Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.


332 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
138. C. Detavernier, C. Lavoie, and F. M. d’Heurle, J. Appl. Phys., 93:2510
(2003)
139. M. Kh. Rabadanov and M. B. Ataev, Inorg. Mater., 38:120 (2002)
140. D. F. Wilson and O. B. Cavin, Scripta Metall. Mater., 26:85 (1992)
141. D. Z. Chi, D. Mangelinck, S. K. Lahiri, P. S. Lee, and K. L. Pay, Appl. Phys.
Lett., 78:3256 (2001)
142. P. S. Lee, K. L. Pey, D. Mangelinck, J. Ding, D. Z. Chi, and L. Chan, IEEE
Electr. Dev. Lett., 22:568 (2001)
143. V. Theodorescu, L. Nestor, H. Bander, H. Steegen, A. Lawers, K. Maex, and
J. V. Landuyt, J. Appl. Phys., 90:167 (2001)
144. T. G. Finstad, Phys. Status Solidi, 63:223 (1981)
Ch_06.qxd 11/12/04 4:06 PM Page 332

7 Metal Diffusion in Polymers and
on Polymer Surfaces
Franz Faupel and Vladimir Zaporojtchenko
Lehrstuhl für Materialverbunde, Technische Fakultät der
Universität Kiel, Germany
Axel Thran
Philips Research Hamburg, Hamburg, Germany
Thomas Strunskus
Ruhr-Uni Bochum, Lst.f. Physikalische Chemie I, Bochum, Germany
Michael Kiene
AMD Saxony LLC&Co.KG, Dresden, Germany
7.1 Introduction
Metal diffusion plays a crucial role in polymer metallization, which is
indispensable in applications ranging from food packing to microelec-
tronics.
[1, 2]
Diffusion of metal atoms, and sometimes also of small clus-
ters, along the polymer surface determines nucleation and growth of metal
films on polymers and hence has a strong effect on the resulting
microstructure.
[3]
Recently, there is also great interest in nano-size metal
clusters, which form by surface diffusion in the initial stage of polymer
metallization if the metal is not too reactive. This interest not only arises
from quantum size effects and single-electron tunneling phenomena
[4]
but
is also triggered by applications in medicine
[5]
and as substrates for bio-
molecules.
[6]
Above the polymer glass transition, which can be depressed
at the surface,
[7, 8]
metal clusters are generally embedded in the polymer
and, depending on their size and the polymer viscosity, may perform a
Brownian motion.
[9, 10]
In microelectronics, diffusion of metal atoms into the polymer bulk is
also a concern. This field has stimulated intensive research throughout
recent decades.
[1–3, 11–14]
Polymers have been used extensively as low-
permittivity (low-k) dielectrics in packaging and are now even used in on-
chip interconnects.
[13–16]
Both processing and operation of microelectronics
components involve exposure to elevated temperatures. In chip applica-
tions, diffusion of even very small amounts of copper, acting as a deep-
level impurity, from the polymer into silicon would lead to device failure.
[14]
Transport may be further enhanced by the very strong electrical fields
resulting from the small feature sizes. Therefore, much effort has been
made to control the microstructure and thermal stability of metal-polymer
Ch_07.qxd 11/12/04 4:10 PM Page 333

334 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
interfaces, especially with the aim to block diffusion by suitable barriers,
improve adhesion, and prevent degradation.
[1–3, 9, 11–14, 17–24]
Meanwhile, polymers are not only used as passive dielectrics in
conventional microelectronics; they are increasingly being used as
active semiconducting components in low-cost organic electronic devices
such as light-emitting diodes and displays, solar cells, and field effect
transistors.
[25–27]
Here direct contact between the organic semicoductor and the
metal contacts generally cannot be avoided because the metals, which are
selected to adjust their work functions to the highest occupied and the
lowest unoccupied molecular orbitals, are needed for charge injection.
Metal diffusion into the organic semiconductor has particularly been
observed for metal-on-polymer deposition, whereas the polymer-on-metal
interface seems to be quite sharp.
[27]
Massive diffusion can be expected for
alkali metals and other metals like In that are able to reduce the organic
molecules and to diffuse as highly mobile cations.
[28–30]
The repulsion of
the ions prevents aggregation, which usually gives rise to strong immobi-
lization of diffusing neutral metal atoms.
[9]
From the fundamental point of view, metal diffusion in polymers can
only be understood by taking into account the strongly contrasting prop-
erties of both materials. While metals are densely packed crystalline
solids with a high cohesive energy, noncrosslinked polymers are made up
of large covalently bonded macromolecules held together by very weak,
mostly only van-der-Waals-type interactions in an open structure. The
cohesive energy of metals is typically two orders of magnitude higher
than the cohesive energy of polymers. Furthermore, the interaction
between moderately reactive metals and polymers is generally much
weaker than the strong metal-metal binding forces. As a consequence,
these metals are expected to exhibit a strong aggregation tendency, and
their solubility in polymers should be extremely low under equilibrium
conditions. Hence, practically no intermixing should occur when a piece
of metal of low reactivity is brought into close contact with a polymer sur-
face.
[9]
For the same reasons, metals of low reactivity do not wet untreated
polymer surfaces. They form clusters during polymer metallization, which
finally coalesce and form a continuous film (Volmer-Weber growth).
In view of the extremely low solubility of metals in polymers, it is obvi-
ous that the aforementioned embedding of metal clusters in polymers above
the glass transition temperature is a process being entirely different from
ordinary dissolution, for example, of gas molecules in polymers. Apparently,
there is a driving force for embedding of metal clusters; that is, the Gibbs free
energy of a metal particle inside the polymer is lower than that of the parti-
cle at the surface. Once more this is related to the high cohesive energy of
metals, which gives rise to a correspondingly high surface Gibbs free
energy of metal particles. The surface Gibbs free energy can be reduced by
Ch_07.qxd 11/12/04 4:10 PM Page 334

embedding if the surface tension g
M
of the metal particles exceeds the sum of
the interfacial tension g
MP
and the polymer surface tension g
P
:
[10, 31]
g
M
g
MP
g
P
. (1)
Since the cohesive energy of polymers is so much lower than that of met-
als, g
P
is very small in comparison to g
M
.
When a metal particle is covered by polymer, there is still a net van
der Waals force driving it deeper into the bulk, and an entropic force, due
to the confinement of the polymer chains near the metal particle, pushing
it back to the surface.
[10, 31]
This results in a size-dependent potential minimum
pinning large particles below the surface. Clusters of the order of 10 nm
or less can overcome the potential barrier by thermal activation.
[9, 10, 31, 32]
While the above considerations on metal solubility and the absence of
significant metal diffusion into polymers are based on equilibrium ther-
modynamics, the conditions during the initial stage of polymer metalliza-
tion are far from thermodynamic equilibrium. Here, the virgin polymer
surface is exposed to isolated metal atoms that do not have to overcome
the strong metallic cohesive force to become mobile. Therefore, signifi-
cant diffusion of metal atoms into polymers is only expected to take place
during the early metallization process or when the metal is deposited at a
very low rate. Unfortunately, despite the many investigations that have
been carried out on metal diffusion in polymers, the reported conclusions
are still strongly conflicting.
[9, 24]
The origin of the controversial views lies
in the complicated interplay of metal atom diffusion and aggregation. This
behavior contrasts diffusion of metals in polymers strongly with the ordi-
nary diffusion and may give rise to gross misinterpretations of diffusion
experiments carried out by surface analytical tools.
This chapter is mainly concerned with diffusion of metals in fully
cured polymers and on their surfaces. The general characteristics of the
polymer-on-metal interfaces are discussed by Kowalczyk et al.
[33]
and
Godbey et al.
[34]
Emphasis throughout is placed on investigations per-
formed by the Kiel group, but other important investigations
[1–3, 9–12, 14, 17]
are also addressed, although no attempt is made to give a fully compre-
hensive literature review. We treat metal diffusion during the early depo-
sition stages, involving metal condensation, nucleation, and growth. In
Sec. 7.3, metal-polymer interaction is addressed as a key factor determin-
ing atomic mobility. Section 7.4 is devoted to metal diffusion in the bulk
of polymers. Here, the interplay of atomic diffusion and aggregation,
which is observed for metals of not-too-high mobility, is discussed. This
interplay leads to a strong aggregation-induced metal immobilization. We
will see that the degree of aggregation and hence the extent of diffusion
strongly depend on the metal deposition conditions. Diffusion profiles of
METAL DIFFUSION IN POLYMERS, FAUPEL ET AL. 335
Ch_07.qxd 11/12/04 4:10 PM Page 335
336 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
metal atoms in polymer are generally non-Gaussian, and different ranges
are related to diffusion of clusters of different sizes. Therefore it is diffi-
cult to extract quantitative diffusion data that can be attributed unambigu-
ously to single atoms or clusters of known size. Section 7.4 also briefly
covers the diffusion behavior of reactive metals whose mobility is usually
blocked by strong chemical interactions with the polymer. Moreover,
alkali and other metals that are able to form highly mobile ions in poly-
mers are addressed. In this connection, the role of interfacial oxygen in
ion formation is also discussed for copper and other metals. The chapter
closes with a summary and important conclusions.
7.2 Diffusion During Nucleation and Growth of
Metal Films on Polymers
As discussed in Sec. 7.1, the early stages of polymer metallization are
far from thermodynamic equilibrium since isolated metal atoms impinge
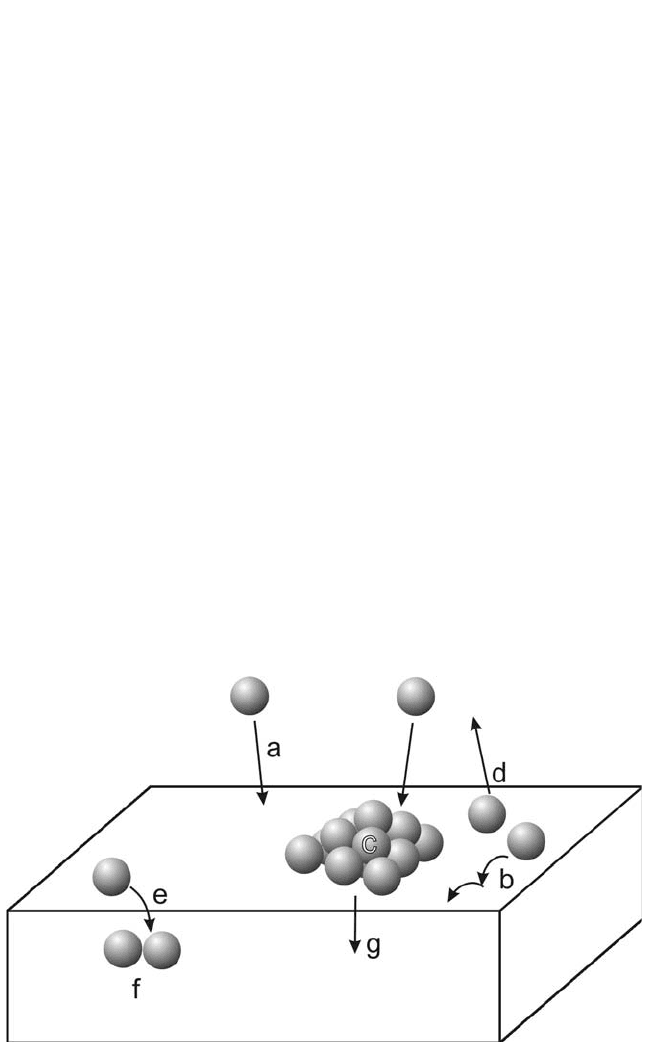
on the polymer surface. Various competing processes, illustrated in
Fig. 7.1, have to be considered.
[35]
Following deposition, the arriving
atoms (a) may perform a random walk on the surface (b), or diffuse into
the polymer (e). Metal atoms encountering each other on their diffusion
path may form aggregates at the surface (c) and in the polymer bulk (f).
These aggregates are stable if their size exceeds the size of a critical
Figure 7.1 Processes taking place during the initial deposition stage where iso-
lated metal atoms impinge on the polymer surface.
Ch_07.qxd 11/12/04 4:10 PM Page 336

nucleus. Above the glass transition temperature, metal clusters may also
be embedded into the polymer. Moreover, metal atom reemission into the
vacuum (d) has to be taken into account.
[36]
The sticking or condensation coefficient of metal atoms on metal sur-
faces is generally very close to unity, even at elevated temperatures. In
contrast, using two novel techniques based on radiotracer measure-
ments
[36]
and x-ray photoelectron spectroscopy (XPS),
[37, 38]
we have
recently demonstrated that the tendency of metals of low reactivity not to
wet polymer surfaces can be accompanied by a very low condensation
coefficient. The condensation coefficient C is defined as the ratio of the
number of adsorbed metal atoms to the total number of metal atoms arriv-
ing at the surface. Depending on polymer-metal combination and temper-
ature, C varies by several orders of magnitude. For example, condensation
coefficients close to unity are observed for Cu on the PMDA-ODA
(pyromellitic dianhydride-oxydianiline) and SiLK
®
up to temperatures as
high as 200 and 300°C, respectively.
[39]
Above these temperatures, how-
ever, C decreases drastically. On a polystyrene surface, the condensation
coefficient of Cu is only 0.26 at room temperature and drops further at
higher temperatures.
[38]
Finally, for Teflon AF
®
, C is as low as 0.02.
[38]
This means that only 2 of 100 atoms impinging on the surface of this
important low-k polymer stick to the polymer even at room temperature.
At elevated temperature, the fraction is much smaller still.
The condensation coefficients for Ag and Au are smaller than those
for Cu on all polymers. The differences are most prominent for strongly
incomplete condensation. For example, we found a C value as low as
0.002 for Ag on Teflon AF
®
,
[36, 37]
which is about an order of magnitude
smaller than the value for Cu.
The extreme differences in the metal condensation coefficients of the
polymers are the subject of ongoing investigations. We note, however, that
C follows the trend of the surface energies of the polymers. Since a low
surface energy impedes macroscopic wetting of the surface, it does not
appear unreasonable that a low surface energy also impedes atomic con-
densation. The increase of the condensation coefficient from Ag and Au to
Cu seems to be related to the higher reactivity of Cu. Adetailed discussion
of condensation of metals on polymers and a compilation of C values for
various metal-polymer systems is given by Zaporojtchenko et al.
[37–40]
In connection with diffusion, it is interesting to note that results from
the radiotracer technique, which measures the integral condensation coef-
ficient, and photoelectron spectroscopy, which exhibits a high surface sen-
sitivity, generally proved to be in good agreement.
[37]
This shows that even
for the low evaporation rates used in the measurements of C (typically 1
monolayer per min.), most of the metal atoms do not diffuse into the poly-
mer bulk. This can be attributed to the general behavior
[41]
that surface
METAL DIFFUSION IN POLYMERS, FAUPEL ET AL. 337
Ch_07.qxd 11/12/04 4:10 PM Page 337

338 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
diffusion is much faster than bulk diffusion. Therefore, most metal atoms
get trapped in metal clusters at the surface before they are able to “escape”
into the polymer bulk, as discussed in Sec. 7.4. Consequently, to a good
approximation, metal diffusion into the polymer bulk can be neglected during
nucleation and growth of metal films. This is not expected to hold for the
aforementioned alkali metals and other metals that reduce polymers, thus
forming highly mobile and mutually repelling ions.
At higher metal coverages, metal atoms increasingly impinge on metal
clusters, and finally the condensation coefficient approaches unity, which is
typical for condensation of metals on metals at moderate temperatures.
[36]
Note that even in the case of extremely low sticking coefficients, the
metal atoms are not backscattered directly but perform a random walk
prior to reemission. This conclusion is based on the measured cosΦ angu-
lar distributions of reemitted atoms, which is a “fingerprint” of random
emission and turned out to be independent of the angle of incidence.
[36]
Apparently, a fraction (1 C) of the metal atoms diffusing on the surface
desorbs thermally before it finds a nucleation site.
Two possibilities have to be taken into account for the nucleation. In
so-called preferred nucleation, metal atoms are trapped at preferred sites;
in random nucleation, nuclei are formed by metal atom encounters. Both
processes have been observed in polymer metallization.
[36, 37, 40]
Preferred
nucleation was shown for Ag on trimethylcyclohexane (TMC) polycar-
bonate, for example.
[36]
Here the Ag condensation coefficient is independent
of the metal evaporation rate. This clearly allows random nucleation to be
ruled out.
[36]
Apparently, nucleation takes place at special surface sites.
The nature of these sites is not known yet; one can, for example, think of
terminal groups of the polymer chains, impurities, or attractive local
arrangements of the chains. The number of these surface defects, and
hence the condensation coefficient, can be strongly increased by even
moderate ion-beam treatment.
[36, 40]
Preferred nucleation at defect sites is
particularly expected for low-condensation coefficients and at the initial
deposition stage. In all other cases, random nucleation dominates.
For a detailed quantitative treatment of metal nucleation on polymers
in terms of nucleation theory, we refer to Zaporojtchenko et al.
[38, 40]
Here
we only outline an approach for random nucleation, which also yields val-
ues for the activation enthalpy of surface diffusion.
The approach is based on measurements of the deposition rate and tem-
perature dependence of the maximum cluster density N
max
in the regimes of
incomplete and complete condensation. The latter regime is always accessi-
ble at sufficiently low temperatures, and cluster densities can be determined
from transmission electron microscopy (TEM) measurements.
[37]
The cluster
density quickly reaches a maximum during the nucleation period, shows lit-
tle changes thereafter, and finally drops as a result of cluster coalescence.
Ch_07.qxd 11/12/04 4:10 PM Page 338

The dependence of the maximum cluster density on the deposition rate
R can be used to evaluate the size of the critical cluster, which is important
to understand the interplay of diffusion and aggregation. For random nucle-
ation in the regime of complete condensation, nucleation theory predicts:
[42]
N
max
(RD
s
)
i
i
2.5
, (2)
where the integer quantity i is the critical cluster size and D
s
is the diffu-
sivity of metal atoms on the surface. A fit to experimental data for noble
metals was only possible with i 1.
[37]
This means that noble metal
dimers already form stable clusters on polymers. This is in accord with the
high cohesive energy of metals and the calculated binding energies for
dimers, for example, 77 kJ/mol for Ag.
[43]
With i 1 and the Arrhenius law for surface diffusion, Eq. (3) imme-
diately yields the temperature dependence of the maximum cluster density:
N
max
exp[E
d
(3.5kT)]. (3)
Thus, measurements of the maximum cluster density as a function of tem-
perature allow us to determine the activation enthalpy E
d
of surface diffusion.
In the regime of incomplete condensation, there is a competition
between thermal desorption with a rate t
exp(E
a
(kT)) and diffusion-
controlled nucleation. Here nucleation theory predicts:
[42]
N
max
exp[(23)(2E
a
E
d
)(3.5kT)]. (4)
Based on Eqs. (3) and (4), we have determined adsorption enthalpies E
a
and activation energies of surface diffusion E
d
for various noble metal
polymer systems. Representative plots of ln (N
max
) against 1/T are shown
in Fig. 7.2. A compilation of E
a
and E
d
values from Zaporojtchenko
et al.
[40]
is given in Table 7.1.
The adsorption enthalpies of Cu on polyimide, 58 ± 10 kJ/mol, and on
SiLK
®
, 67 ± 10 kJ/mol, turned out to be surprisingly high.
[39]
They reflect
a substantial interaction of the noble metal with these polymers, in accord
with conclusions drawn from bulk diffusion studies discussed in Sec. 7.4.
Ag and Au exhibit a much weaker interaction (29 kJ/mol for Ag on poly-
imide, for example). The adsorption energies are also much lower for
noble metals on polymers like polystyrene and Teflon AF
®
.
The activation enthalpies of surface diffusion are substantially lower
than the adsorption enthalpies, for example, 19 ± 5 kJ/mol for Cu on poly-
imide and 29 ± 5 kJ/mol on SiLK
®
.
[39]
This reflects nonlocalized binding
forces, which make it much easier to displace an atom at the surface than
to remove it completely. Similar to the adsorption enthalpies (E
a
), E
d
also
METAL DIFFUSION IN POLYMERS, FAUPEL ET AL. 339
Ch_07.qxd 11/12/04 4:10 PM Page 339
340 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
decreases following change of the metal species (from Cu to Ag and Au
or the polymers themselves) from polyimide and SiLK
®
to polystyrene
and Teflon AF
®
. E
d
values are also given by Zaporojtchenko et al.
[40]
Based on the activation enthalpies of surface diffusion, we also esti-
mated the ratio of the surface diffusivities D
s
of Ag, Au, and Cu, assuming
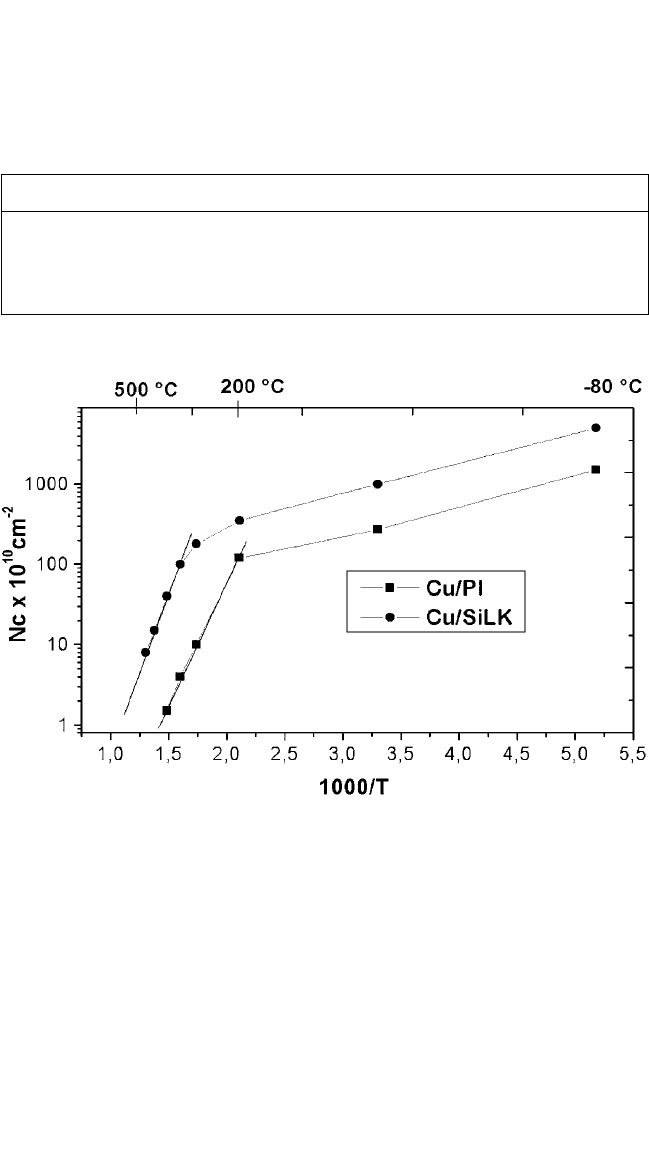
Figure 7.2 Maximum cluster density N
max
as a function of reciprocal absolute tem-
perature on a semi-logarithmic scale for Cu evaporated onto the polyimides
PMDA-ODA, DuPont PI-2545, and DuPont SiLK
®
. The regimes of complete con-
densation at low temperatures and incomplete condensation at higher tempera-
tures are clearly distinguished by two linear ranges of different slopes. The slopes
are related to E
a
and E
d
via Eqs. (3) and (4), and allow these important micro-
scopic quantities to be determined.
Table 7.1. Activation Enthalpies of Surface Diffusion E
d
and Adsorption
Enthalpies E
a
for Metals on Polymers
Metal/Polymer E
d
(kJ/mol) E
a
(kJ/mol) Reference
Ag/PMDA-ODA 8 ± 3.0 29 ± 10 [37]
Ag/TMC-PC 6 ± 3.0 17 ± 10 [36]
Cu/PMDA-ODA 20 ± 5.0 58 ± 10 [39]
Cu/ SiLK
®
29 ± 5.0 67 ± 10 [39]
Ch_07.qxd 11/12/04 4:10 PM Page 340

that the pre-exponential factors do not differ much. For polyimide at room
temperature, D
s
ratios for Ag/Au/Cu are 100/2/1.
[37]
The larger surface dif-
fusivity of Ag compared to Au is unexpected, judging from the metal reac-
tivity. Apparently, the general trend of the reactivity does not account for
the interaction with the polymer. Deviations from the general trend are
also seen in the complex formation behavior of Ag and Au.
[44]
The higher
surface diffusivity of Ag compared to Cu also calls for further comment,
because the opposite behavior has been observed in radiotracer measure-
ments of bulk diffusion.
[9, 45]
The slower diffusion of Ag in the bulk was
attributed to the larger size of Ag atoms, which should reduce the bulk dif-
fusivity substantially in a glassy polymer. However, diffusion at the sur-
face should not be affected significantly by size effects because factors
like availability of free volume and distortion of the polymer do not come
into play. This supports the view that the reported Ag bulk diffusion coef-
ficients do not reflect atomic diffusion but are diffusivities of very small
clusters (see Sec. 7.4).
7.3 Metal-Polymer Interaction
It is obvious that the mobility of metal atoms in polymers and on
their surfaces is correlated with the extent of chemical interaction.
Surprisingly, irrespective of extensive research throughout recent
decades, our present knowledge of metal-polymer interaction is still
rather incomplete, particularly with respect to the details of the interac-
tion mechanisms and the early deposition stages.
[17, 28, 46, 47]
This is partly
due to the strong aggregation tendency of moderately reactive metals on
polymers. Here, the chemical interaction at room temperature and above
occurs between metal clusters and the polymer. This not only leads to a
significant drop of the detectable interfacial area but also may change
the mode of interaction significantly because the chemistry of clusters
and single atoms is not expected to be the same. The observations
of chemical interactions of isolated noble metal atoms and polymer
surfaces require the deposition at much lower temperatures.
[48]
An addi-
tional complication arises because of the frequently low sticking coeffi-
cients discussed in Sec.7.2.
[36–38]
In most studies, the sticking coefficient
was assumed to be unity, and the absolute metal coverage was deter-
mined by means of a quartz balance. This procedure strongly overesti-
mates the metal coverage if the sticking coefficient on the polymer
surface is low.
Nevertheless, consensus exists that Au, Ag, Cu, and Pd interact
weakly with polymers.
[48, 50–58]
For highly reactive metals such as the
transition metals Cr
[17, 46, 52, 58, 59]
and Ti,
[60–62]
the rare-earth metal Ce,
[63]
and
Al,
[64–67]
the available experimental data show clear signs of strong chemical
METAL DIFFUSION IN POLYMERS, FAUPEL ET AL. 341
Ch_07.qxd 11/12/04 4:10 PM Page 341
