Gary Nichols. Sedimentology and Stratigraphy(Second Edition)
Подождите немного. Документ загружается.


3
Biogenic, Chemical and
Volcanogenic Sediments
In areas where there is not a large supply of clastic detritus other processes are impor-
tant in the accumulation of sediments. The hard parts of plants and animals ranging from
microscopic algae to vertebrate bones make up deposits in many different environments.
Of greatest significance are the many organisms that build shells and structures of
calcium carbonate in life, and leave behind these hard parts when they die as calcareous
sediments that form limestone. Chemical processes also play a part in the formation of
limestone but are most important in the generation of evaporites, which are precipitated
out of waters concentrated in salts. Volcaniclastic sediments are largely the products of
primary volcanic processes of generation of ashes and deposition of them subaerially or
under water. In areas of active volcanism these deposits can swamp all other sediment
types. Of the miscellaneous deposits also considered in this chapter, most are primarily
of biogenic origin (siliceous sediments, phosphates and carbonaceous deposits) while
ironstones are chemical deposits.
3.1 LIMESTONE
Limestones are familiar and widespread rocks that
form the peaks of mountains in the Himalayas, form
characteristic karst landscapes and many spectacular
gorges throughout the world. Limestone is also impor-
tant in the built environment, being the construction
material for structures ranging from the Pyramids of
Egypt to many palaces and churches. As well as being
a good building stone in many places, limestone is
also important as a source of lime to make cement,
and is hence a component of all concrete, brick and
stone buildings and other structures, such as bridges
and dams. Limestone strata are common through
much of the stratigraphic record and include some
very characteristic rock units, such as the Late Cre-
taceous Chalk, a relatively soft limestone that is found
in many parts of the world. The origins of these rocks
lie in a range of sedimentary environments: some
form in continental settings, but the vast majority
are the products of processes in shallow marine envi-
ronments, where organisms play an important role in
creating the sediment that ultimately forms limestone
rocks.
Calcium carbonate (CaCO
3
) is the principal com-
pound in limestones, which are, by definition, rocks

composed mainly of calcium carbonate. Limestones,
and sediments that eventually solidify to form them,
are referred to as calcareous (note that, although
they are carbonate, they are not ‘carbonaceous’: this
latter term is used for material that is rich in carbon,
such as coal). Sedimentary rocks may also be made of
carbonates of elements such as magnesium or iron,
and there are also carbonates of dozens of elements
occurring in nature (e.g. malachite and azurite are
copper carbonates). This group of sediments and rocks
are collectively known as carbonates to sedimentary
geologists, and most carbonate rocks are sedimentary
in origin. Exceptions to this are marble, which is a
carbonate rock recrystallised under metamorphic
conditions, and carbonatite, an uncommon carbon-
ate-rich lava.
3.1.1 Carbonate mineralogy
Calcite
The most familiar and commonest carbonate mineral
is calcite (CaCO
3
). As a pure mineral it is colourless or
white, and in the field it could be mistaken for quartz,
although there are two very simple tests that can be
used to distinguish calcite from quartz. First, there is a
difference in hardness: calcite has a hardness of 3 on
Mohs’ scale, and hence it can easily be scratched with
a pen-knife; quartz (hardness 7) is harder than a knife
blade and will scratch the metal. Second, calcite
reacts with dilute (10%) hydrochloric acid (HCl),
whereas silicate minerals do not. A small dropper-
bottle of dilute HCl is hence useful as a means of
determining if a rock is calcareous, as most common
carbonate minerals (except dolomite) will react with
the acid to produce bubbles of carbon dioxide gas,
especially if the surface has been powdered first by
scratching with a knife. Although calcite sometimes
occurs in its simple mineral form, it most commonly
has a biogenic origin, that is, it has formed as a part
of a plant or animal. A wide variety of organisms use
calcium carbonate to form skeletal structures and
shells and a lot of calcareous sediments and rocks
are formed of material made in this way.
Magnesium ions can substitute for calcium in the
crystal lattice of calcite, and two forms of calcite are
recognised in nature: low-magnesium calcite (low-Mg
calcite), which contains less than 4% Mg, and high-
magnesium calcite (high-Mg calcite), which typically
contains 11% to 19% Mg. The hard parts of many
marine organisms are made of high-Mg calcite, for
example echinoderms, barnacles and foraminifers,
amongst others (see 3.13). Strontium may substitute
for calcium in the lattice and although it is in small
quantities (less than 1%) it is important because
strontium isotopes can be used in dating rocks
(21.3.1).
Aragonite
There is no chemical difference between calcite and
aragonite, but the two minerals differ in their
mineral form: whereas calcite has a trigonal crystal
form, aragonite has an orthorhombic crystal form.
Aragonite has a more densely packed lattice structure
and is slightly denser than calcite (a specific gravity of
2.95, as opposed to a range of 2.72–2.94 for calcite),
and is slightly harder (3.5–4 on Mohs’ scale). In
practice, it is rarely possible to distinguish between
the two, but the differences between them have some
important consequences (18.2.2). Many invertebrates
use aragonite to build their hard parts, including
bivalves and corals.
Dolomite
Calcium magnesium carbonate (CaMg(CO
3
)
2
)isa
common rock-forming mineral which is known as
dolomite. Confusingly, a rock made up of this mineral
is also called dolomite, and the term dolostone is now
sometimes used for the lithology to distinguish it from
dolomite, the mineral. The mineral is similar in
appearance to calcite and aragonite, with a similar
hardness to the latter. The only way that dolomite can
be distinguished in hand specimen is by the use of the
dilute HCl acid test: there is usually little or no reac-
tion between cold HCl and dolomite. Although dolo-
mite rock is quite widespread, it does not seem to be
forming in large quantities today, so large bodies of
dolomite rock are considered to be diagenetic
(18.4.2).
Siderite
Siderite is iron carbonate (FeCO
3
) with the same
structure as calcite, and is very difficult to distinguish
between iron and calcium carbonates on mineralogi-
cal grounds. It is rarely pure, often containing some
magnesium or manganese substituted for iron in the
Limestone 29

lattice. Siderite forms within sediments as an early
diagenetic mineral (18.2).
3.1.2 Carbonate petrography
All of these carbonate minerals have similar optical
properties and it can be difficult to distinguish
between them in thin-section using the usual optical
tests. Their relief is high, and the birefringence col-
ours are high-order pale green and pink. The cleavage
is usually very distinct, and where two cleavage
planes are visible they can be seen to intersect to
form a rhombohedral pattern. Dolomite can be iden-
tified by adding a dye to the cut surface before a glass
cover slip is put on the thin-section: Alizarin Red-S
does not stain dolomite, but colours the other car-
bonates pink. A second chemical dye is also com-
monly used: potassium ferricyanide reacts with
traces of iron in a carbonate to stain it blue, and
on this basis it is possible to distinguish between
ferroan calcite/aragonite/dolomite and non-ferroan
forms of these minerals. The two stains may be used
in combination, such that ferroan calcite/aragonite
ends up purple, ferroan dolomite blue, non-ferroan
calcite/aragonite pink and non-ferroan dolomite
clear.
There is an alternative to making thin-sections of
rocks made up primarily of carbonate minerals. It is
possible to transfer the detail of a cut, flat surface of a
block of limestone onto a sheet of acetate by etching the
surface with dilute hydrochloric acid, then flooding the
surface with acetone and finally applying the acetate
film. Once the acetone has evaporated, the acetate is
peeled off and the imprint of the rock surface can then be
examined under the microscope. These acetate peels
are a quick, easy way to look at the texture of the
rock, and distinguish different clast types: the rock
can also be stained in the same way as a thin-section.
3.1.3 Biomineralised carbonate sediments
Carbonate-forming organisms include both plants
and animals. They may create hard parts out of cal-
cite, in either its low-Mg or high-Mg forms, or arago-
nite, or sometimes a combination of both minerals.
The skeletal fragments in carbonate sediments are
whole or broken pieces of the hard body parts of
organisms that use calcium carbonate minerals as
part of their structure (Figs 3.1 & 3.2). Some of
them have characteristic microstructures, which can
be used to identify the organisms in thin-sections
(Adams & Mackenzie 1998).
single crystals - crinoids, echninoids,
calcareous sponges
homogeneous - trilobites, ostracods,
some molluscs
microgranular - foraminifers,
some molluscs
nacreous - some molluscs
lamellar - some molluscs
prismatic - some molluscs
foliated - brachiopods,
some molluscs
radial - belemnites
spherulitic - corals
Fig. 3.1 Types of bioclast commonly
found in limestones and other sedimen-
tary rocks.
30 Biogenic, Chemical and Volcanogenic Sediments

Carbonate-forming animals
The molluscs are a large group of organisms that
have a fossil record back to the Cambrian and com-
monly have calcareous hard parts. Bivalve molluscs,
such as mussels, have a distinctive layered shell struc-
ture consisting of two or three layers of calcite, or
aragonite, or both. Of the modern forms, some such
as oysters and scallops are calcitic, but most of the rest
are aragonitic: aragonite shells may have been the
norm throughout their history, but no pre-Jurassic
bivalve shells are preserved because of the instability
of the mineral compared with the more stable form of
calcium carbonate, calcite. Gastropods are molluscs
with a similar long history: they also have a calcite or
aragonite layered structure, and are distinctive for
their coiled form (Fig. 3.3). The cephalopod molluscs
include the modern Nautilus and the coiled, cham-
bered ammonites, which were very common in Meso-
zoic times. Most cephalopods have a layered shell
structure, and, in common with most other molluscs,
this is a feature that may be recognisable in fragments
of shells under the microscope. There is an important
exception in the belemnites, a cephalopod that had a
cigar-shaped ‘guard’ of radial, fibrous calcite: these
can be preserved in large numbers in Mesozoic sedi-
mentary rocks.
The brachiopods are also shelly organisms with
two shells and are hence superficially similar to
bivalves. They are not common today but were very
abundant in the Palaeozoic and Mesozoic. The shells
are made up of low-magnesium calcite, and a two-
layer structure of fibrous crystals may be completely
preserved in brachiopod shells. The exoskeletons of
arthropods, such as the trilobites, are made up of
microscopic prisms of calcite that are elongate per-
pendicular to the edges of the plates. Although they
may appear to be quite different, barnacles are also
arthropods and have a similar internal structure to
their skeletal material.
Another group of shelly organism s, the echinoids
(sea urchins), can be easily recognised because they
construct their hard body parts out of whole low-
magnesium calcite crystals. Individual plates of
echinoids are preserved in carbonate sediments.
Crinoids (sea lilies) b elong to the same phylum as
echinoids (the Echinodermata) and are similar in the
sense that they too construct their body parts out of
whole calcite crystals, with the discs that make up
the stem of a crinoid forming sizeable accumulations
in Carboniferous sediments. In life the individual
crystals in echinoid and crinoid body p arts are per-
forated, but the pores are filled with growths of
calcite that may also extend beyond the original
limits of the skeletal element as an overgrowth
(18.2.2). These large single crystals that make up
echinoderm fragments make them easily recognis-
able in thin-section.
Foraminifera are small, single-celled marine
organisms that range from a few tens of microns in
diameter to tens of millimetres across. They are either
floating in life (planktonic) or live on the sea floor
(benthic) and most modern and ancient forms have
hard outer parts (tests) made up of high- or low-
magnesium calcite. Both modern sediments and
ancient limestone beds have been found with huge
concentrations of foraminifers such that they may
form the bulk of the sediment.
Fig. 3.2 Bioclastic debris on a beach consisting of the hard
calcareous parts of a variety of organisms.
Fig. 3.3 Fossil gastropod shells in a limestone.
Limestone 31

Some of the largest calcium carbonate biogenic
structures are built by corals (Cnidaria) which
may be in the form of colonies many metres across
or as solitary organisms. Calcite seems to have been
the main crystal form in Palaeozoic corals, with ara-
gonite crystals making the skeleton in younger corals.
Hermatypic corals have a symbiotic relationship
with algae that require clear, warm, shallow marine
waters. These corals form more significant build-ups
than the less common, ahermatypic corals that do
not have algae and can exist in colder, deeper water.
Another group of colonial organisms that may con-
tribute to carbonate deposits are the bryozoa. These
single-celled protozoans are seen mainly as encrusting
organisms today but in the past they formed large
colonies. The structure is made up of aragonite,
high-magnesium calcite or a mixture of the two. The
sponges (Porifera ) are a further group of sedentary
organisms that may form hard parts of calcite,
although structures of silica or protein are also com-
mon. Stromatoporoids are calcareous sponges that
were common in the Palaeozoic. Other calcareous
structures associated with animals are the tubes of
carbonate secreted by serpulid worms. These are a
type of annelid worm that encrusts pebbles or the
hard parts of other organisms with sinuous tubes of
calcite or aragonite.
Carbonate-forming plants
Algae and microbial organisms are an important
source of biogenic carbonate and are important con-
tributors of fine-grained sediment in carbonate envi-
ronments through much of the geological record
(Riding 2000). Three types of alga are carbonate
producers. Red algae (rhodophyta) are otherwise
known as the coralline algae: some forms are found
encrusting surfaces such as shell fragments and peb-
bles. They have a layered structure and are effective at
binding soft substrate. The green algae (chloro-
phyta) have calcified stems and branches, often seg-
mented, that contribute fine rods and grains of
calcium carbonate to the sediment when the organ-
ism dies. Nanoplankton are planktonic yellow-
green algae that are extremely important contribu-
tors to marine sediments in parts of the stratigraphic
record. This group, the chrysophyta, include cocco-
liths, which are spherical bodies a few tens of
microns across made up of plates. Coccoliths are an
important constituent of pelagic limestone, including
the Cretaceous Chalk.
Cyanobacteria are now classified separately to
algae. The ‘algal’ mats formed by these organisms
are more correctly called bacterial or microbial
mats. In addition to sheet-like mats, columnar and
domal forms are also known. The filaments and sticky
surfaces of the cyanobacteria act as traps for fine-
grained carbonate and as the structure grows it
forms layered, flat or domed structures called stro-
matolites (Fig. 3.4), which are some of the earliest
lifeforms on Earth. In contrast to stromatolites,
thrombolites are cyanobacterial communities that
have an irregular rather than layered form. Oncoids
are irregular concentric structures millimetres to cen-
timetres across formed of layers bound by cyanobac-
teria found as clasts within carbonate sediments.
Other cyanobacteria bore into the surface of skeletal
Fig. 3.4 Mounds of cyanobacteria form stromatolites,
which are bulbous masses of calcium carbonate material at
various scales: (top) modern stromatolites; (bottom) a cross-
section through ancient stromatolites.
32 Biogenic, Chemical and Volcanogenic Sediments
debris and alter the original structure of a shell into a
fine-grained micrite (micritisation).
3.1.4 Non-biogenic constituents of limestone
A variety of other types of grain also occur commonly in
carbonate sediments and sedimentary rocks (Fig. 3.5).
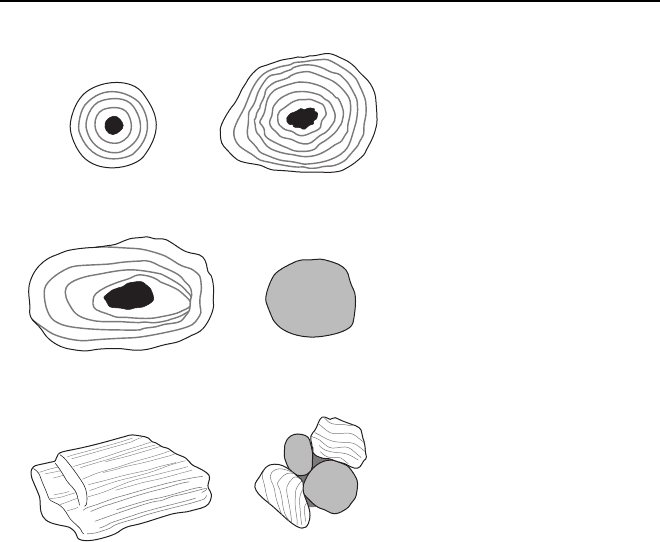
Ooids are spherical bodies of calcium carbonate less
than 2 mm in diameter. They have an internal struc-
ture of concentric layers which suggests that they
form by the precipitation of calcium carbonate around
the surface of the sphere. At the centre of an ooid lies
a nucleus that may be a fragment of other carbonate
material or a clastic sand grain. Accumulations of
ooids form shoals in shallow marine environments
today and are components of limestone throughout
the Phanerozoic. A rock made up of carbonate ooids is
commonly referred to as an oolitic limestone,
although this name does not form part of the Dunham
classification of carbonate rocks (3.1.6). The origin of
ooids has been the subject of much debate and the
present consensus is that they form by chemical pre-
cipitation out of agitated water saturated in calcium
carbonate in warm waters (Tucker & Wright 1990). It
is likely that bacteria also play a role in the process,
especially in less agitated environments (Folk & Lynch
2001). Concentrically layered carbonate particles
over 2 mm across are called pisoids: these are often
more irregular in shape but are otherwise similar in
form and origin to ooids.
Some round particles made up of fine-grained cal-
cium carbonate found in sediments do not show any
concentric structure and have apparently not grown
in water in the same way as an ooid or pisoid. These
peloids are commonly the faecal pellets of marine
organisms such as gastropods and may be very abun-
dant in some carbonate deposits, mostly as particles
less than a millimetre across. In thin-section these
pellets are internally homogeneous, and, if the rock
underwent some early compaction, they may have
become deformed, squashed between harder grains,
making them difficult to distinguish from loose mud
deposited as a matrix.
Intraclasts are fragments of calcium carbonate
material that has been partly lithified and then
broken up and reworked to form a clast which is
incorporated into the sediment. This commonly
occurs where lime mud dries out by subaerial expo-
sure in a mud flat and is then reworked by a current.
A conglomerate of flakes of carbonate mud can be
formed in this way. Other settings where clasts of
lithified calcium carbonate occur are associated with
reefs where the framework of the reef is broken up by
wave or storm action and redeposited (15.3.2). Car-
bonate grains consisting of several fragments cemen-
ted together are aggregate grains, which when they
comprise a collection of rounded grains are known as
grapestones.
3.1.5 Carbonate muds
Fine-grained calcium carbonate particles less than
4 microns across (cf. clay: 2.4) are referred to as
lime mud, carbonate mud or micrite. The source
of this fine material may be purely chemical preci-
pitation from water saturated in calcium carbo-
nate, from the breakdown of skeletal fragments, or
have an algal or bacterial origin. The small size of
the particles usually makes it very difficult to deter-
mine the source. Lime mud is found in many
Intraclast
Pisoid
(> 2 mm)
Peloid
(< 1 mm)
Oncoid
(> 2 mm)
Ooid
(< 2 mm)
Aggregate
(grapestone)
Fig. 3.5 Non-biogenic fragments that occur in limestones.
Limestone 33

carbonate-forming environments and can be the
main constituent of limestone.
3.1.6 Classification of limestones
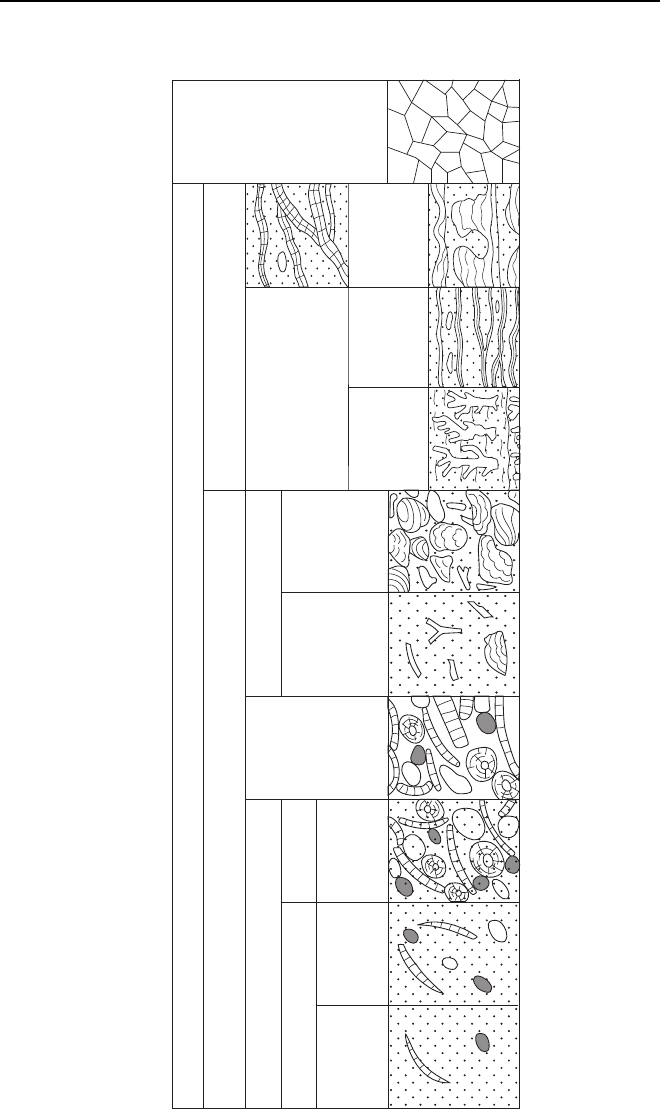
The Dunham Classification is the most widely
used scheme for the description of limestone in the
field, in hand specimen and in thin-section. The pri-
mary criterion used in this classification scheme is the
texture, which is described in terms of the proportion
of carbonate mud present and the framework of the
rock (Fig. 3.6). The first stage in using the Dunham
classification is to determine whether the fabric is
matrix- or clast-supported. Matrix-supported lime-
stone is divided into carbonate mudstone (less than
10% clasts) and wackestone (with more than 10%
clasts). If the limestone is clast-supported it is termed a
packstone if there is mud present or a grainstone if
there is little or no matrix. A boundstone has an
organic framework such as a coral colony. The origi-
nal scheme (Dunham 1962) did not include the sub-
division of boundstone into bafflestone, bindstone
and framestone, which describes the type of organ-
isms that build up the framework. These categories,
along with the addition of rudstone (which are clast-
supported limestone conglomerate) and floatstone
(matrix-supported limestone conglomerate) were
added by Embry & Klovan (1971) and James &
Bourque (1992). Note that the terms rudstone and
floatstone are used for carbonate intraformational
conglomerate made up of material deposited in an
adjacent part of the same environment and then
redeposited (e.g. at the front of a reef: 15.3.2). These
should be distinguished from conglomerate made up
of clasts of limestone eroded from older bedrock and
deposited in a quite different setting, for example on
an alluvial fan (7.5).
The nature of the grains or framework material
forms the secondary part of the classification. A rock
consisting entirely of ooids with no matrix would
be an oolitic grainstone, one composed of about
75% broken shelly fragments in a matrix of carbo-
nate mud is a bioclastic packstone, and rock com-
posed mainly of large oyster shells termed a
bioclastic rudstone. Naming a limestone using the
combination of textural and compositional criteria
in the Dunham scheme provides information about
the likely conditions under which the sediment
formed: for example, a coral boundstone forms
under quite different conditions to a foraminiferal
wackestone.
3.1.7 Petrographic analysis
of carbonate rocks
Thin-section analysis of limestones and dolostones
can reveal a great deal of information about the en-
vironment in which the sediment was deposited.
Assessment of the proportions of carbonate mud and
larger fragments provides an indication of the environ-
ment of deposition: a high proportion of fine-grained
carbonate material suggests a relatively low-energy
setting, whereas an absence of mud characterises
higher-energy environments. The mud to fragmental
component ratio is also the basis for classification
using the Dunham scheme of carbonate mud-
stones, wackestones, packstones and grainstones. If it
is not already evident from hand specimen, thin-sections
will also reveal the presence of framework organ-
isms such as corals and algae that form a bound-
stone fabric.
The nature of the fragmental material provides
further evidence of the conditions under which the
sediment was deposited: for example, high concentra-
tions of ooids indicate shallow, wave-dominated
coastal settings (15.3.1) whereas a rock composed of
biogenic material that is all from the same group of
organisms, such as bivalves or gastropods, is an indi-
cator of a lagoonal setting (15.2.2). The degree to
which the shelly material is broken up also reflects
the energy of the setting or the amount of transport
and reworking of the sediment. It is usually possible to
determine the fossil group to which larger bioclasts
belong from their overall shape and the internal struc-
ture (Fig. 3.1). Additional clues may also come from
the mineral that the original bioclast was made of
(Fig. 3.7): shells originally composed of aragonite
tend to recrystallise and the primary fabric is lost;
similarly, high-magnesium calcite commonly recrys-
tallises and also results in bioclasts with a recrystal-
lised fabric. Organisms such as many brachiopods and
bivalves that were formed of low-magnesium calcite
tend to retain their primary structure.
It should be noted, however, that all carbonate
rocks are susceptible to diagenetic alteration (18.4)
that can change both the mineralogy and the struc-
ture of the fragments and the carbonate mud. Diage-
netic alteration can vary from a simple cementation of
34 Biogenic, Chemical and Volcanogenic Sediments
Mudstone Wackestone Packstone Grainstone
Boundstone
(may be divided into
three types below)
Floatstone Rudstone
Bindstone
Framestone
Crystalline
Less than
10% grains
More than
10% grains
Mud-supported
Grain-
supported
Contains mud
(clay and fine silt-size carbonate)
Lacks mud
and is grain-
supported
Original components not bound
together during deposition
Supported
by >2mm
component
Matrix-
supported
>10% grains >2mm
Original components organically
bound during deposition
Depositional texture recognisable
Depositional
texture not
recognisable
Bafflestone
By organisms
which act
as baffles
By organisms
which encrust
and bind
By organisms
which build a
rigid framework
Fig. 3.6 The Dunham classification of carbonate sedimentary rocks (Dunham 1962) with modifications by Embry & Klovan (1971). This scheme is
the most commonly used for description of limestones in the field and in hand specimen.
Limestone 35

the sediment with little alteration of the material to
complete recrystallisation that obliterates all of the
depositional fabric (18.4.3).
3.2 EVAPORITE MINERALS
These are minerals formed by precipitation out of
solution as ions become more concentrated when
water evaporates. On average, seawater contains
35 g L
1
(35 parts per thousand) of dissolved ions,
mainly chloride, sodium, sulphate, magnesium, cal-
cium and potassium (Fig. 3.8). The chemistry of lake
waters is variable, often with the same principal ions
in different proportions. The combination of anions
and cations into minerals occurs as they become con-
centrated and the water saturated with respect to a
particular compound. The least soluble compounds
are precipitated first, so calcium carbonate is first
precipitated out of seawater, followed by calcium sul-
phate and sodium chloride as the waters become more
concentrated. Potassium and magnesium chlorides
will only precipitate once seawater has become very
concentrated. The order of precipitation of evaporite
minerals from seawater and the loss of water required
for them to form are listed in Fig. 3.9, along with the
mass formed per unit volume of seawater and the
chemistry of the mineral.
3.2.1 Gypsum and anhydrite
The most commonly encountered evaporite minerals
in sedimentary rocks are forms of calcium sulphate,
Bivalves
Gastropods
Cephalopods
Brachiopods
Echinoderms
Foraminifera
Corals
Bryozoans
Sponges
Rhodophyta (algae)
Chlorophyta (algae)
Chrysophyta (algae)
Aragonite
Low-Mg calcite
High-Mg calcite
Aragonite+calcite
dominant
less common
Mineralogy of major
fossil groups
Fig. 3.7 The calcareous hard parts of organisms may be
made up of aragonite, calcite in either its low- or high-
magnesium forms, or mixtures of minerals.
other
55% Cl
-
1.1% K
+
1.2% Ca
2+
7.7%
SO
4
2-
31% Na
+
3.5% Mg
2+
Fig. 3.8 The proportions of the principal ions in seawater of
normal salinity and ‘average’ river water. (Data from
Krauskopf 1979).
0
100
20
40
60
80
Percentage
90
10
30
50
70
Marine evaporites
0.3%
3.5%
18%
CaCO
3
CaSO
4
e.g. KCl
calcium
carbonate
calcium
sulphate
potash
salts
78%
NaCl
sodium
chloride
Fig. 3.9 The proportions of minerals precipitated by the
evaporation of seawater of average composition.
36 Biogenic, Chemical and Volcanogenic Sediments

either as gypsum or anhydrite. Calcium sulphate is
precipitated from seawater once evaporation has con-
centrated the water to 19% of its original volume.
Gypsum is the hydrous form of the mineral
(CaSO
4
.2H
2
O). It precipitates at the surface under all
but the most arid conditions but may become dehy-
drated to anhydrite on burial (18.5). Anhydrite has
no water in the crystal structure (CaSO
4
) and forms
either by direct precipitation in arid shorelines
(15.2.3) or as a result of alteration of gypsum by
burial. It may become hydrated to gypsum if water
is introduced. Primary gypsum occurs as elongate
crystals of selenite when it forms from precipitation
out of water. If it forms as a result of the rehydration
of anhydrite it has a fine crystalline form in nodules of
alabaster. Gypsum also occurs as a fibrous form in
secondary veins.
Gypsum is readily distinguished from calcium car-
bonate minerals in the field because it is softer (hard-
ness 2, easily scratched with a fingernail) and does
not react with dilute HCl: it can be distinguished from
halite by the fact that it does not taste salty. Crystals of
gypsum have a low relief when they are viewed under
the microscope, cleavage is usually well developed
and the birefringence colours are low-order greys.
Anhydrite is a harder (hardness 3.5), denser mineral
than gypsum: it is commonly white in hand specimen,
and is not easily scratched by a fingernail. In thin-
section the high density means crystals have a rela-
tively high relief; birefringence colours are moderate,
higher-order colours than gypsum.
3.2.2 Halite
Halite (NaCl) precipitates out of seawater once it has
been concentrated to 9.5% of its original volume
(Fig. 3.10). It may occur as thick crystalline beds or
as individual crystals that have a distinctive cubic
symmetry, sometimes with a stepped crystal face
(a hopper crystal). The high solubility of sodium
chloride means that it is only preserved in rocks in
the absence of dilute groundwater, which would dis-
solve it. Surface exposures of halite can be found in
some arid regions where it is not removed by rain-
water.
Naturally occurring halite is rock salt, so the sim-
plest test to confirm the presence of the mineral
is taste: the only mineral it might be confused with
on this basis is sylvite (below), but this potassium
chloride mineral has a more bitter taste than ‘normal
salt’ and is much less common. Halite is soft (hardness
2.5, slightly more than gypsum but still scratched by
a fingernail), white or colourless. In thin-section
halite crystals may show a strong cleavage with
planes at right angles and, being a cubic mineral, it
is isotropic.
3.2.3 Other evaporite minerals
Evaporation of seawater can yield other minerals,
which are rarely found in large amounts but can be
economically important. In particular, potassium
chloride, sylvite (KCl), is an important source of
industrial potash that occurs associated with halite
and is interpreted as the product of extreme evapora-
tion of marine waters. However, evaporation of mod-
ern waters results in a number of different magnesium
sulphate (MgSO
4
) minerals rather than sylvite, and
this has led to suggestions that the chemical composi-
tion of seawater has not been constant over hundreds
of millions of years (Hardie 1996). Variations in the
relative importance of meteoric waters (run-off from
land) and hydrothermal waters (from mid-ocean ridge
vents) are thought to be the reason for these varia-
tions in water chemistry, which either favour KCl or
MgSO
4
precipitation at different times.
Saline lakes (10.3) generally contain the same dis-
solved ions as seawater, but the proportions are
usually different, and this results in suites of evap-
orite minerals characteristic of different lake chem-
istries. Most of these minerals are sulphates,
Fig. 3.10 White halite precipitated on the shores of the
Dead Sea, Jordan, which has a higher concentration of ions
than normal seawater.
Evaporite Minerals 37
