FTA (изд-во). Flexography: Principles And Practices. Vol.1-6
Подождите немного. Документ загружается.

32 FLEXOGRAPHY: PRINCIPLES & PRACTICES
• and ambient conditions, e.g., tempera-
ture or atmospheric pressure.
To prevent imbalance, any solvent blend
added as a replacement should be of a simi-
lar nature to the escaping solvents. Failure
to use a compatible replacement may also
result in resin kick-out or ink souring, loss of
gloss, increasing ink viscosity or lack of
adhesion. Such problems are more notice-
able in jobs where ink usage is limited, such
as process work or small spot colors and it is
the responsibility of the ink formulator to
identify a suitable “balanced solvent” to pre-
vent this from occurring.
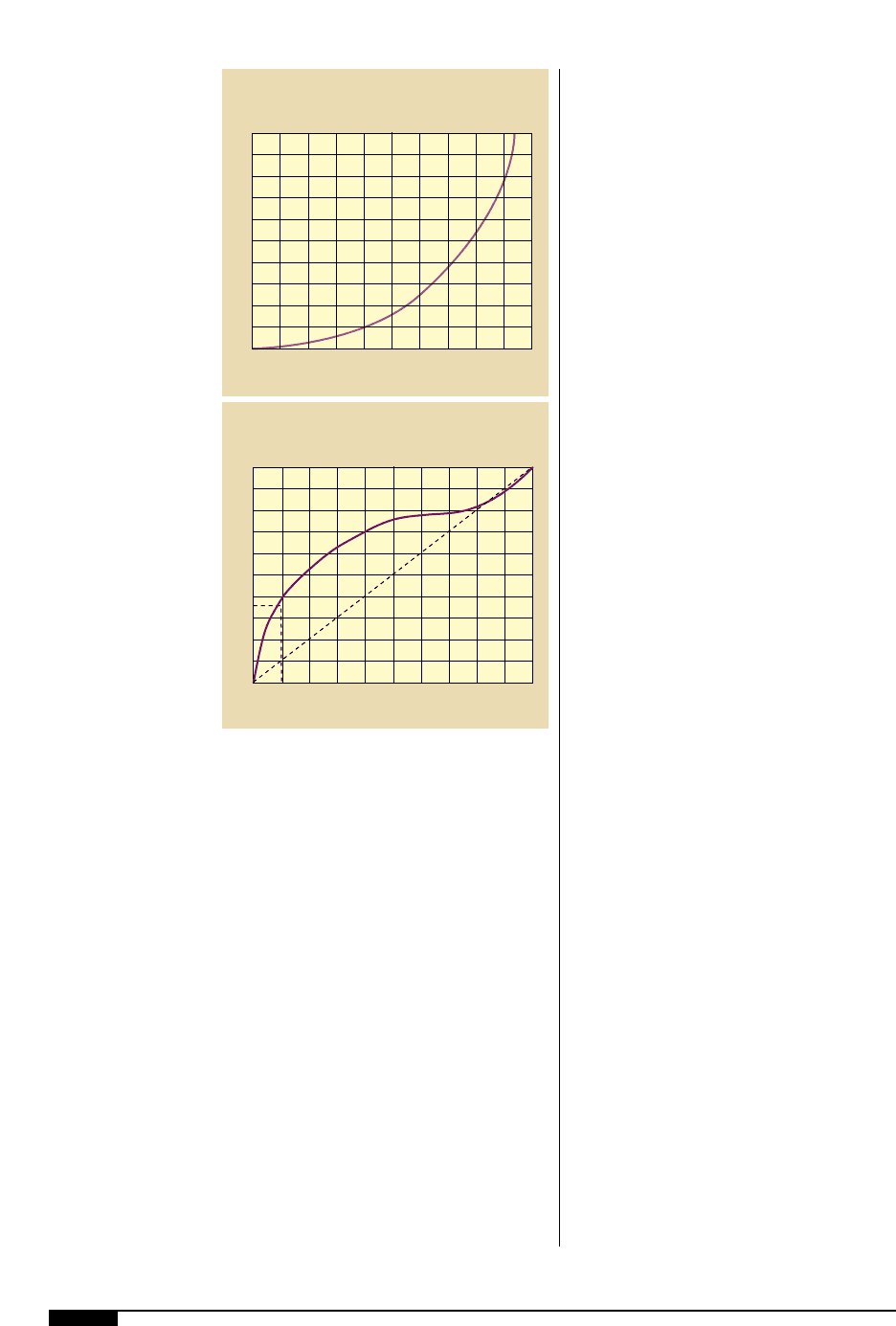
The importance of solvent balance is
demonstrated in Figure
2*
. The horizontal
axis represents the percentage of n-propyl
acetate in an ink fountain that contains a
blend of n-propyl acetate and n-propyl alco-
hol. The vertical axis represents the percent
of n-propyl acetate in the solvent evaporating
from the fountain. The solid curve represents
the composition of the solvent vapors from
the various mixtures. For example, if you
start with a fountain blend of 10% n-propyl
acetate, it follows that the solvent vapors
contain 35% n-propyl acetate. This excessive
loss of n-propyl acetate will shift the solvent
balance and result in a leaner, less acetate
rich solvent resulting in print problems. In
this case, the problem can be avoided by
using a 35:65 blend of n-propyl acetate/n-
propyl alcohol to replace the solvents lost by
evaporation from the ink fountain.
In summary, the original ink and any fresh
ink added to the fountain should be diluted
to target viscosity with the 10:90 blend of n-
propyl acetate/n-propyl alcohol. Any subse-
quent manual viscosity reduction, while on
press, should be carried out with the 35:65
blend of n-propyl acetate/n-propyl alcohol to
maintain solvent balance.
Similarly, Figure
2(
shows a plot of a gly-
col ether solvent and n-propyl alcohol. In
this case, the percent of glycol ether evapo-
rating is significantly lower than the level of
glycol ether in the fountain. It would be
extremely dangerous to replace any evapo-
rating solvent with the same blend used to
make the initial cut. The fountain would
become increasingly richer in glycol ether,
leading to poor drying, blocking in the
rewind, retained odor, and lamination prob-
lems such as blistering, tunneling and poor-
bond strengths.
Most solvents present a fire hazard, and it
is important to take note of flash points and
explosive limits. In addition, some solvents
are considered hazardous to health or the
environment above certain concentrations.
The properties of a number of common sol-
vents used in flexographic inks are detailed
in Table 5.
2*
The importance of
solvent balance is
shown in this graph,
where the solid curve
represents the
composition of the
solvent vapors from
the various mixtures.
2(
A comparison of
glycol ether solvent and
n-propyl alcohol levels
shows the percent of
glycol ether evaporating
is significantly lower
than the level of glycol
ether in the fountain.
010 9080706050403020 100
100
90
80
70
60
50
40
30
20
10
Percent Glycol Ether in Fountain
Percent Glycol Ether in
Evaporating Solvent
2*
010 9080706050403020 100
100
90
80
70
60
50
40
30
20
10
Percent Normal Propyl Acetate in Fountain Ink
Percent Propyl Acetate in
Evaporating Solvent
2(

Additives
Although the pigments, resins and solvents
chosen provide the ink formula “skeleton,” it
is often necessary to enhance or modify cer-
tain ink characteristics to achieve the neces-
sary performance. While various additives
are used to modify the performance of the
ink, it is important to recognize that with a
correctly formulated vehicle, the use of addi-
tives will be minimized. Poorly formulated
vehicles will require material additions that
may cause secondary problems, which in
turn require the use of further additives. The
additives typically used in modifying flexo-
graphic ink fall into many categories. The
most common, briefly detailed below:
• Plasticizers. The main function of a plasti-
cizer in an ink film is to make the dried print
more flexible and elastic. They do this by
acting as non-volatile solvents for the film,
forming resins. Certain plasticizers are used
to promote specific properties in the printed
ink film, such as increased gloss, improved
flexibility or increased adhesion on difficult
substrates.
• Waxes. Different chemical types of wax can
be incorporated into flexographic inks (1–3%
dry weight) to achieve mar resistance, reduce
blocking or set-off, and improve slip and
water repellency. Keep usage as low as possi-
ble as excess wax levels may lead to reduced
gloss, poor ink rheology and reduced transfer
characteristics.
• Silicones. This class of materials find use at
low levels (0.1–1.0%) as substrate- or pig-
ment-wetting agents, additives to improve
mar/slip, anti-foams and release agents.
While it appears they have wide utility, they
do have drawbacks. Excessive use can lead
to print defects such as pinholing or crawl-
ing. Also the presence of low-molecular-
weight silicones (<50 centiStokes) in inks
can lead to the poisoning of catalytic beds
within incineration units.
• Surfactants. These additives are used to
improve wetting and spreading. Surfactants
are regarded as high performance deter-
gents, finding use as dispersion aids, flow
promoters and wetting aids on difficult sub-
strates. Care has to be exercised in surfac-
INK 33
Table 5
DRYING BOILING POINT SPECIFIC FLASH POINT FLAMMABILITY LIMITS
SOLVENT RATE
a
°F °C GRAVITY °F °C LOWER% UPPER%
Ethyl alcohol 4.4 173° 78° 0.79 55° 13° 4.3% 19.0%
n-Propyl Alcohol 2.39 208° 98° 0.80 77° 25° 2.1% 13.5%
n-Propyl Acetate 5.78 215° 102° 0.89 58° 14° 2.1% 8.0%
Isopropyl Acetate 9.5 191° 88° 0.87 40° 4° 1.8% 8.0%
Heptane 13.5 209° 98° 0.68 39° 4° 1.8% 6.7%
VM&P Naptha 4.1 212°–320° 100°–160° 0.67 20°–50° -7°–10° 1.1% 6.7%
Dowanol PM 2.3 250° 121° 0.92 97° 36° 0.9% 13.1%
Propylene Glycol 0.03 370° 188° 1.04 210° 99° 1.1% 9.2%
Acetone 15.7 134° 57° 0.79 0° 15° 0.9% 12.8%
Methyl Ethyl Ketone 10.6 176° 80° 0.80 20° 7° 2.6% 10.0%
Toluene 5.7 232° 111° 0.87 40° 4° 1.8% 7.1%
Water 1.0 212° 100° 1.0 ——1.2% —
Ammonia — 232° 111° 0.9 ————
a
Water=1
SOLVENT PROPERTIES
34 FLEXOGRAPHY: PRINCIPLES & PRACTICES
tant choice. Watch for problems with foam-
ing, adhesion or reduced water resistance.
• Defoamers. Foaming is a problem that most
commonly occurs in water-based inks and is
evident under conditions of high agitation.
Obviously, prevention is better than cure,
but where foaming is present it can be dealt
with by the addition of defoaming agents.
Such materials work by dramatically reduc-
ing surface tension in the system, causing
existing bubbles to burst, and preventing
unstable foams from forming. These materi-
als are usually derived from mineral oils or
silicones.
• Pure Chemicals. This category of additives
covers a diverse group of materials including
acids, alkalis, metal chelates, polyols, metal
salts and anti-oxidants. Such materials can
function as adhesion promoters, fixatives,
drying aids, stabilizers and cure agents, as
determined by the ink chemistry and formu-
lation.
INK CHARACTERISTICS
There are a number of fundamental prop-
erties required of flexographic inks that are
determined by the nature and demands of
the printing process and the final application
of the print:
• rheology;
• transfer;
• color and strength;
• print appearance;
• adhesion; and
• functional properties.
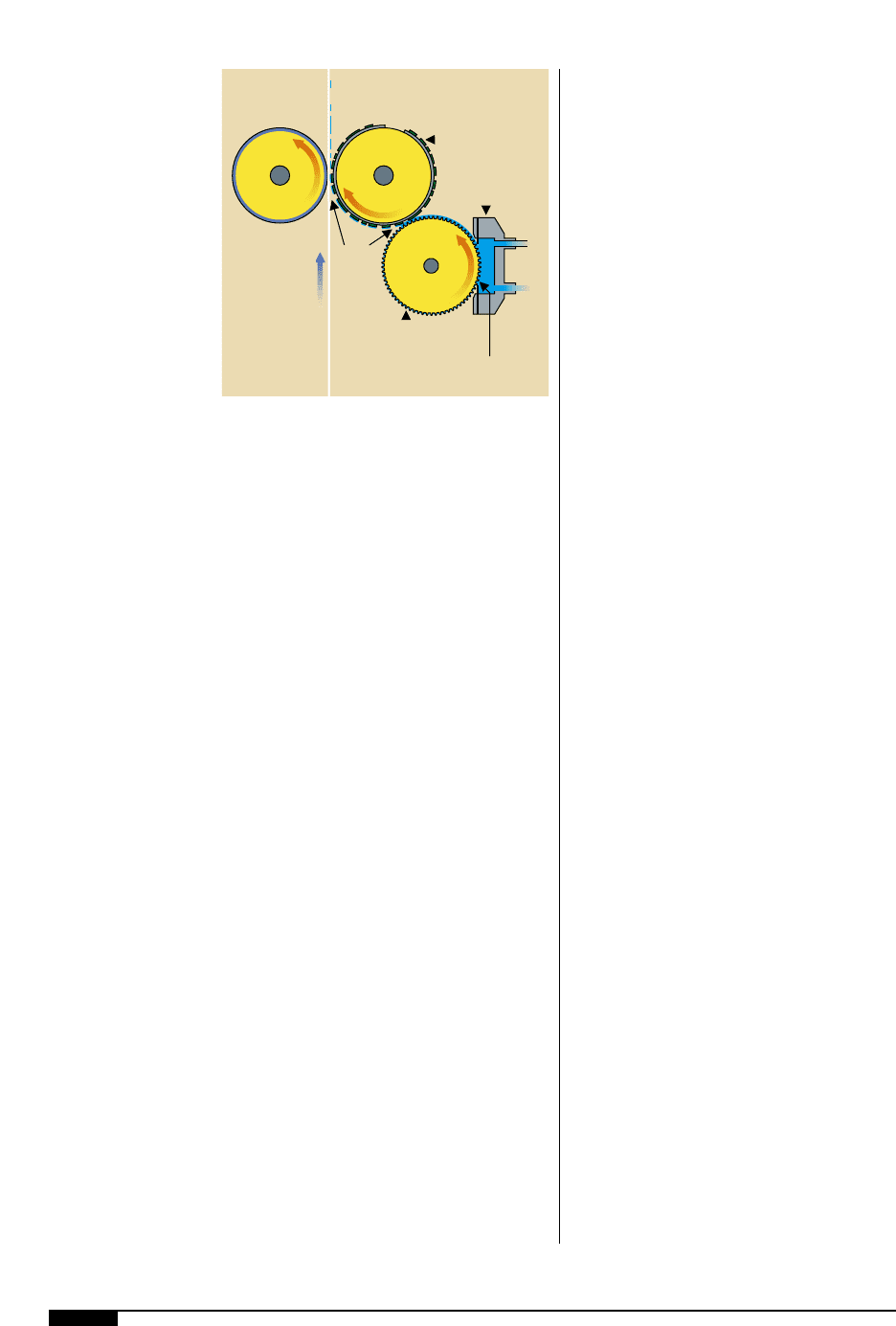
The fundamental property required of an
ink is that it prints well. To do this, it must
possess the rheology or fluidity to be trans-
ported through the inking system onto the
anilox. It has to have sufficient wet tack to
transfer from the anilox onto the plate and
then finally to the substrate (Figure
3)
). The
drying speed of the ink needs to be such that
it remains fluid while on the press, but dries
rapidly after application to the substrate.
The ink must be of suitable shade and
strength. It must print cleanly, adhere to the
chosen substrate and provide the properties
necessary to meet customer specifications.
Rheology. Fluidity and low viscosity are cru-
cial to the flexographic process. While on
press, the ink is required to be pumped and
transported through various pieces of equip-
ment and subjected to extreme shear forces.
Maintaining a fluid ink at a low viscosity
reduces the wear and tear on press compo-
nents and can be achieved by the incorpora-
tion of suitable solvents. The actual viscosi-
ty chosen for printing is determined by a
variety of factors including the metering sys-
tem, substrate characteristics, press speed
and print requirements.
Transfer. Of all the ingredients present in the
ink, the binder system impacts most heavily
on transfer. In general, as the molecular
weight of the chosen resin and the resin
solids present in the ink increase, so does
the transfer. Transfer properties of differing
formulations or resin systems can be easily
compared in the laboratory by applying the
test inks – after ensuring the strength and
viscosities are equal – side by side using a
hand anilox proofer.
Color and Strength. The color and strength of
a flexographic print are largely determined
3)
In order for an ink
to print well, it must
possess the rheology to
be transported through
the inking system onto
the anilox. It has to have
sufficient wet tack to
transfer from the anilox
onto the plate and then
finally to the substrate.
Impression
Cylinder
Plate
Cylinder
Anilox
Roll
Web
Doctor
Blade
Assembly
Ink Drying on Plate
Ink Drying
into Anilox Cells
Resolubility
Points
Ink
Film
Split
3)
by the type and concentration of the col-
orant used within the ink and the thickness
of the wet film applied. Other factors such as
substrate and converting processes can also
affect appearance. The thickness of the wet
film laid down is determined largely by the
metering system employed and can vary in
different applications from 2–12 microns.
Since this variation is so large, it is difficult
to formulate an ink consistently with the
precise amount of colorant present to meet
requirements. To combat this, the ink maker
commonly supplies the ink at a higher color
strength and viscosity to allow adjustments
to be made on press.
Print Appearance. In addition to basic color
requirements, the ink may have to be glossy,
matte, transparent or opaque as determined
by the design and substrate. The ink should
also print clean, smooth solids, fine type and
process screens. To achieve these properties,
it is desirable that the ink display good flow,
wetting and resolubility. Most inks today are
formulated with pigments, due to required
end-use application. Care has to be taken
with pigment selection to prevent poor “ink
working” and flow properties. This caution
also extends to higher performance binders
like high molecular-weight polyamides that
exhibit low solvent solubility, which can lead
to dirty printing.
After printing, it is critical that the ink
dries quickly. This need is particularly
noticeable when printing a multiple “trap”
job on a common impression press. In such
cases, the minimal gap between subsequent
printing units and the speed of the press
necessitate the rapid drying of the ink.
Drying rate should not be so rapid that it
causes the ink to dry on the plate or anilox.
A balance must be struck. Any ink that dries
on either the anilox or plate should be easily
resolubilized on the next revolution, mini-
mizing the risk of poor image reproduction
or dirty working. Care has to be exercised
when “trapping” or printing color-on-color to
prevent solvents from the overprinting color
resolubilizing the first layer down. This
rewetting can lead to solvent retention, loss
of print quality or print blocking.
Adhesion. This property is particularly
important with non-absorbent substrates
such as polyethylene or polypropylene. With
paper or board printing, adhesion problems
with an ink seldom arise.
The binder is the most important primary
constituent in determining ink adhesion. It is
common for an ink to require a blend of
resins to achieve the desired level of adhe-
sion and other performance properties. Each
substrate presents different challenges, and
in general the more inert the substrate, the
more difficult it is to obtain adhesion. In
practice, this means that resins conferring
INK 35
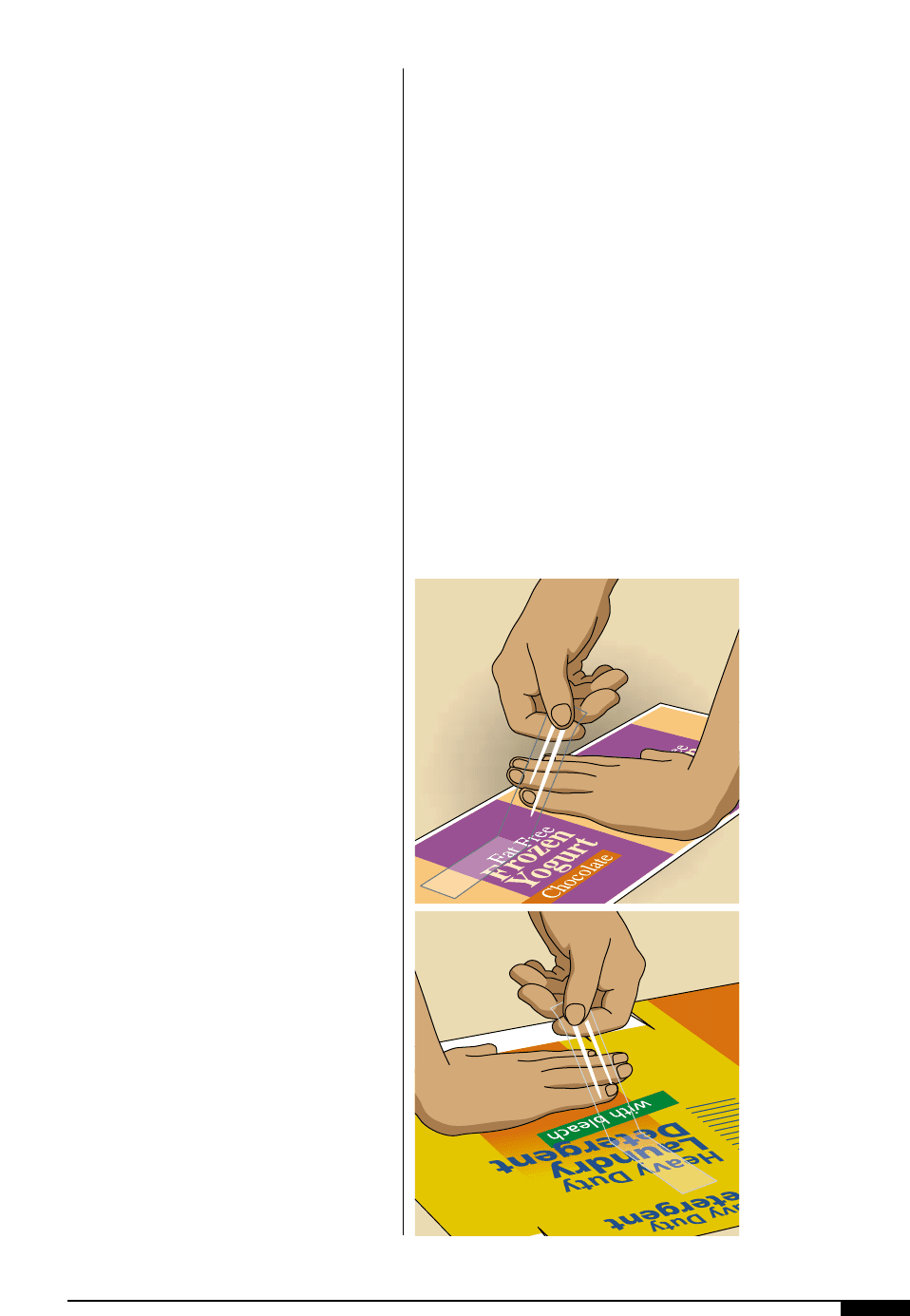
3!
In this figure, common,
everyday cellophane
tape is used for the
adhesion test by
placing it on the
printed substrate.
3@
To test for adhesion,
the tape is peeled off
and examined for the
effect on both tape and
substrate.
3!
3@

36 FLEXOGRAPHY: PRINCIPLES & PRACTICES
adhesion on the myriad of available sub-
strates must be identified on a case-by-case
basis (Figures
3! 3@
).
End-use Application. This requirement has a
particular bearing on the formulation of an
ink. There may be requirements during pro-
cessing, as outlined in the previous chapter,
for heat resistance, the ability to be laminat-
ed and certain slip or scuff characteristics.
The package may have to show product
resistance to fats, oils, detergents, solvents
acids or alkalis. Consideration of the printed
product’s end use can be used to determine
the gross performance needs. However, all
technical requirements for a particular appli-
cation should be fully specified by the cus-
tomer prior to formulation. Table 6 shows a
few flexographic end-use markets and the
general properties required.
The enormity of formulating an ink capa-
ble of complying with all these requirements
should not be underestimated. One also
needs to keep in mind the added complica-
tions of converting needs, regulatory con-
cerns and quality performance objectives.
Converting Needs. The converting processes
in operation have a dramatic influence on
the formulation of an ink. Changes in the ink
may require changes in the process. For
example, while solvent-based inks may not
require a corona treatment unit to print suc-
cessfully on polyolefinic films, it is almost
certainly required with water-based inks.
Some of these process parameters are out-
lined in Table 7.
Regulatory Controls. The composition of an
ink has to account for local, state and gov-
ernment regulations covering:
• air remissions;
• metal content;
• material toxicity; and
• food and drug standards.
Sweeping environmental legislation within
the last 20 years has necessitated major
reformulation efforts to remove, replace or
reduce harmful materials, and require even
tighter controls over incoming raw materials.
This situation is becoming ever more com-
plex since federal legislation is increasingly
enacted differently on a regional basis,
depending on local conditions and needs.
The changes ultimately require the ink man-
ufacturer to supply different ink formula-
tions for identical applications, based on geo-
graphic environmental need. This topic is
expanded upon in a following section.
Table 6
END USE SUBSTRATE CONSTRUCTION INK PERFORMANCE REQUIREMENTS
■Bakery Ink-LDPE High performance, low COF, low retained solvent,
ice water crinkle resistance
■Milk Carton Ink-LDPE-Board Product resistance, alkali resistance,
wet/dry rub resistance
■Snack Package OPP/Polyester-Ink Low retained solvent
Adhesive polyethylene High bond strength
■Confectionery Coex. OPP-Ink Low odor, low solvent retention
Cold seal adhesive Cold-seal release
■Display, Corrugated Ink-bleached Kraft High gloss, rub/scruff resistance
END-USE APPLICATIONS
INK FORMULATION
AND SELECTION
Having an understanding of the properties
of the materials used within inks, the print
and conversion processes utilized, and the
end-use application, allows inks to be for-
mulated to meet all required specifications.
As mentioned previously, flexographic inks
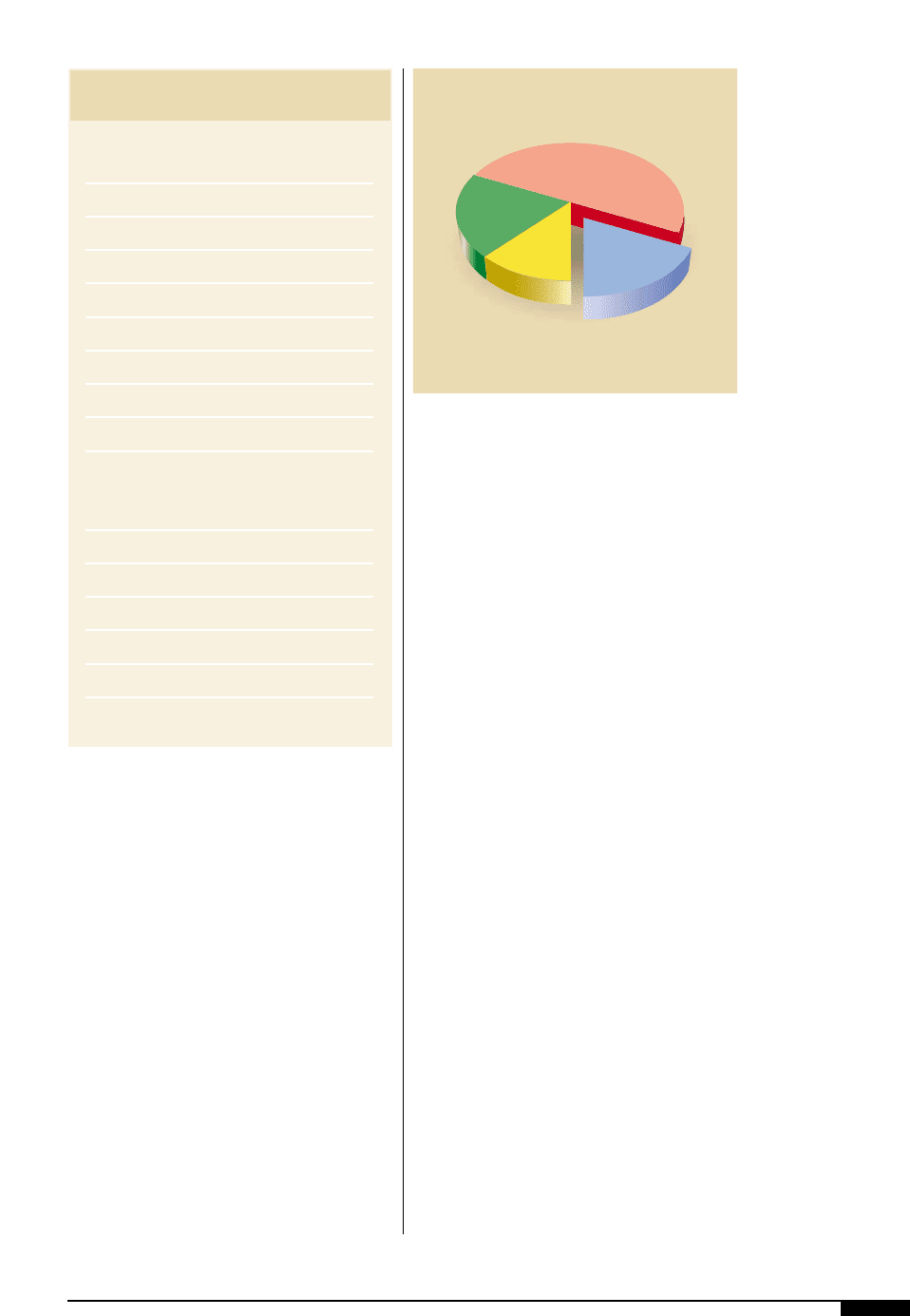
consist of four main ingredients: colorant,
binder, solvent and additives. The composi-
tion varies widely depending on the choice
of substrate, press conditions, and final use.
Figures
3#
illustrates the ingredients which
make up a typical ink.
It is normal to classify inks into product
types according to formulation, substrate
used and performance properties, rather
than have discrete inks for individual situa-
tions. This helps avoid product proliferation
and inventory problems both at the ink man-
ufacturer and converter.
Product categorization eases ink selection
and benefits manufacturing and product
quality through improved inventory control
and material planning. The system has to
maintain flexibility since flexographic indus-
try is subject to increasingly rapid changes
that may require ink modification. A selec-
tion of characteristic formulations is out-
lined with respect to the substrate printed
and end-use needs (Table 8).
Water-based Inks
Water-reducible flexo inks, based on
casein, were first used in the 1940s on corru-
gated cartons. During the 1950s and 1960s, as
printability improved, their use in paper/
paperboard printing increased. The imple-
mentation of the Clean Air Acts during the
early 1980s spurred the development of
water-based technology. Today, water-based
inks are used successfully in all areas of flex-
ographic printing. Conversion to water-based
inks, particularly in demanding applications,
has been a success where this change has
been accompanied by an increase in press-
drying capacity, improved ink-metering sys-
tems, and the replacement of press compo-
INK 37
3#
Four primary
ingredients constitute
flexographic inks:
colorant, binder,
solvent and additives.
Table 7
PRINTING
1. Corona Treaters.
2. BC and OH Dryer Capacities.
3. Line Speeds.
4. Tension Controls.
5. Cooling Rolls.
6. Ink Pumps/Viscosity Controls.
7. Run Times.
8. Design Parameters: % Coverage, Traps.
9. Press Room Temperature and Humidity.
LAMINATIONS
1. Ink-to-Substrate Adhesion.
2. Ink-to-Laminate Adhesion.
3. Corona Treaters.
4. Use of Primers.
5. Wetting of Ink by Adhesive.
6. Extrudate Temperature.
7. Extrudate Compositions.
CONVERTING NEEDS –
PERFORMANCE ISSUES
Resin
10%–30
Solvents
40%–60%
Additives
10%–25%
Colorant
4%–20%
3#

38 FLEXOGRAPHY: PRINCIPLES & PRACTICES
Table 9
HIGH-GLOSS INK FOR LDPE (low density polyethylene).
HEAT-RESISTANT, HIGH-ABRASION INK FOR POLYPROPYLENE.
SOLVENT INK FOR FOLDING CARTON
Color strength is a primary concern along with
good print quality, rapid drying, good flexibility
(folding) and scuff for sheeting equipment. Wax
and plasticizers improve scuff and flexibility.
Isopropyl acetate and ethanol speed up drying for
less absorbent coated stock
s.
MAKE UP %
calcium 2B pigment 14.0
titanium dioxide 6.0
maleic resin (dry) 8.0
nitrocellulose (dry) 11.5
n-propyl alcohol 25.0
ethanol 18.0
isopropyl acetate 10.0
plasticizer 5.0
wax compound 3.5
100.0
Inert nature of polypropylene film requires use of
adhesion promoter. Nitrocellulose has necessary
heat reistance and hardness. Urethane provides
adhesion and flexibility. PTFE and silicone provide
scuff resistance. For use on VFFS and HFFS filling
machinery. Low odor needed if used for food pack-
aging.
MAKE UP %
organic pigment 12.0
nitrocellulose (dry) 10.0
polyurethane 14.0
n-propyl alcohol 36.0
ethanol 10.0
n-propyl acetate 15.8
adhesion promoter 1.0
PTFE wax 1.0
silicone 0 . 2
100.0
Used for bakery and deep-freeze applications.
Requires fast drying because of press speeds, low
odor for food packaging. Deep freeze and water
resistance require careful selection of pigments.
Waxes required to prevent blocking and sticking in
roll and wickets.
The traditional ink for polyethylene is based on
polyamide, although at one time it was shellac. To
achieve acceptable adhesion, the film must have a
surface energy of 38 to 42 dynes/cm. Below this
level, poor ink adhesion will be observed. Above
this energy level, the water resistance of the printed
film and its heat sealability may be compromised.
MAKE UP %
organic pigment 12.0
alcohol-soluble polyamide 22.0
nitrocellulose (dry) 4.0
n-propyl alcohol 34.0
ethanol 13.0
n-propyl acetate 12.0
polyethylene wax 2.0
amide wax 1.0
100.0
INK FORMULATIONS
Table 8

nents based on “electrically” different metals
(e.g., copper, iron) that come into contact
with the ink. Installing corona-discharge
equipment for film printing, and retraining
press operators also must be part of the pic-
ture. While water-based inks bear some simi-
larity to their solvent-based counterparts,
there are some differences, particularly in
the chemistry of the resins used and their
handling.
Colorants. The colorants available for use are
largely identical to those used in solvent
inks. Many have been specially treated or
prepared to make them suitable for use in
water systems where alkali or water solubil-
ity are concerns.
Vehicles. The primary vehicles used can be
classified either as alkali-soluble vehicles,
emulsions or colloidal-dispersion vehicles.
The fundamental requisite in all these is that
they retain water solubility while being print-
ed but become water insoluble after printing
and drying. These apparently contradictory
requirements are largely achieved by using
water-insoluble carboxylated (acid-contain-
ing) resins and converting them to their
water-soluble salts, using volatile alkaline
materials such as ammonia or organic
amines. Organic amines such as monoethan-
olamine evaporate more slowly than ammo-
nia, so resins solubilized with an organic
amine dry more slowly and take more time to
achieve water and product resistance.
Just as solvent balance in solvent inks gov-
erns print performance, the alkalinity or
amount of alkali present in the water-based
ink determines performance. The amount of
alkali present can be measured using a pH
meter, which measures acidity or alkalinity
and is associated with water-based inks. The
pH value can vary in range from 1.0 (battery
acid) to 14.0 (household ammonia solution),
where 7.0 is neutral – equivalent to distilled
water. A pH higher than 7.0 indicates
increased alkalinity. Maintaining a level of
alkalinity, typically between pH 8.6–9.4, is
vital to controlling the performance of an ink
on press. Such control can be a problem
with long runs of water-based inks, particu-
larly with printing a low coverage design in
hot weather.
A comparison of the properties of these
vehicles is given in Table 9.
Auxiliary Solvents. A variety of alcohols, gly-
cols and glycol ethers are used to control
drying speed, improve resolubility or aid
film formation. Caution should be exercised
when using these materials to prevent fire
and health hazards.
Additives. The additives used within water-
based inks include waxes, plasticizers and
defoamers and are largely similar to those
used in solvent inks, with the exception that
the chemistries used have been modified to
make them suitable for an aqueous environ-
ment.
INK 39
Table 9
SOLVENT SOLUTION EMULSION COLLOIDAL
POLYMER POLYMER DISPERSION
Printability E F P
Drying Speed P G VG
Product P G E
Resistance
Resolubility E P VP
Applied Solids L H VH
Freeze Stability G F P
Foaming G P P
Pigment E P NR
Dispersion
KEY:
E=Excellent
G=Good
VG=Very Good
P=Poor
L=Low
H=High
VH=Very High
NR=Not Recommended
VEHICLE PROPERTIES

40 FLEXOGRAPHY: PRINCIPLES & PRACTICES
Using Water-based Inks
The problems encountered in handling
water-based inks typically stem both from
the physical properties of water and the
chemistry of the ink and include:
• pH control;
• volatility of water;
• specific heat capacity of water;
• conductivity; and
• surface energy.
Water-based inks are commonly regarded
as easier to handle than solvent-based inks
because of their lower volatility. However,
this is a misconception since pH control in
water-based inks is just as vital as maintain-
ing solvent balance in solvent-based inks.
Water-based inks are most stable within cer-
tain pH limits. After prolonged use, the pH
can drop below the lower limit leading to
false heavy body and resin kick-out (similar
to solvent-based ink souring). A pH reading
higher than the suggested upper limit can
lead to both drying, odor and product resis-
tance problems (similar to solvent retention).
Caution has to be exercised in reducing
the viscosity of water-based inks as they
tend to lose viscosity faster than solvent-
based inks. Over-reduction often leads to
poor print quality, e.g., crawling and poorer
performance properties.
Water dries much more slowly than typical
flexographic solvents like propyl alcohol or
propyl acetate (See Table 5, Solvent Proper-
ties). To compensate for this, water-based
inks are often formulated to have higher
resin and pigment solids. The higher solids
allow a thinner wet-ink film to be printed,
which speeds up drying – especially on non-
absorbent substrates – while maintaining
performance properties (Table 10). Since
inks on non-absorbent substrates dry solely
by evaporation, the drying capacity of the
press is very important. It is critical that the
inter-unit and overhead drying units operate
under negative pressure with an air velocity
of approximately 3,500 cu.ft/min. The higher
specific heat capacity of water also requires
the ovens to be set at slightly higher settings
to achieve the same web temperature as
with solvent-based inks.
In the case of absorbent substrates, the
need for efficient driers is lessened. On high-
ly absorbent stocks, the free water is drawn
into the surface, often by capillary action,
leaving the pigment and resin solids deposit-
ed on the surface. This can occur in as quick-
ly as 0.1 second. Drying can also be en-
hanced on acidic paper stocks where the
acid neutralizes the solubilizing amines.
Although no longer a major problem with
ceramic aniloxes, water-based inks are often
associated with increased chrome anilox
wear for two reasons. The first is mechani-
cal: water-based inks have less lubricity and
cause mechanical breakdown of the chrome
anilox from frictional wear. The second is
chemical: the inks aid the generation of a
galvanic cell by functioning as an electrical
conductor between dissimilar metals.
Ink will only transfer or “wet out” on a sub-
strate when the surface tension of the ink is
lower than that of the substrate to be print-
Table 10
Typical water-based flexographic formulations
are as follows:
For For
Nonabsorbent Absorbent
Substrate Substrate
35% pigment dispersion 50.0 40.0
acrylic solution polymer 10.0 30.0
acrylic emulsion 30.0 12.5
water 5.0 13.0
organic amine 1.0 1.0
polyethylene wax compound 3.0 3.0
surfactant 0.5 —
organic anti-foam 0.5 0.5
TOTALS 100 100
WATER-BASED INK FORMULATION

ed. Pure water has a surface tension of 72
dynes/cm, while the surface energy of most
untreated polyolefin films lies in a range of
31 to 35 dynes/cm. To facilitate the printing
of water-based inks on film substrates, it is
necessary to reduce the surface tension of
the inks by incorporating wetting agents and
boosting the surface energy of the film using
corona discharge or flame treatment. Film
treatment, while aiding ink adhesion, also
helps in “burning off” the migratory fatty
acid amides which are used as a slip agent in
the film. Such slip agents commonly migrate
to the surface of the film, and at high levels
can cause wetting problems like pinholing or
reticulation.
Catalytic Inks. The resistance properties of
prints produced from conventional inks that
dry by evaporation are dictated by the prop-
erties of the resins employed. With the flex-
ographic process, the choice of resins is lim-
ited by the available range of solvents, which
in turn places limits on resistance properties
achievable with regular flexographic inks.
This problem can be overcome by utilizing
ink systems that undergo specific chemical
reactions upon drying. The principle types
are usually based on epoxy amine or aziri-
dine acid chemistry and rely on a reaction to
crosslink the individual reactive elements
together to generate a composite polymer
with improved heat, solvent and product
resistance and more gloss and adhesion than
a conventional ink. The inks do have some
disadvantages:
• the need to mix two components;
• limited pot life after mixing; and
• specific cure conditions.
Such inks typically have discrete curing
conditions requiring a high temperature and
long-drying time to complete reaction. Care
has to be taken to ensure that the materials
used to produce the ink and those inks or
materials in contact with the printed catalyt-
ic material do not act as cure inhibitors.
UV- and Electron Beam-Cured Inks
Drying vs. Curing. In simplest terms, drying of
an ink film occurs with conventional inks
when the ink vehicle (solvent or water) evap-
orates or is absorbed, leaving behind the
solids (pigments, resins, waxes, etc.) to form
a film on the substrate surface. In radiation
curing, on the other hand, all of the compo-
nents in the ink or coating remain on the sur-
face of the substrate, but are chemically
transformed into a hard film through expo-
sure to ultraviolet (UV) lights or a concentrat-
ed beam of highly energized electrons (EB).
The difference lies in the chemistry of the
materials in the inks and coatings and in the
pressroom equipment needed to “energize”
the curing process.
Rudimentary Ink Chemistry. The materials
used in radiation-curable ink formulas are
considerably more “user friendly” today than
ever before. Advancements in raw materials
will continue to make radiation-curable inks
and coatings more commonplace in the years
ahead. The major components of UV and EB
inks and the function of each of these chem-
icals are as follows:
• Reactive Diluent (Monomer). A reactive dilu-
ent monomer is a simple, lightweight chemi-
cal similar to a solvent in its ability to thin
down the ink. Monomers help determine the
characteristics of the ink, such as gloss,
hardness and flexibility. These low-viscosity
monomers, which can be readily absorbed
by unprotected skin, are also the chemicals
which give uncured UV and EB inks their
most hazardous characteristics. The poten-
tial of a monomer to cause skin irritancy or
sensitization problems can be gauged from a
given Draize value. The Draize value is a 1 to
4 numeric measure of its irritation or sensiti-
zation potential. The higher the Draize value,
the more hazardous the material. Most com-
mercially available UV or EB flexographic
products utilize materials with a Draize val-
ues of 2 or less and do not pose any major
health and safety concerns, providing of
INK 41
