Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.


302 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
heating that may occur. The modalities of nucleation-controlled formation
in the silicides are detailed in the literature;
[26]
some consequences are
summarized below.
With thin films, and experiments conducted at relatively low tem-
peratures, difficult nucleation can affect the order of phase formation. A
case in point is offered by comparing the sequences of phases in Rh and
Ir reactions with Si. The two metals are quite similar and are found just
below one another in the same column of the periodic table. Hence the
respective equilibrium diagrams display remarkable similarities yet are
not totally identical. With either metal, one step in the sequence of phase
formation is the monosilicide (either RhSi or IrSi); then the similarity
ends. With Ir, we observe the formation of Ir
3
Si
5
, bypassing two other
phases: Ir
4
Si
5
and Ir
2
Si
3
. In this case, the ∆G for the formation of Ir
3
Si
5
is sufficiently large to allow easy nucleation and the diffusion-controlled
formation of that phase at temperatures lower than necessary to nucle-
ate the two missing phases, with smaller composition changes with
respect to IrSi, and therefore also smaller ∆G changes. With Rh, how-
ever, the list of silicon-rich silicides ends before Rh
3
Si
5
, which does not
exist. Then, after the formation of RhSi, we observe the nucleation-
controlled formation of Rh
3
Si
4
, curiously with very few nucleation sites,
some 2 or 3 mm apart, visible with the naked eye. Thus we can conclude
that the existence of Ir
3
Si
5
prevents the formation of the other phases,
Ir
4
Si
5
and Ir
2
Si
3
.
A somewhat similar effect is observed in the sequence of phases
during the reaction of Ni films with Si. The sequential formations of
Ni
2
Si and NiSi were considered at the beginning of this section.
Another phase exists, Ni
3
Si
2
, that does not appear in the sequence of
phases experimentally observed. In the absence of an explanation for
the failure of that phase to form after Ni
2
Si, it might be tempting to
assign this failure to some nucleation process; that would at least please
nucleation afficionados. But we do not know. However, once NiSi has
began to form from the reaction of Ni
2
Si with Si, Ni
3
Si
2
would form
from the reaction:
Ni
2
Si NiSi Ni
3
Si
2
, (22)
with a ∆H 0 ± 9 kJ/mol,
[24]
nearly infinitely small. To the extent that
the phase is thermodynamically stable, the value of ∆G for Eq. (22) has
to be negative. By artificially preparing thin-film samples with an over-
all composition corresponding to Ni
3
Si
2
, but composed of adjacent lay-
ers of Ni
2
Si and NiSi, it has been shown
[47]
that the nucleation of Ni
3
Si
2
under the conditions where the two adjacent phases preexist occurs only
Ch_06.qxd 11/12/04 4:05 PM Page 302

REACTIVE PHASE FORMATION, D’HEURLE ET AL. 303
at temperatures above those necessary to form NiSi. In bulk samples
where observations are carried out at temperatures on the order of
800°C, rather than 400°C with thin films, nucleation effects are hardly
noticeable. All anticipated phases are observed to grow, more or less
simultaneously, at least at times sufficiently long to allow observations
in the optical microscope. Yet singularities are observed even then.
[48, 49]
Figure 6.6 is the cross section of a bulk Ni-Si sample heat treated
at 800°C for 6 hours. Anumber of phases are distinguishable, but atten-
tion should be focused on Ni
3
Si
2
. The other phases have more or less
planar interfaces, as anticipated in binary systems, but not Ni
3
Si
2
,
which displays a morphology similar to that of the jaws of Tyrannosaurus
rex. It might be tempting to attribute such a singular appearance to
anisotropic diffusion, but that is quite unlikely since this would require
extremely large anisotropy factors. Tentatively, we may propose the
attractive alternative that the cause of the extremely irregular interface
is stress and stress relaxation that occur in very different conditions,
whether at the valley between teeth or at the peak of the teeth. Because
the equilibrium ∆G is so small, even minor changes in elastic (and
possibly plastic) energy would make considerable differences in the
Figure 6.6 Cross section of a Si-Ni diffusion couple after 6 hours at 800°C. Note
the very irregular interface between Ni
3
Si
2
and NiSi. From Gülpen.
[48]
Ch_06.qxd 11/12/04 4:05 PM Page 303

304 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
driving force for growth; hence the unexpected morphology. Initially,
differences in orientation and consequently, differences in anisotropic
stress or diffusion, might be the cause of irregularities that increase in
magnitude as growth proceeds, with stress assuming the primary role.
Because of the small ∆G’s, we anticipate that stress would affect these
nucleation-controlled reactions, so that one of us (d’Heure) has been on
the lookout for such effects for some 30 years, in vain. The bulk growth
of Ni
3
Si
2
might have finally provided the smoking gun. We may won-
der whether some strong crystalline anisotropy is necessary for the
development of interfaces such as those observed with Ni
3
Si
2
.
Otherwise, we might expect the same behavior for NiSi
2
, although in
this case, the chemical (equilibrium) ∆G could be too large to be
affected significantly by variations in ∆H
elas
. In multiphase growth,
however, as difficult as it might be to know small values of ∆G, it is
clear that the value is zero at a eutectic or peritectic point, even if, as a
result, the exact eutectic or peritectic temperature is not known pre-
cisely. In such cases, the nucleation of the corresponding phase (or
phases) is likely to be very difficult. In an otherwise excellent discus-
sion of V-Si reactions,
[10]
that point is not so clearly outlined with
respect to the formation of V
6
Si
5
.
6.2.8 Amorphous and Other Metastable Phases,
Quasicrystals, Ternary Systems
So far, nothing in this discussion has excluded the formation of
metastable phases as a result of reactive diffusion. It has been emphasized
that in the competition for the growth of different phases from reacting
elements, the respective ∆G
f
’s vary little from each other, at least in com-
parison with the respective D’s. This is also true, for example, of amor-
phous phases, with free energies of formation ∆G only slightly higher than
the corresponding values for the crystalline phases. As a first approxima-
tion, the excess free energy ∆G
excs
∆T ∆S
melt
, where ∆T is the differ-
ence between the temperature T and the melting temperature T
melt
, and
∆S
melt
is the entropy of melting ∆H
melt
T
melt
. Samwer et al.
[50]
and Herr
et al.
[51]
review this subject. Two criteria are usually put forward for the
formation of amorphous phases. The first appears to be thermodynamic, a
high ∆G
f
of formation for the corresponding crystalline phases; the sec-
ond is kinetic, a high difference in the diffusion coefficients of the two or
three species present in the phase. The second criterion seems to be the
most important and the most direct because crystallization in a binary
system requires that the two elements be mobile. If one element is not
Ch_06.qxd 11/12/04 4:05 PM Page 304

REACTIVE PHASE FORMATION, D’HEURLE ET AL. 305
mobile, the phase can grow via the motion of the mobile element, but once
grown, it will remain in an amorphous state until the sample is brought to
a temperature high enough to allow the motion of the slow-moving
element. This is well typified by the growth of thermal SiO
2
, which grows
via the very fast interstitial diffusion of molecular oxygen in the SiO
2
net-
work, while the Si atoms with an activation energy for motion of the order
of 5 eV are totally immobile at temperatures below 1100°C. That is sim-
ple enough.
The first criterion, a high ∆G
f
of formation, has a direct thermody-
namic implication: The sum ∆G
f
T∆S
melt
has to remain negative for
the phase to grow, even in a metastable amorphous condition. But this
high ∆G
f
of formation has two important kinetic implications as well.
Ahigh ∆G
f
of formation implies, in general, a high T
melt
, so that at ordi-
nary temperatures (much below T
melt
), the atomic mobilities will be
small, making any crystallization difficult. To be sure, a high T
melt
implies a high driving force for crystallization at a low temperature T,
as the force increases linearly with the difference T
melt
T, while the
atomic mobility necessary for crystallization decreases as some expo-
nential function of that difference. Moreover, there is a second aspect
to this: A high ∆G
f
of formation implies almost necessarily a large dif-
ference in atomic mobilities in any compound that is not equiatomic
(type AB). This is so because, while the majority of atoms can usually
diffuse along their own network without undue interference, the motion
of the minority atoms often requires that these occupy the sites of
majority atoms. This requires the local destruction of the structure of
the growing phase and an excess activation energy for motion equal to
at least a fraction of the free energy of formation; hence, the higher the
latter, the bigger the difference in mobilities between the two atomic
species.
[21]
This is the prime criterion for the growth of an amorphous
phase.
A brief exploration of the literature shows that the mostly kinetic
conditions that govern the reactive formation of amorphous phases do
not apply for the formation of other metastable phases. The formation of
the metastable Ni
2
Al
9
already discussed
[36, 37]
seems to depend not on
kinetic details but on the complex details of the nucleation process. That
a compound with a corresponding composition is a stable phase in the
Co-Al system confirms suspicions that Ni
2
Al
9
is not far from being sta-
ble also, so that even a minor decrease in surface or elastic energy would
be enough to stabilize that phase in preference to the stable NiAl
3
. The
formation of NiSi with the NiAs structure epitaxial on (111) Si is clearly
dictated by the low interfacial energy,
[52]
not by considerations about the
relative mobilities of Ni and Si. The same is true of the formation of
FeSi
2
and other Fe silicides with the CaF
2
structure.
[53]
These phases
Ch_06.qxd 11/12/04 4:05 PM Page 305

306 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
tend to transform to the stable form beyond a critical thickness where
the constant “gain” due to a low interface energy becomes lower than
the increasing “loss” (proportional to the thickness) due to the
metastable phase.
Some of these metastable phases do not form initially, but after other
phases have already formed, for example, Ni
2
Si before NiSi. Two
metastable phases were observed in the reaction of Al with Pt, after an
amorphous phase was formed initially.
[54]
We have already alluded to the
formation of quasicrystalline phases.
[25]
Further information is contained
in the proceedings of symposia.
[55, 56]
In Al-Pd reactions,
[37]
the stable
phase Al
3
Pd
2
was observed to grow prior to the decagonal quasicrystalline
Al
3
Pd. During the reaction of Co with Al, the stable Co
2
Al
9
grows first,
then a decagonal phase Co
4
Al
13
.
[57]
For this phase, it has been shown
[57–59]
that 1) the enthalpy released during its formation is within experimental
errors equal to that expected for the stable phase; 2) changes in thickness
do not affect the phase sequence and do not suppress its formation; 3) an
increase in temperature causes the formation of the stable phase. A low
interface energy and, therefore, preferential nucleation are presumed to be
at the origin of the formation of such quasicrystalline phases.
[55, 56]
Few
words are more randomly misused in the literature than the word “stable.”
In the same vein, care should be taken with the expression metastable: In
the reaction of Au with amorphous Si,
[60]
we can form compounds that are
metastable with respect to thermodynamic equilibrium, yet are stable with
respect to amorphous Si, since they form with a negative enthalpy of
formation.
Reactions in ternary systems deserve more attention; they constitute
a subject in themselves. Reactions between Ni and SiC are considered
by Lien et al.
[46]
and Gas et al.
[47]
Aspects of the reaction of Ti with Si-Ge
are analyzed by Thomas et al.
[61]
and Aldrich et al.
[62]
Kodentsov et al.
[63]
and Loo
[64]
discuss ternary reactions in general. All that has been writ-
ten, of a more or less theoretical character, implies that researchers are
working with very pure materials. Impurities even in small quantities
may change the character of the reactions from that of a binary to a ter-
nary or higher order system, with serious consequences. Impurities may
affect kinetics by forming more or less porous diffusion barriers, or by
reducing grain boundary diffusion. In the reaction of Si with commer-
cial Fe, different results are obtained with different grades of Fe (see
Shimozaki et al.,
[65]
Fig. 2). Single-phase growth (Fe
3
Si) is observed in
one case, and multiple-phase growth in the other. (The explanation
given by the authors may or may not be correct.) Similarly, the reaction
between Mo and Si should lead to multiphase growth, contrary to the
report of the formation of MoSi
2
only.
[9]
Ch_06.qxd 11/12/04 4:05 PM Page 306

REACTIVE PHASE FORMATION, D’HEURLE ET AL. 307
6.2.9 Other Effects: Grain Boundaries and
Impurities, Diffusion Coefficients
Varying with Composition
In general, with binary systems, the interfaces remain planar and par-
allel to themselves. This has been observed to be true with silicide phases
such as NiSi and PtSi, and less true with phases whose formation is
nucleation-controlled, such as NiSi
2
. Effects due to grain boundary dif-
fusion can often be neglected, but not always. Astudy of Au-Cu interac-
tions at about 200°C in thin films
[66]
did not lead to publication because
the interactions were dominated by the very fast intergranular diffusion
of Cu inside the Au film, which made any kinetic interpretation
extremely ambiguous. A similar effect with Al films had serious practi-
cal implications for electromigration. We can delay electromigration fail-
ures in Al thin-film conductors, by forming a continuous intermetallic
layer
[67, 68]
of Al with some transition metal in parallel. Because of the
considerably smaller diffusion coefficients in such layers, they remain as
an electrical bridge in those regions where failure has already occurred in
the adjacent Al film with its relatively high diffusion. When attempting
to form a thin continuous layer of an intermetallic compound between,
for example, Al and Hf, it was observed that the compound formed pref-
erentially along the grain boundaries of the Al film, and thus could not
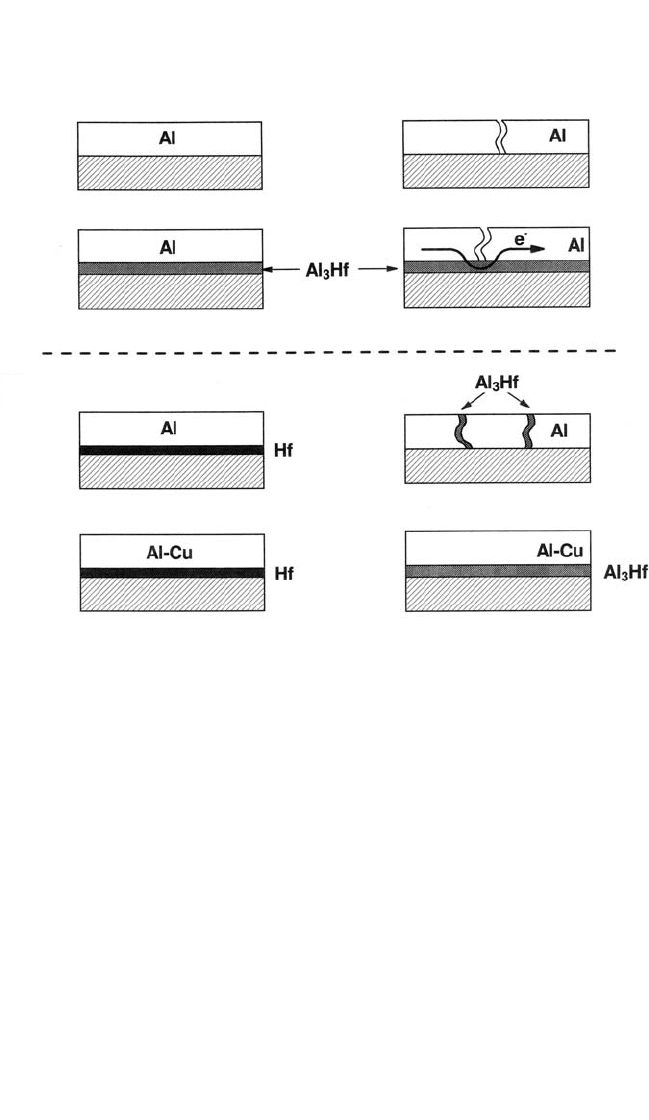
act later as the desired electrical bridge (see Fig. 6.7). Continuous inter-
metallic layers, however, could be formed if, instead of using pure Al,
one uses Al alloyed with Cu, which is known to reduce grain boundary
diffusion in Al. Indeed, this alloy addition is used to retard electromigra-
tion failure in Al thin film conductors by a factor of about 50.
[69, 70]
The
grain boundary impurity effect being considered here is quite general:
Impurities segregate to grain boundaries, thereby lowering the grain
boundary energy, and simultaneously increasing the activation energy for
grain boundary diffusion and decreasing the diffusion coefficient.
[71]
This
effect is mentioned again later. There is also an interesting experimental
mode where diffusion does not proceed in a direction normal to the inter-
face, as in ordinary diffusion couples; instead, it occurs along the lateral
dimension of a thin film. A very good example referring to Al-Ni reac-
tions is provided by Liu et al.
[72]
This discussion of the reactive phase formation has assumed that the
diffusion coefficient in the growing layer is either constant in each grow-
ing layer from one interface to the other, or that some average value pro-
vides a valid approximation. In some cases, this assumption leads to an
erroneous interpretation. In the thin-film reaction of Al with Ni at about
100°C,
[73]
it was observed that the central phase, NiAl, did not appear as a
Ch_06.qxd 11/12/04 4:05 PM Page 307
308 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
single phase. Instead, it was divided into two regions, each with a com-
position corresponding to the extreme limits of stability, about 45 and 55
at.% Ni. This effect is not actually due
[74]
to some thermodynamically
mandated phase separation; it is the simple result of kinetics, because the
diffusion coefficients at these two extreme compositions are immensely
higher than at the central composition (50 at.%). Since phases grow only
at interfaces, the flux of atoms, proportional to D dmdx, must be con-
stant from one interface to the other. If in some region of the growing
phase D is very small, a constant flux is achieved by making the gradient
dmdx very large. For example, in the case of NiAl, the phase does not
grow at the stoichiometric composition where the diffusion coefficient is
Figure 6.7 Schematic representation of the effect of a layer of transition metal
compound on electromigration in Al thin-film conductors. From left to right: In
(A), the presence of a layer of intermetallic compound provides an electrically
continuous path, even after failure of the Al conductor itself. In (B), with pure Al
the product of the reaction between Al and a transition metal tends to be scat-
tered along the grain boundaries of the Al film, but a continuous layer is
obtained if the Al is alloyed with Cu, which reduces grain boundary diffusion in
Al. Not to scale.
(A)
(B)
Ch_06.qxd 11/12/04 4:06 PM Page 308

REACTIVE PHASE FORMATION, D’HEURLE ET AL. 309
the smallest. Another example, Fe-Si, has been discussed by d’Heurle
et al.
[75]
At least in some phases with very narrow composition limits, the
diffusion coefficient is likely to be a very strong function of composition,
but the resulting effect may not be observable.
Following considerations of a more or less theoretical character, some
very practical effects in silicide thin films are examined in Section 6.3.
Before doing so, let us recall some topics of general interest. Thin-film
studies of interactions of Al or Au with transition metals
[76, 77]
demonstrate
the following: (1) The first phase to form (there as well as elsewhere) is
one that is rich in the metal with low melting point (either Al or Au).
(2) This phase grows by the motion of the species with the low melting
point, as mandated by the ordered Cu
3
Au rule.
[21]
This rule (1) does not
apply so clearly to silicides because during reaction, Si undergoes a state
transformation (from covalent with coordination number 4 to a much
more metallic state with coordination number 10). For example, in TiSi
2
,
Si has little similarity with elemental Si (unlike, for example, Ni in Ni
3
Al).
For more information, it would be worthwhile to consult
Schmalzried’s general book,
[78]
although it is a little heavy on ionic solids
for our purpose. The general equations for multiple phase growth have
already been mentioned.
[7, 8]
There are several good reviews of thin-film
silicides formation.
[21, 79, 80]
For reactions in bulk samples, there are some
earlier investigations on the formation of Al-Ti
[81]
and Ni-Al
[82]
inter-
metallics, and some recent ones on Ni-Si
[49]
and Co-Si
[83]
silicides.
Diffusion in silicides
[84]
and in intermetallic compounds in general
[85–87]
have also been discussed.
6.3 Practical Problems in Electronic
Technology
This section discusses some practical aspects of the use of com-
pounds formed via reactive diffusion in the electronics industry. Quite a
few of the problems currently encountered with these materials directly
illustrate some of the issues discussed in Section 6.2. Others comple-
ment aspects that were not fully developed. For example, for VSi
2
or
WSi
2
, the mode of reaction does not fit neatly in the other two categories
of diffusion-controlled and nucleation-controlled reactions. Considerations
about the formation of TiSi
2
will allow examination of a class of sili-
cides once called “ill-behaved”.
[16]
Although the appellation has not met
with great success, there are good reasons to believe that it remains
valid.
Ch_06.qxd 11/12/04 4:06 PM Page 309

310 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
6.3.1 Titanium Disilicide, Activation Energy for the
Motion of the Interface Between the C49 and
C54 Phases, Nucleation of the C49 Structure
For many years, the interconnections between devices in integrated cir-
cuits were made of highly doped polycrystalline Si. This material has a num-
ber of serious advantages. For one thing, it was ideally suited for processing
on Si substrates from which active devices are fabricated. Moreover, it was
also quite ideally suited as electrode material over the SiO
2
gate of insulated
gate field effect transistors (FETs). About 25 years ago, with device dimen-
sions approaching 1 mm, the resistance of even highly doped polycrystalline
Si became too high, causing unacceptable IR (current × resistance) drops and
RC (resistance × capacitance) time delays. Substitute materials must also be
compatible with existing fabrication processes, which ideally might include
exposure to high temperatures (of the order of 1000°C) for diffusion, or the
activation of dopants in the active part of the devices. Silicides fulfilled most
of the requirements: resistance to oxidation, a conductivity considerably
higher than that of polycrystalline Si, resistance to some etching solutions,
and the ability to withstand exposure to high temperatures without suffering
serious damage to their shape and physical properties. One of the first sili-
cides used was WSi
2
. However, following the discovery that the resistivity of
TiSi
2
(20 mΩ.cm) was about one-third to one-fourth that of WSi
2
,
[88]
this com-
pound became and remained for many years the favored silicide for use in
integrated circuits. (The resistivity values referred to here are those that are
encountered in thin-film applications, not necessarily the values obtained
with single crystals of high purity.) Because of the technical applications, the
literature on TiSi
2
films is enormous. Yet in spite of much effort, a good
understanding of the behavior of TiSi
2
remains wanting. Two aspects of this
behavior are analyzed here.
Unfortunately, when forming TiSi
2
from the reaction of Ti films with
Si, or during the crystallization of amorphous TiSi
2
obtained from the
codeposition of Ti and Si, it is not the low-resistivity structure of TiSi
2,
C54, that forms first, but the C49 structure, with a resistivity about three
or four times higher. The reason for this is not clear, but it may be due to
the fact that the mobile species in TiSi
2
, Si (in agreement with the ordered
Cu
3
Au rule), has a higher mobility in the C49 than in the C54 phase.
[89]
Also, the elastic constants of the C49 phase have been reported
[90]
to be
lower than those of the C54 phase, which would lead to smaller stress
effects (∆H
elas
). It is likely that the C49 structure is metastable, although it
is the stable form of the closely related ZrSi
2
and HfSi
2
. The low resistiv-
ity phase is the desired one, and it requires heat treatment at a temperature
sufficiently high to allow the nucleation and growth of the C54 phase via
Ch_06.qxd 11/12/04 4:06 PM Page 310

REACTIVE PHASE FORMATION, D’HEURLE ET AL. 311
a paramorphic transformation (the term allotropic being reserved for the
transformations of elemental phases) driven by a small difference in free
energy. The experimentally determined enthalpy change for this transfor-
mation is reported to be 1.5 kJ/mol.
[91]
This is commensurate with the
driving force for the nucleation of the C54 phase, and then for its growth.
The point has been made that in the reaction between phases, if the com-
position difference is small, for example, Eq. (22), the corresponding ∆G
is also small. Here, the limit is reached when, in a paramorphic transfor-
mation, there is no change in composition at all. With respect to nucle-
ation, the condition remains the same as before; namely, from a small ∆G
it ensues that nucleation is difficult and the density of nuclei small. That
is no problem with blanket films that transform at temperatures in the
vicinity of 700°C, about 150 K above the temperature required for the for-
mation of C49. However, when the width of the thin-film conductors
decrease below about 0.3 mm, it is observed that for a given heat treat-
ment, some conductors do not transform at all, while others may trans-
form only partially.
[92]
That is not tolerable. Apartial remedy is to increase
the heat treatment temperature, but engineering constraints prevent this
approach. One of the constraints, which is built into the conductors them-
selves, is that with films 10 nm thick, exposure to high temperatures
causes the film to agglomerate into individual islands to minimize the sur-
face energy. The temperature at which this occurs may fall below that
required for nucleation. Many recipes have been used to try to alleviate
these difficulties. Since a good account of these is given elsewhere,
[93]
they are not considered here. Rather, some quantitative aspects of the
nucleation and growth process are discussed. The increase in temperature
(akin to superheating) necessary to nucleate the C54 form of TiSi
2
has a
close equivalent in the classical example of the solidification of liquid
metals, where the solidification of mercury requires increased undercool-
ing as the liquid metal is divided into droplets of decreasing size.
[94]
Many attempts have been made to understand the intricacies of the
nucleation and growth of the C54 phase of TiSi
2
. Different authors arrive
at the same conclusion: a very high activation energy of about 4 eV. The
most recent and complete study is based on mapping the new grains via
micro-Raman scattering as well as electrical resistance measurements.
[95]
(The two coincide quite nicely.) The analysis was carried out according to
a modified form of the Johnson-Mehl-Avrami method because the direct
JMAmethod did not provide a good account of the evolution of the nucle-
ating centers during the process of nucleation and growth. The study led
to values of apparent activation energies, ∆G*, ∆G*
kin
, and ∆G
mot
, for
growth (the motion of the interface between C49 and C54) of the same
order of magnitude, about 4 eV. Therefore, ∆G*
th
should be quite small,
perhaps about 0.5 eV. Avalue of ∆G
mot
of 4 eV for a material with a melting
Ch_06.qxd 11/12/04 4:06 PM Page 311
