Gottstein G., Shvindlerman L.S. Grain Boundary Migration in Metals: Thermodynamics, Kinetics, Applications
Подождите немного. Документ загружается.

304 3 Grain Boundary Motion
D’
op
δ
[m
3
/s]
10
-17
10
-18
10
-19
10
-20
0 5 10 15 2520
V* [cm
3
/mol]
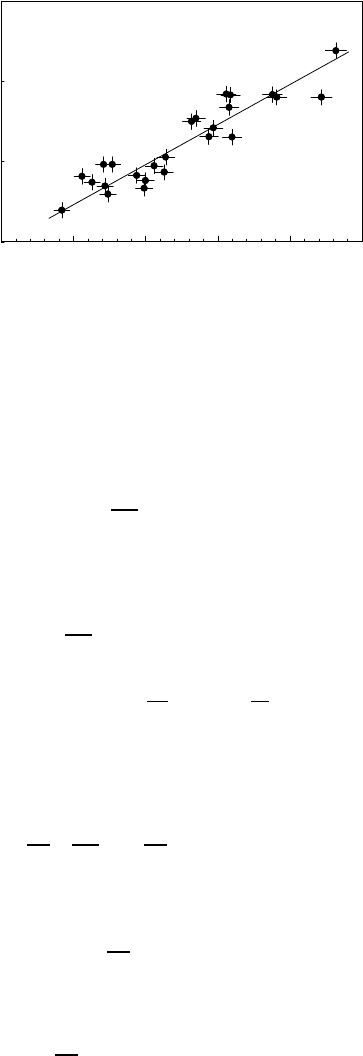
FIGURE 3.122
Dependence of the product under zero pressure on activation volume at 161
◦
C.
an example, the case of grain boundary motion under a constant driving force
P . The rate of reduction of the free energy of the system dG/dt,
dG
dt
= −vP (3.205)
where v = m
b
P is the velocity of grain boundary motion, m
b
the grain bound-
ary mobility. Accordingly,
dG
dt
= −vP = −m
b
P
2
(3.206)
and
m
b
= m
0
e
−
H
∗
kT
; m
0
= e
S
∗
k
(3.207)
where G
∗
= E
∗
−TS
∗
+pV
∗
= H
∗
−TS
∗
is the Gibbs free energy of activation.
Let us consider the extremum of the function in Eq. (3.206) with respect
to an arbitrary state parameter λ:
d
dλ
dG
dt
=
d
dλ
−m
b
P
2
= 0 (3.208)
and since P is independent of λ
d
dλ
(m
b
) = 0 (3.209)
With λ = λ
0
at the maximum of dG/dt and using Eqs.(3.206) and (3.207)
d
dλ
(H
∗
− TS
∗
)
λ=λ
0
= 0 (3.210)
© 2010 by Taylor and Francis Group, LLC
3.7 Compensation Effect in Grain Boundary Motion 305
Again we obtain the compensation temperature
T
c
=
dH
∗
dS
∗
λ=λ
0
(3.211)
As a result, the CE is in agreement with the principles of nonequilibrium
thermodynamics and yields the previously derived CT.
It is noted that there are several atomistic approaches of the activation
process. Either the energy dissipation into the adjoining finite volume is con-
sidered [384]–[386], or, alternatively, the transition state is examined as a
heterophase fluctuation [387].
3.7.4 Applications
3.7.4.1 The Island Model of Grain Boundary Migration
To reveal the meaning and significance of the CE and the CT we will study
a simple model system, namely grain boundary migration according to the
“island” model as proposed by Mott long ago [162]. The model assumes that
groups of n atoms “melt” on the side of the vanishing grain and become
attached to the side of the growing grain. The activated, metastable state is
identified with a frozen liquid state. This permits one to determine the entropy
and enthalpy of activation, namely
S
∗
=
nL
T
m
,H
∗
= nL (3.212)
where L is the heat of melting per atom, and T
m
is the melting temperature.
The velocity v of the grain boundary according to this model reads
v = bνn
ΔG
kT
exp
S
∗
k
exp
−
H
∗
kT
(3.213)
where b is the atomic spacing and ν the Debye frequency.
It is obvious from Eq. (3.213) that the model complies with the compensa-
tion effect: the enthalpy of activation is proportional to the activation entropy.
H
∗
= T
m
S
∗
(3.214)
and, therefore,
T
c
=
dH
∗
dS
∗
= T
m
(3.215)
An identical result can be obtained in terms of irreversible thermodynamics
according to Eq. (3.211).
© 2010 by Taylor and Francis Group, LLC
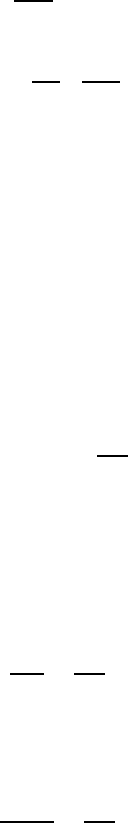
306 3 Grain Boundary Motion
3.7.4.2 Brown-Ashby Relations
Brown and Ashby [388] analyzed diffusion data for a wide range of solids
(metals and ceramics) and, as a result, found three correlations for a given
structure and bond type
D (T
m
)=κ
1
(3.216)
H
∗
kT
0
m
= κ
2
(3.217)
and
V
∗
=
H
∗
T
0
m
dT
0
m
dp
(3.218)
where κ
1
and κ
2
are constants, which in first order are independent of the
pressure p; T
0
m
is the melting point at atmospheric pressure and k is the
Boltzmann constant.
We will show that Eqs. (3.216)–(3.218) are in full agreement with the con-
cepts of the CE as developed above [389]. If “for a given structure and bond
type” [388] the compensation effect is obeyed, the compensation tempera-
ture necessarily coincides with the melting point, as for pure elements not
undergoing a phase transition in solid state, T
m
is the only temperature for
a first-order phase transition in the temperature range discussed. Then, in
accordance with Eq. (3.191) we obtain for bulk diffusion
H
∗
= kT
m
ln D
0
+ β (3.219)
and at T = T
m
D (T
m
)=e
−
β
kT
m
(3.220)
Apparently Eq. (3.220) exactly corresponds to the empirical relation given by
Eq. (3.219). We shall not discuss Eq. (3.217), which is a special case of the more
general relation (3.218). Most interesting is the third relation, Eq. (3.218). Let
us consider this relation in terms of the “activation-barrier-phase.” The change
in the transition point (compensation temperature T
c
) with pressure p is given
by the Clausius-Clapeyron equation
ΔV
ΔS
=
dT
c
dp
(3.221)
With
ΔV = V
− V
0
= V
∗
and ΔS = S
− S
0
= S
∗
(3.222)
we obtain
V
∗
(λ)
S
∗
(λ)
=
dT
c
dp
(3.223)
Since
T
0
c
S
∗
(λ)=E
∗
(λ) (3.224)
© 2010 by Taylor and Francis Group, LLC

3.7 Compensation Effect in Grain Boundary Motion 307
Eq. (3.223) can be rewritten as
V
∗
· T
0
c
E
∗
=
dT
c
dp
(3.225)
If the compensation temperature coincides with the melting point we arrive
at the Brown-Ashby relation
V
∗
=
E
∗
T
0
m
dT
m
dp
(3.226)
Therefore, the Brown-Ashby relation can be considered a direct consequence
of the CE [389].
3.7.4.3 Impact of the CE on Thermally Activated Processes
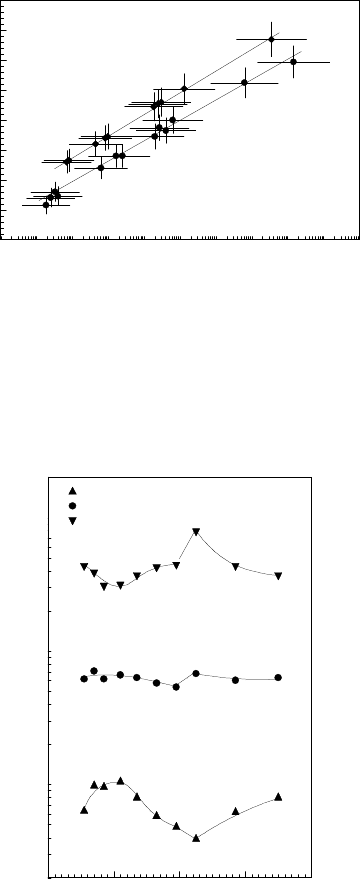
Fig. 3.50 gives the measured rate of boundary migration vs. the misorien-
tation angle about a common 111 tilt axis [264]. The maximum velocity
is attained for a misorientation angle of ∼ 41
◦
. Over many years this result
worried scientists (including the authors themselves [390]). The point is that
close to the 41
◦
111 tilt grain boundary there is the special grain boundary
Σ7: 38.2
◦
111 with apparently high mobility and low activation energy of
migration [190, 275, 280, 299]. Therefore, the 41
◦
111 boundary was tacitly
assumed to represent a scatter of the Σ7 boundary. Nevertheless, numerous
growth selection experiments provided strong experimental evidence that the
maximum mobility was attained at misorientation angles above 40
◦
, i.e. close
to 41
◦
and not 38.2
◦
.
The misorientation dependence of activation enthalpy for migration of 111
tilt grain boundaries in the vicinity of the special grain boundary Σ7 (38.2
◦
)
as obtained from bicrystal experiments [190] is given in Fig. 3.123. Obviously,
the activation enthalpy is at maximum for a misorientation angle close to 41
◦
.
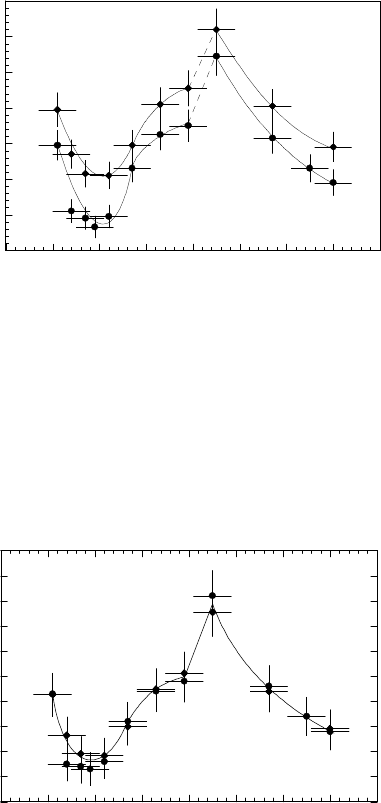
However, the misorientation dependence of the preexponential factor behaves
the same way, i.e. attains a maximum for 41
◦
misorientation (Fig. 3.124). In
fact, the CE with a CT of ∼ 450
◦
C (Fig. 3.125) causes the grain boundaries
with the lowest activation energy to be most mobile at T<CT,whereasfor
T>CTthe opposite is the case (Fig. 3.126). As a result, due to the CE,
which boundary moves fastest depends on the temperature range (relative
to CT), and this reconciles the contradiction between recrystallization and
growth selection experiments mentioned above.
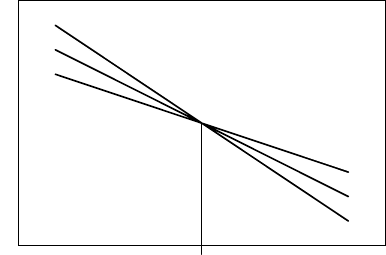
As a general rule the compensation temperature divides the temperature
range into two regimes with different relations between the magnitude of re-
action rate and energy of activation. When the experiments are conducted
below T
c
, then the processes with low energies of activation prevail. The re-
verse is also true; if the measurements are taken above T
c
, then the processes
with high energies of activation dominate the kinetics (Fig. 3.127).
The example given above also elucidates another consequence of the CE,
namely the drastically mitigated influence of the activation enthalpy. For
© 2010 by Taylor and Francis Group, LLC
308 3 Grain Boundary Motion
H [eV]
2.3
2.5
2.1
1.7
1.9
1.3
1.5
1.1
36 4038
42 44
ϕ
[deg]
FIGURE 3.123
Activation enthalpy H
m
for migration of 111 tilt grain boundaries as a
function of misorientation angle ϕ for two different aluminum charges: Al I -
0.4 ppm of impurity; Al II - 1.0 ppm of impurity.
36 37 38 39 40 41 42 43 44
ϕ
[deg]
10
00
10
02
10
04
10
06
10
08
10
10
A
0
[
m
2
/
s
]
36 37 38 39 40 41 42 43 44
36
3
7
38
39
40
41
42
43
44
1
0
1
0
1
0
1
0
1
0
1
0
1
0
1
0
1
0
1
0
1
0
A
0
[m
2
/s]
10
10
10
8
10
4
10
2
10
6
10
0
36 38 40 42 44
ϕ
[deg]
FIGURE 3.124
Reduced mobility preexponential factor A
0
of 111 tilt grain boundaries as
a function of misorientation angle ϕ for two different aluminum charges: (• -
Al I; -AlII).
© 2010 by Taylor and Francis Group, LLC
3.7 Compensation Effect in Grain Boundary Motion 309
H
m
[eV]
2.6
2.2
1.8
1.4
10
0
1.0
10
4
10
2
10
8
10
6
10
10
A
0
[m
2
/s]
FIGURE 3.125
Dependence of migration activation enthalpy H
m
on the preexponential re-
duced mobility factor A
0
for the investigated 111 tilt grain boundaries in
high-purity aluminum of two different charges: (• -AlI; -AlII).
430°C
485°C
540°C
A
b
[m
2
/s]
430°C
485°C
540°C
10
-7
10
-8
10
-9
36
ϕ
[deg]
38 40 4442
FIGURE 3.126
Misorientation dependence of grain boundary reduced mobility A for 111
tilt boundaries in Al II at different temperatures.
© 2010 by Taylor and Francis Group, LLC
310 3 Grain Boundary Motion
1
2
3
In m
2
3
1
T
C
1/T
FIGURE 3.127
Schematic sketch demonstrating the impact of the CE on grain boundary
mobility m above and below the CT. (H
∗
1
>H
∗
2
>H
∗
3
; m
01
>m
02
>m
03
;
T>T
c
: m
1
>m
2
>m
3
; T<T
c
: m
1
<m
2
<m
3
.)
instance, in the case of two different 111 tilt grain boundaries (Σ7 and
40.5
◦
111) in Al the activation enthalpies for grain boundary migration are
∼ 1.24 eV and 2.1 eV, respectively. If we take into account the difference of
the activation enthalpy only, then at 480
◦
C the mobilities of the two bound-
aries would differ by six orders of magnitude, while, in reality, due to the CE,
they differ by not more than a factor of 3!
However, very often conclusions are drawn only on the basis of the mag-
nitude of the activation energy. For instance, on this basis it is contended a
small amount of impurities (0.001% Mn) in Al reduces the rate of boundary
migration by many orders of magnitude (up to 10
11
times) [390, 391]. Be-
cause of the CE this is very unlikely to happen and has never been observed.
In essence, the compensation effect controls the kinetics of the processes (at
least, interfacial reactions), restricting and compensating the influence of the
involved activation parameters, in particular of the activation enthalpy.
3.7.4.4 The CE in Thermally Activated Bulk Processes
The CE is not restricted to interface phenomena. Brief mention has already
been made of CE observations on bulk activation processes [336]–[338]. But
nowhere is the role of the CE as important as in grain boundary activation
processes. As an example let us consider bulk diffusion. In this case the preex-
ponential factor is predicted by Zener’s theory [392], which assumes that the
Gibbs free energy G
∗
of an atom in the transition state — i.e. in the saddle
© 2010 by Taylor and Francis Group, LLC
3.8 Mechanisms of Grain Boundary Migration 311
point configuration — is defined by the work of elastic deformation
G
∗
=
1
2
με
2
0
(3.227)
where ε
0
is some effective elastic strain, μ the relevant elastic modulus [392].
Since G
∗
m
is a free energy, the corresponding entropy
S
∗
= −
∂G
∗
∂T
p
(3.228)
and, taking into account the very weak temperature dependence of the elastic
modulus μ
S
∗
∼
=
−G
∗
0
d (μ/μ
0
)
dT
= −H
∗
0
d (μ/μ
0
)
dT
(3.229)
where G
∗
0
= H
∗
0
is the Gibbs’ free activation energy and enthalpy and μ
0
the
shear modulus at 0K, respectively.
Introducing χ = d(μ/μ
0
)/d(T/T
m
), where T
m
is the melting temperature of
the solvent: S
∗
∼
=
(χH
∗
)/(T
m
). According to Zener’s theory the preexponen-
tial factor for bulk diffusion can change by less than 2–3 orders of magnitude.
This is negligible compared to the 7–10 orders of magnitude (!!) for grain
boundary diffusion or migration. It is emphasized again that this large vari-
ance in the preexponential factor is a consequence of the collective nature of
grain boundary processes. This is why the CE plays such an important role
in interfaces and especially grain boundary kinetics.
3.8 Mechanisms of Grain Boundary Migration
In Sec. 3.1 we mentioned that there is actually no theory of grain bound-
ary migration and hitherto, we have no detailed information on the atomic
mechanism of grain boundary migration, except from preliminary results of
molecular dynamics computer simulations [309] or high-resolution TEM stud-
ies of boundary migration [393]. Because of the importance of this issue to
the major topic of this text, we at least want to review and critically assess
proposals made in the past.
Early models [162, 178] of grain boundary structure assumed that the
grain boundary comprised a certain volume of thickness δ b, i.e. much
broader than the spacing of atoms in the adjacent lattices, in other words
a wide boundary (Fig. 3.128). Corresponding phenomenological theories of
grain boundary motion have to consider that for steady-state motion the
three fluxes
j
1b
= j
c
= j
b2
(3.230)
© 2010 by Taylor and Francis Group, LLC
312 3 Grain Boundary Motion
where j
1b
is the flux of atoms detaching from the consumed crystal, j
b2
is the
flux of atoms attaching to the growing crystal and j
c
= v/Ω
a
is the convec-
tion flux in the boundary, i.e. a flux of atoms that an observer attached to the
boundary would see. The attachment and detachment processes will be ther-
mally activated and may occur in a specific respective process (Fig. 3.128b)
j
b1
= c
11
b
Ω
a
ν exp
−
G
m
kT
− c
12
b
Ω
a
ν exp
−
G
m
+ P Ω
a
/2
kT
(3.231)
j
b2
= c
21
b
Ω
a
ν exp
−
G
m
− P Ω
a
/2
kT
− c
22
b
Ω
a
ν exp
−
G
m
kT
j
c
=
v
Ω
a
where c
ij
are geometrical constants, G
m
the activation free energy of the
diffusive atomic motion. Assuming similar structural conditions on both shores
of the boundary: c
11
= c
22
, c
12
= c
21
yields c
11
= c
12
and with Ω
a
≈ b
3
v = c
11
bν
exp
−
G
m
− P Ω
a
/2
kT
− exp
−
G
m
+ P Ω
a
/2
kT
(3.232)
∼
=
c
11
b
4
ν
kT
e
−
G
m
kT
· P
which is the fundamental equation already derived in Sec. 3.1.
The detachment and attachment process may be composed of several steps,
each of which may be associated with a specific flux equation, which will com-
plicate the system of Eq. (3.231) but yield a similar solution to contain the
activation energies for the elementary steps considered. Also, vacancies may be
involved in the diffusion process at the boundary edges so that the boundary
vacancy concentration can be introduced in Eq. (3.232). Such an approach
allows us to introduce crystallography in the modeling of grain boundary
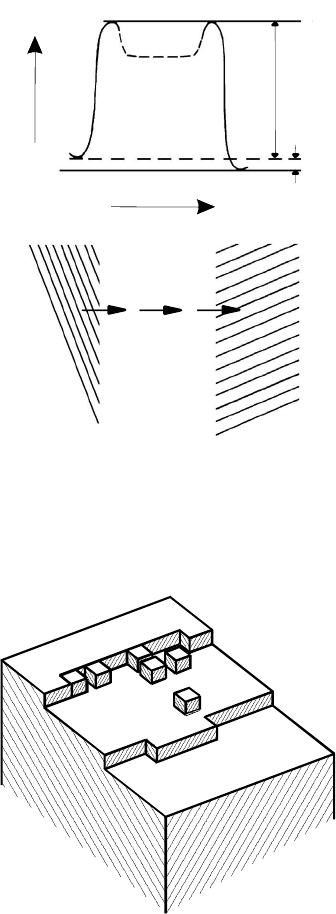
migration. This was first proposed by Gleiter [177], who treated grain bound-
ary migration like an evaporation/condensation process on the internal crys-
tal surfaces (Fig. 3.129) and, therefore, tacitly assumed the existence of a
wide grain boundary. This concept can, in principle, account for the observed
anisotropy of grain boundary migration. Unfortunately, this theory lacks pre-
dictive power since most involved crystallographic parameters are unknown.
A more serious objection to the theory, however, is the assumption of a wide
grain boundary, which is in obvious contradiction to the narrow structure of
real grain boundaries observed (Chapter 2).
Mott [162] assumed in his theory of boundary migration (Island model)
that the boundary contains small patches of perfect crystal structure which
become detached and attached to the crystal surfaces by partial melting and
solidification processes. The migration process can be modeled in analogy to
Eq. (3.232), except that the free activation energy is the energy of melting of a
cluster of n atoms and correspondingly the attachment of the island will move
the boundary by a distance nb. While this model is also based on unrealistic
© 2010 by Taylor and Francis Group, LLC
3.8 Mechanisms of Grain Boundary Migration 313
free energy
boundary
distance
lattice 1 lattice 2
Δ
G
m
Pb
3
j
1b
j
c
j
b2
(a)
(b)
free energy
boundary
distance
lattice 1 lattice 2
Δ
G
m
Pb
3
j
1b
j
c
j
b2
(a)
(b)
FIGURE 3.128
Wide grain boundary (schematically); (a) free energy barrier; (b) fluxes across
the boundary.
FIGURE 3.129
Model of grain boundary motion according to Gleiter [177]. Atoms are re-
moved from ledges on the surface of the shrinking grain and become attached
to ledges on the growing grain surface.
© 2010 by Taylor and Francis Group, LLC
