actual curves for the various ∆G
f
’s as a function of composition. So
the message is that the approach used here, based on table values of
∆H’s, provides a simple tool to differentiate diffusion-controlled from
nucleation-controlled reactions. Where possible, refer to tables that
give the free energies of compounds (and elements). According to the
most recent thermodynamic evaluation of the Ti-Si system,
[120]
the free
energy change at 900 K for Eq. (23) would be 20.8 kJ, including the
correction for the C49 to C54 transition.
[91]
The same reference explic-
itly mentions that the phase Ti
5
Si
3
can contain significant amounts of
excess Si substitutionally located on one of the two different Ti sites, so
that for our purpose (in the presence of excess Si), its formula should
be (Ti,Si)
2
Si
3
Ti
3
. For Eq. (24), assuming stoichiometric Ti
5
Si
3
, ∆G
would be 53.5 kJ. This quantity would be much reduced, in absolute
value, for Ti
5
Si
3
saturated with Si and/or contaminated with interstitial
impurities.
Ch_06.qxd 11/12/04 4:06 PM Page 316

REACTIVE PHASE FORMATION, D’HEURLE ET AL. 317
6.3.2 Cobalt Disilicide, Entropy of Mixing,
(Possible) Enthalpy and Density-of-State
Effects in Ternary Reactions
With conductor dimensions reduced below about 0.3 mm, the operat-
ing window between the temperatures necessary to nucleate the C54
form of TiSi
2
and that at which surface energy effects cause the films to
break into separate islands has become intolerably small. Thus attention
was turned to CoSi
2
, with a similar or even somewhat smaller resistivity,
which does not suffer from the kind of paramorphic transformation that
plagues users of TiSi
2
. Because f.c.c Co and Ni are so similar from a met-
allurgical point of view, it is not surprising that the sequence of metal-
silicon reactions is the same with both metals: first Co
2
Si, then CoSi and
CoSi
2
. Although it is not especially relevant to what follows, we should
note that the Co-Si phase diagram is much simpler than that of Ni-Si,
with no phase corresponding to Ni
3
Si
2
, and without several metal-rich
phases. Although the phase sequences are similar with the two metals, on
a temperature scale the formation of CoSi
2
is not so distinct from that of
CoSi as the formation of NiSi
2
from that of NiSi. Yet early measurements
showed that during isothermal heat treatments, the growth of CoSi
2
ceased to be parabolic as the temperature was lowered towards 500°C,
[121]
and indeed the formation of CoSi
2
becomes practically impossible below
that temperature. By comparison with NiSi
2
, the effect was correctly
attributed to a difficult nucleation. Recent results give a ∆H
f
of 95 ± 4
kJ/mol for the formation of CoSi and 99 ± 6 kJ/mol for CoSi
2
,
[122]
resulting in a difference of 4 ± 10 kJ/mol for the nucleation of that
phase, a value commensurate with 5 ± 20 kJ/mol for the nucleation of
NiSi
2
[Eq. (21)]. The two values are nearly equal, as anticipated within
the limits of accuracy.
One problem with CoSi
2
is that on account of its nucleation-controlled
formation, its surface is rough. Attempts made to reduce the roughness by
alloying reveal some interesting aspects of the control of ∆G, the driving
force that enters into ∆G*
th
(∆s
3
∆G
2
) and thus controls the density of
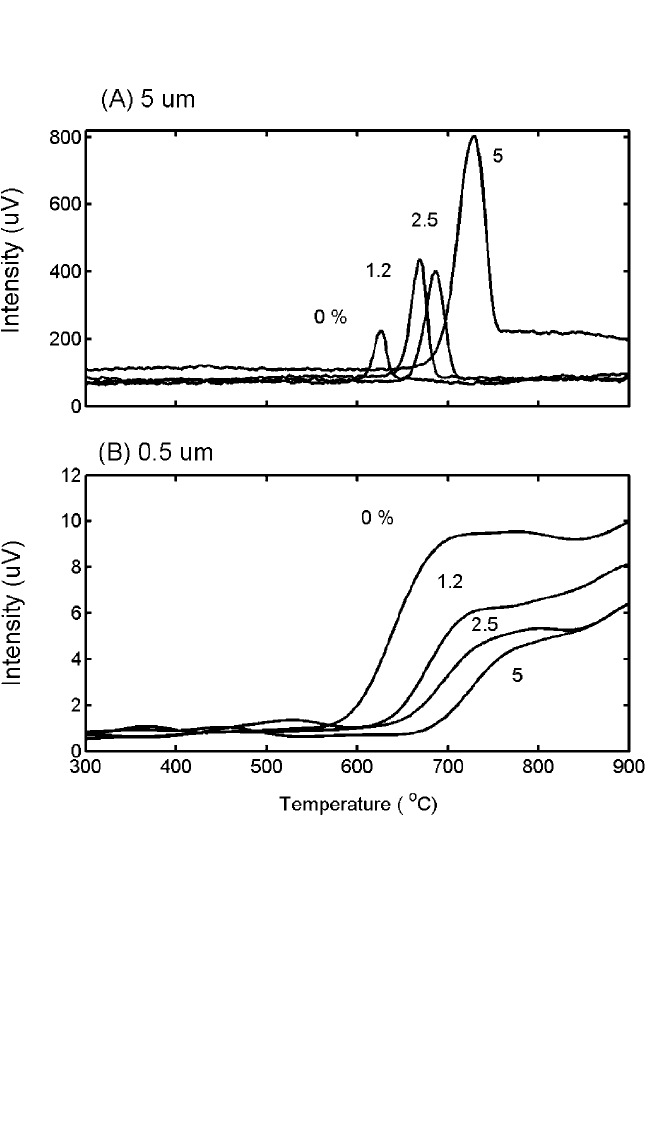
critical nuclei. The effect is well illustrated in Fig. 6.9, where the nucle-
ation temperature for the formation of CoSi
2
is seen to increase when the
Co film is alloyed with different amounts of Ge. (See Lavoie et al.
[123]
for
this and other alloying elements.) An opposite effect had been observed
with the use of Co additions to Ni, where alloying with 50 at.% reduced
the nucleation temperature to about 350°C,
[124]
below what is required to
nucleate pure CoSi
2
. In that case, the two preceding phases, NiSi and
CoSi, with different structures (respectively, MnPtype: orthorhombic, and
FeSi type: cubic, complex) did not mix and were clearly separated. In
Ch_06.qxd 11/12/04 4:06 PM Page 317
318 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
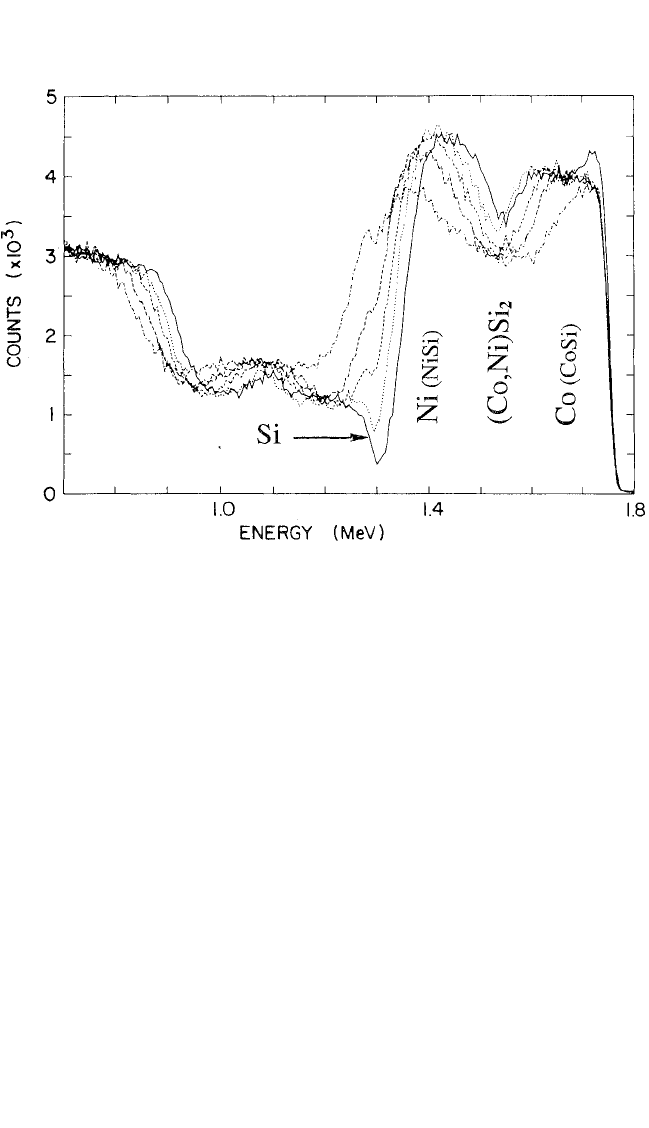
Fig. 6.10, the NiSi and CoSi monosilicides are seen to form in the same
sequence near the Si substrate and at the surface, regardless of the order
of the metal film depositions, Co then Ni, or vice versa, or alloyed solid
solution. The new solid solution (Co, Ni)Si
2
forms at the interface between
the two phases, clear evidence of the role of nucleation control. The key
Figure 6.9 Elastic scattering of light for two different lateral scales, (A) 5 mm and
(B) 0.5 mm, for a Co film with various amounts of Ge during heating on Si (100) at
3 K/s. The sharp features correspond to the nucleation and formation of CoSi
2
.
There is very little evolution of roughness below 600°C during the formation of
Co
2
Si and then CoSi. Both of these reactions are diffusion-controlled.
Ch_06.qxd 11/12/04 4:06 PM Page 318
REACTIVE PHASE FORMATION, D’HEURLE ET AL. 319
point here is the new phase, a single-disilicide phase, made possible by the
nearly total similarity of NiSi
2
with CoSi
2
, which have the same structure
(CaF
2
) and the same lattice parameters; namely, a solid solution of the
two. The reaction then becomes:
12 CoSi 12 NiSi Si (Co, Ni)Si
2
. (26)
For such a reaction, in comparison with Eq. (21) for the formation of NiSi
2
and identically for CoSi
2
, the ∆G is increased by a quantity proportional
to the entropy of mixing. For one mole of (Co, Ni)Si
2
, that quantity would
be kT[xlnx (1x)ln(1x)], where x is the metal composition [from
0 to 1, but 12 in Eq. (26)]. The increased ∆G increases the density of crit-
ical nuclei and thereby decreases the temperature at which nucleation is
observed. For the additions of Ge to Co (Fig. 6.9), the effect is opposite
Figure 6.10 Backscattering spectra showing the formation of (Ni, Co)Si
2
at the
interface between NiSi and CoSi, where entropy of mixing effects increase the
free-energy change for the formation of the disilicide solid solution. The position of
the initial transformation underlines the importance of nucleation in opposition to
diffusion as the controlling mechanism.
[91]
The spectra correspond to samples
(Si/Co/Ni) heated at 475°C for 1.5, 3, 7, 12, and 34 hours, respectively. Back-
scattering alone does not enable us to distinguish between Co and Ni; that was
done separately by means of Auger spectroscopy.
Ch_06.qxd 11/12/04 4:06 PM Page 319

320 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
because, in that case, the monosilicide and monogermanide are soluble
(with the same structure and compatible lattice parameters). The diger-
manide and disilicide with different structures appear not to be very mis-
cible. The reaction has the form:
Co[Si
(1x)
, Ge
x
] Si → (1x)CoSi
2
12 xCoGe
2
. (27)
Compared to Eqs. (21) and (26), Eq. (27), going from left to right, entails
a decrease in entropy, a decrease in the absolute value of ∆G, a decrease
in the density of nuclei, and, as observed, an increase in the nucleation
temperature. A number of alloying additions are observed to behave
according to either Eq. (26) or (27); these are nicely systematized by
Detavernier et al.
[125]
If excess Si is present with the product phases in 27,
as is often the case, the digermanide can be reduced to CoSi
2
and Ge sub-
sequent to its formation. This is due to the higher stability of the silicides
in comparison to the germanides (higher heats of formation in absolute
values). The latter effect, however, is not germane to this discussion.
These qualitative considerations are fundamentally correct, and with
Co-Ni alloys, they apply fully with compositions near 50%. However, we
must be careful. Aternary section of the Co-Ni-Si equilibrium phase dia-
gram, with Si at the top, is dominated by two parallel (horizontal) domains
of complete solubilites for the dimetal silicides and the disilicides and in
the middle a third region for the monosilicides, with solid solution
domains extending to about 20% on each side separated by an immisci-
bility gap.
[126]
Between 500 and 800°C, where CoSi
2
or (Co, Ni)Si
2
nucle-
ates, no drastic change is anticipated. Then why should small amounts of
Ni additions, as low as 2%, cause detectable reduction in the nucleation
temperature? Inasmuch as both the parent monosilicide and the product
disilicide phases are solid solutions, an explanation based on the entropy
of mixing fails. It is probable that the effect is due to an additional
enthalpy effect in the disilicide. In general, phases are most stable when
the density of state (DOS) at the Fermi level is low. Other things being
equal, a structure with the Fermi level at a minimum in the DOS curve
implies a maximum in the number of bonding states below the Fermi
level, and low energy levels for those states. This is exemplified by semi-
conductors where the density of states falls to zero in the gap: Compare
diamond to nitrogen, adjacent in the Periodic Table, or Si to Al, or Ge to
Ga. More subtle examples can be found. For the transition metals with
BCC structure, the calculated density of states at the Fermi level is found
in a trough (referred to as pseudo gap) in the BCC but not in the FCC
structure. It is the BCC structure that is stable [127, p. 135]. For HCPmet-
als (Zr, Hf, Ru, and Os), the Fermi level is also found at troughs in the
DOS curves.
[128]
In the Hume-Rothery phases, the b and g brasses in the
Ch_06.qxd 11/12/04 4:06 PM Page 320
REACTIVE PHASE FORMATION, D’HEURLE ET AL. 321
Cu-Zn system, the density of states at the Fermi level is also at a minimum.
[129]
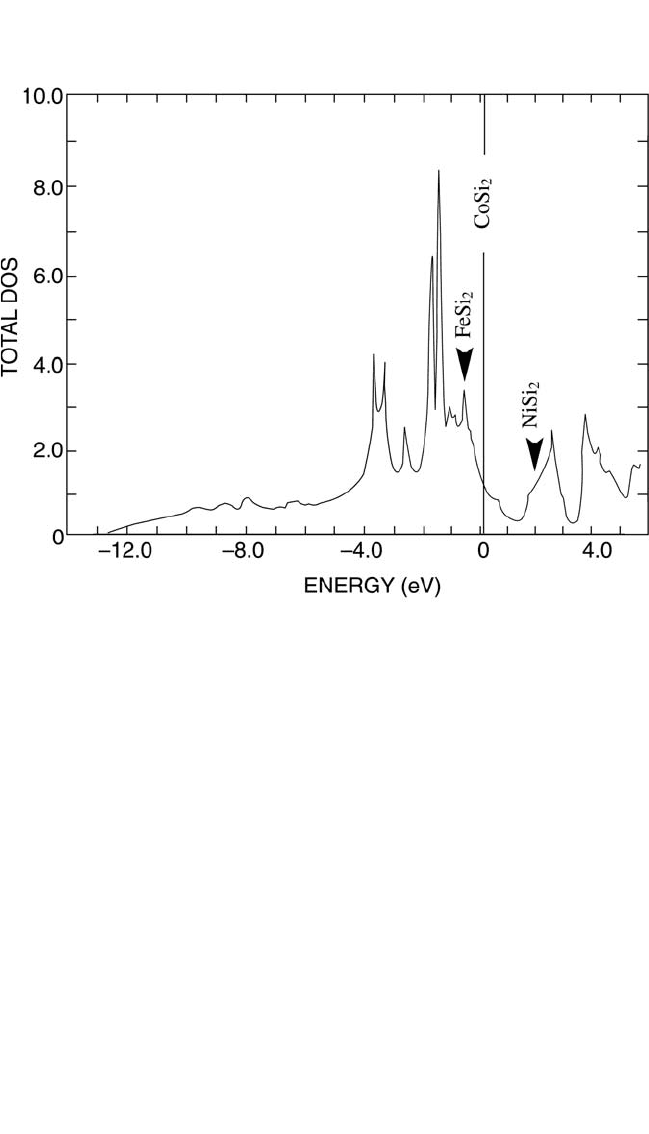
Figure 6.11 displays the DOS versus energy curve for CoSi
2
.
[130]
The
Fermi level is found with bonding states on the low-energy side of a
pseudogap. For NiSi
2
, with one more electron, and for FeSi
2
(in the CaF
2
structure), with one less electron, the curves are nearly identical although
somewhat shifted on the energy scale. However, the Fermi level for NiSi
2
is found on the high-energy side of the pseudogap, in the antibonding
states. For FeSi
2
, on the contrary, the Fermi level moves to low energies
and lies precisely at the peak found at about 0.5 eV in Fig. 6.11.
As a further illustration, the high density of states at the Fermi level
constitutes an unstable condition, so that in equilibrium FeSi
2
, a distortion
Figure 6.11 Density of states versus energy for CoSi
2
, with the Fermi level marked
by a vertical line near zero on the energy scale. The numbers on the vertical scale
are eV per formula unit. For silicides with the same CaF
2
structure, the Fermi lev-
els would be found in the antibonding states for NiSi
2
at the arrow on the right, and
among the bonding states for FeSi
2
at the arrow on the left. The density of states
curves are essentially the same for all three disilicides but would be slightly dis-
placed on the energy scale. Here the positions of the Fermi levels are given in
relation to that of CoSi
2
. According to Jepsen et al.
[128]
Ch_06.qxd 11/12/04 4:06 PM Page 321
