FTA (изд-во). Flexography: Principles And Practices. Vol.1-6
Подождите немного. Документ загружается.


42 FLEXOGRAPHY: PRINCIPLES & PRACTICES
course, that all handling guidelines are fol-
lowed.
• Resin (Oligomer). The resin in radiation-cur-
able inks is actually called an “oligomer.” As
in conventional inks, the resin is the chemi-
cal backbone of the ink. Among others, it
provides the body, wetting ability, binding
and functional properties of the ink.
• Photoinitiators. In UV inks, the photoinitia-
tor is the chemical which becomes “excited”
and starts the curing reaction when exposed
to ultraviolet light. The excited photoinitiator
passes that energy to the other components.
At that point, any component which be-
comes excited has the ability to attract other
components to itself and transfer energy to
the newly attracted component. Photoiniti-
ators are not required in EB inks. The highly
charged energy of the electron beam is suffi-
cient to activate polymerization.
• Additives. These materials include waxes,
wetting agents and rheology modifiers. They
provide the added customizing touches to
the ink.
• Pigments. Pigment particle size and con-
centration can affect the curing rate of a UV
ink. Pigments are selected for color and
wetability, or oil-absorption ratio; and for
their receptivity to UV light. Among process
colors, yellow and magenta are the easiest to
cure, followed by cyan and black. Because
black tends simply to absorb the wave-
lengths of UV light, more energy is required
for a satisfactory cure.
Polymerization. In conventional UV or EB
chemistry, any component that has reacted
is called a “free radical.” It is the free radicals
that have the energy to keep the curing or
“polymerization” chain reaction going. Each
chemical chain continues growing until one
of two things happen: The excited chains
use up all of the available components or the
UV/EB source is removed and a foreign sub-
stance, such as oxygen, quenches or halts
the reaction.
In contrast, irradiation of the photoinitia-
tor used in a cationic UV ink generates a
strong “Bronsted” acid, which reacts with
the other components (aliphatic epoxides
and vinyl ethers). This reaction varies from
free-radical UV chemistry in that removal of
the UV source does not quench the reaction.
The cationic ink or coating continues to cure
for up to 24 hours after UV exposure.
As flexo printing improves in quality and
application, the need for specialized physi-
cal properties also continues to grow.
Increased chemical or product resistance is
the largest attraction to this process. The
additional benefits of low energy cost, mini-
mized downtime and the reduction of VOCs,
will also continue to drive the market.
FLEXOGRAPHIC INK
MANUFACTURING PROCESS
Flexographic inks, whether solvent- or
water-based, are generally manufactured
using the same processes: mixing, dispersing
and filtering.
Many ink companies produce their inks
from scratch using dry pigments for water-
or solvent-based inks, or press-cakes for
water-based inks. Others choose to purchase
predispersed concentrated bases, and let
them down with vehicles of their choice.
These concentrated bases are normally pro-
duced by the same methods as finished inks.
Many ink makers produce their own con-
centrated bases in-house rather than going
outside for them.
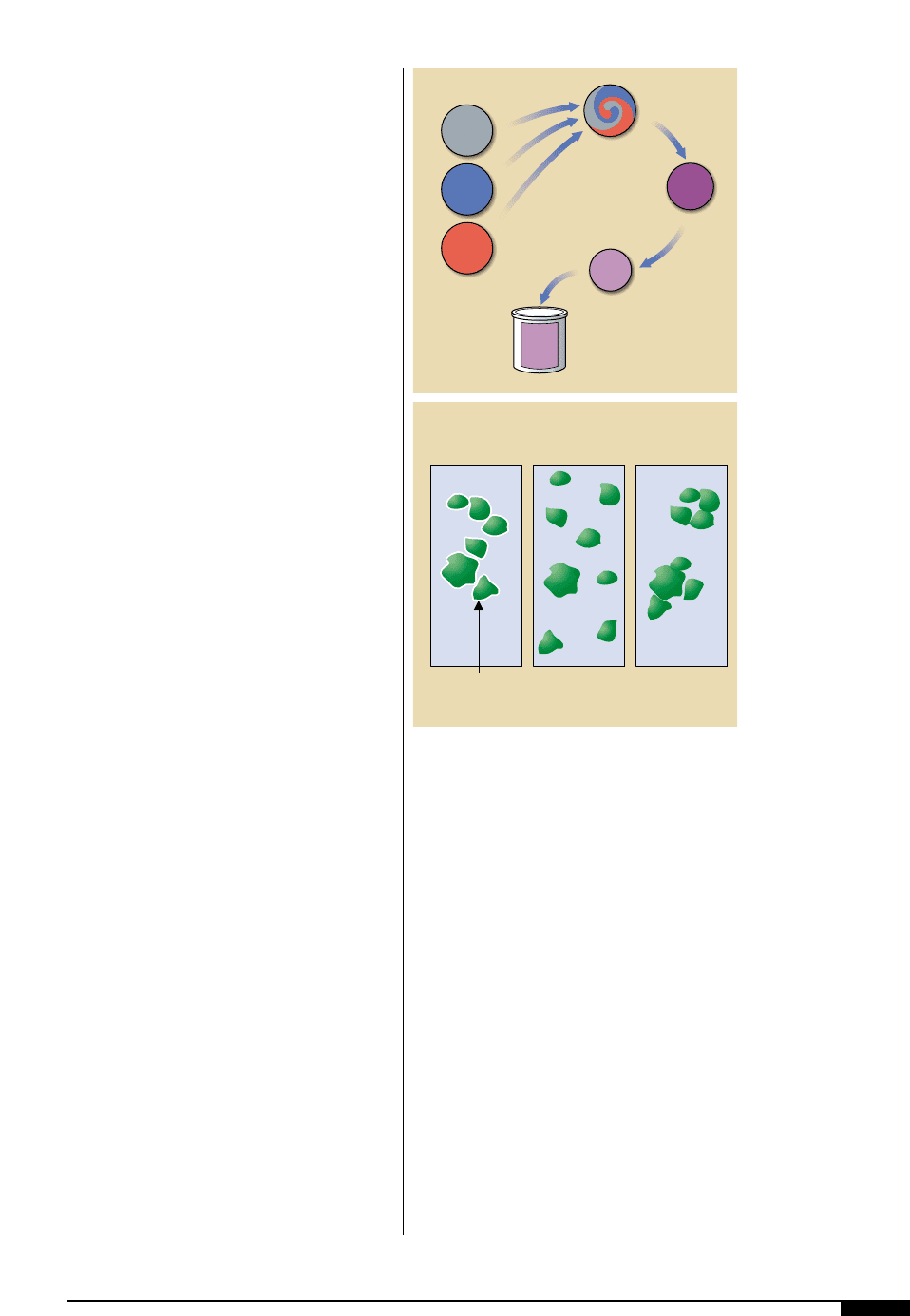
The manufacture of a flexographic print-
ing ink (Figure
3$
) typically begins with the
mixing of the raw materials to produce a uni-
form blend. From there, the product contin-
ues into the dispersion stage where the actu-
al work in breaking up the agglomerates is
completed.
There are a large number of flexographic
ink manufacturers in the United States.
Because of the present health/safety and
environmental regulations, a fair number of
these companies are dedicated to water-
based inks, while other companies still man-
ufacture both water- and solvent-based inks.
In general, the same equipment can be used
for the manufacture of either.
Mixing
The dispersion step begins with a pigment
made up of clusters called agglomerates.
Each agglomerate is made up of smaller
crystals that can have either air or water
absorbed on its surface. Mixing, as the first
step in the dispersion process, separates
these agglomerates into a homogenous
blend with the ink vehicle. The more effi-
ciently this is done, the easier and less
power-consuming the actual dispersion step
becomes. Typically two types of mixers are
employed in the manufacture of flexograph-
ic inks, cavitation and rotor stator mixers.
The cavitation mixer uses an impeller to
produce a vortex. The impeller size and
design varies by manufacturer and vessel
size. Shear is at a minimum with this type of
mixer. Rotor stator mixers have an impeller
rotating in a so-called disintegrating head
(stator). This head is fixed, and there is a
very small clearance between the rotor and
stator. This configuration allows for some
shear to be generated. Some inorganic pig-
ments such as titanium dioxide white can
truly be dispersed during the mixing stage,
but, in general, this process is just a precur-
sor to actual dispersion, separating but not
breaking up the agglomerates.
Dispersion
To achieve dispersion (Figure
3%
), pig-
ment agglomerates are broken up as close to
the individual crystal size as possible. The
degree of dispersion is really how close one
can get to this ideal. The dispersion process
must therefore accomplish three things to be
considered successful:
• It must remove the air or water on the
crystal surface and replace it with the
desired vehicle. This is called wetting.
• The particles must be separated and
uniformly distributed throughout the
vehicle.
• The crystal surface must be stabilized
so that re-agglomeration or flocculation
will not occur.
To accomplish this, a combination of
shear and impact is used. By applying these
forces throughout a viscous liquid, the
agglomerates are literally broken down and
then the absorbed air or water is “wiped off.”
The vehicle then replaces the air and/or
water on the surface of the pigment particle.
Stability is normally accomplished in one of
two ways:
INK 43
3$
The manufacture of a
flexographic printing
ink typically begins
with the mixing of
the raw materials to
produce a uniform
blend. From there, the
product continues into
the dispersion stage
where the actual work
in breaking up the
agglomerates is
completed.
3%
To achieve dispersion,
pigment agglomerates
must be broken up as
close to the individual
crystal size as possible.
Solvent
Resin
Mixing
Dispersion
Filtration
Packing
Pigment
3$
Agglomeration
Air and water
on pigment
particle surface
True Dispersion Flocculation
3%

44 FLEXOGRAPHY: PRINCIPLES & PRACTICES
1.By the introduction of ions or molecules
capable of satisfying the surface charges
on the solid pigment particles. These
ions give each particle a similar, uniform
charge resulting in repulsion, or
2.The use of non-ionic materials that
adsorb onto the pigment surface and
produce steric hindrance. This complex
also results in particle repulsion.
Typically, ink resins will accomplish the
required stability. If they fail, surfactants
specific to the pigments are used in the for-
mulations.
The first real piece of dispersion equipment
used in the manufacture of flexographic inks
was the pebble or ball mill (Figure
3^
). The
mill is a horizontal closed-end cylindrical
vessel filled to about half its height with
porcelain or steel balls. This piece of equip-
ment combines mixing with dispersion. It
has a loading hatch on top and an evacuation
valve on the opposite side. The ball mill is
filled with the raw materials, sealed and
rotated on its horizontal axis. The mill media
is carried up to the top of the mill during the
rotation and cascades down. This action
produces the impact and shear to break up
agglomerates and typically 16 hours will give
the desired results.
These mills are manufactured in a wide
assortment of sizes and use a variety of
media.
There are many advantages obtained using
ball mills for dispersion:
• Dispersion is uniform throughout the
batch.
• Grinding time can accurately control
degree of dispersion.
• Production procedures can be stan-
dardized for the mill used.
• No premix is required.
• There is no loss of volatiles. The vessel
is sealed.
• Highly concentrated bases can be pro-
duced for later letdown.
• The process is not very labor intensive.
The mill is opened, loaded, sealed, run
for 16 hours (normally between 4:00
p.m. and 8:00 a.m.), the degree of dis-
persion checked and unloaded.
There are, however, some major disadvan-
tages to this type of equipment:
• Batch size is limited to mill capacity.
• The time factor is the same regardless
of mill size.
• Power costs are high.
In the early 1950s, it was realized that if
one could produce more impacts per unit of
time, then dispersion could be accomplished
sooner. This improvement was actually
accomplished by combining a premix with a
very small but dense media – sand – and agi-
tating it with an impeller. The problem, how-
ever, was how to separate the dispersion
from the sand. Continuing along these same
lines, in 1958, DuPont’s S. Hockberg patent-
ed the process of sand milling (Figure
3&
).
The premix is pumped upward through the
mill, a vertical cylinder containing the
media. A series of plates is mounted on the
agitator shaft, and the top of the mill is
screened to prevent the media from escap-
ing with the dispersed ink. Flow rate
through the mill is controlled by the pumps
3^
The pebble or ball mill-
combines mixing with
dispersion. This action
produces the impact
and shear to break up
agglomerates. The ball
mill is filled with the
raw materials, sealed
and rotated on its hori-
zontal axis until the mill
media is carried up to
the top of the mill and
then cascades down.
3^

and determines dwell time. This in turn con-
trols the degree of dispersion.
This piece of dispersion equipment over-
came the disadvantages of the ball mill. To
minimize the disadvantages, there have been
numerous innovative improvements made to
the basic sand mill. Among these were the
following:
• Sand has generally been replaced by a
more dense, somewhat larger media.
This media maximizes impact forces
and minimizes the possibility of conta-
mination of the ink with sand grit.
• The screen was closed off to the atmos-
phere, and a true mechanical seal was
added, allowing operation under a
slight positive pressure. This change
minimized any loss of volatile ink com-
ponents and allowed heavier viscosities
to be pumped through the mill without
carrying up the media and overflowing
the mill.
• The vertical mill has evolved into the
horizontal mill (Figure
3*
). This is said
to improve performance by creating
better flow and increasing the media
loading capacity from 50% to 80% of the
mill volume.
• Larger diameter shafts are generally
equipped to carry cooling water. Disk
designs have been modified to increase
the number of impacts in a given time.
Ink companies are utilizing technology to
achieve the highest quality ink possible.
Most equipment is available in lab or pilot
size, so production parameters can be opti-
mized in advance and new raw materials can
be tested under actual conditions.
Filtration
The final process prior to drawing the fin-
ished ink into kits, drums or tote tanks is the
filtration step. Before the use of very fine
screens and low cell volumes, ink manufac-
turers regularly used either cheesecloth or a
fine grade of organdy to filter the finished
inks. This process took out any large parti-
cles that could cause problems on the press.
The anilox cells were large enough so that
any undispersed agglomerates would nor-
mally not cause problems.
Today, the advent of fine-screen halftone
and process printing requires the use of very
fine anilox screens and low volume cells.
This change has driven the need for much
better filtration. The undispersed agglomer-
ates can plug these cells and lodge in doctor-
blade nips, causing the loss of quality print-
ing. There are several types of filters in com-
mon use today. Any one of them, used prop-
erly, will remove any large and fine particu-
late matter that might cause problems.
Bag filters are one of the most common
INK 45
3&
Original sandmilling
process required premix
to be pumped upward
through the mill in a
vertical cylinder. The
media passed through
a series of plates on the
agitator shaft. Pumps
controlled flow rate, set
dwell time and con-
trolled dispersion.
3*
The vertical mill evolved
into the horizontal
mill, where performance
is improved by creating
better flow and
increasing the media
loading capacity from
50% to 80% of the mill
volume.
3&
2*

46 FLEXOGRAPHY: PRINCIPLES & PRACTICES
types of filters used. Felt-type bags with sizes
rated from 100 microns to 25 microns are
available and can be used with gravity flow or
commercial pumping systems. There are cau-
tions to be noted.
• With the use of pumping systems, it is
critical that recommended operating
pressures not be exceeded. If they are,
the filter might be bypassed and unfil-
tered ink will contaminate the batch.
• With gravity flow it is common to see
plant workers “beating” the filters with
ink knives to get faster flow. Beating
deforms the bag and can allow larger
particles through.
The cartridge filter is often used to filter
flexographic ink. Natural or synthetic fibers
are wound around a porous core, and the ink
is pumped through the core and fibers. A
wide variety of micron-size cartridges are
available. Here too, if pressures are exceed-
ed, unfiltered ink can bypass the filter and
contaminate the batch.
A new type of filter that is beginning to see
more use in the manufacture of flexographic
inks is the vibrating screen filter. Ink is
pumped onto a rigid vibrating sieve and the
large particles are retained on the screen. A
large number of sieve sizes are available,
some with new innovations like self-clean-
ing filters.
Most flexographic ink manufacturers
today are using 100-micron filters as their
standard size. For special inks, 50-micron or
even 25-micron filters are used. Also,
mechanical systems are equipped with mag-
netic filters to ensure that no contamination
from metal particles may have been intro-
duced during the shot mill stage.
INK 47
Ink Prepress
3(
Ink is a small part of the
total cost of the printed
product.
4)
The purpose of any
ink-blending operation
is to deliver the right
amount of the right ink
to the right press at the
right time.
I
n the past 10 years, there has been a
growing trend in the prepress area
toward treating ink management as a
key process. Converters have found
that attention to this area pays high div-
idends in in the form of reduced waste
and increased pressroom productivity.
Professional management of the inkroom is
now an essential part of running a competi-
tive converting operation. The days of the
dingy, dirty manual inkroom are rapidly dis-
appearing and being replaced by computer-
managed information systems and automat-
ed product dispensing. What goes on in the
inkroom today?
Most printing operations have one or two
people controlling the department. Besides
providing ink to the presses, the inkroom
usually controls color standards, ink pur-
chases, inventory, waste, waste reports,
usage records and the very important volatile
organic compound (VOC) reports. In addi-
tion, the inkroom is a management resource,
having essential input into purchases of new
equipment and in many process improve-
ments.
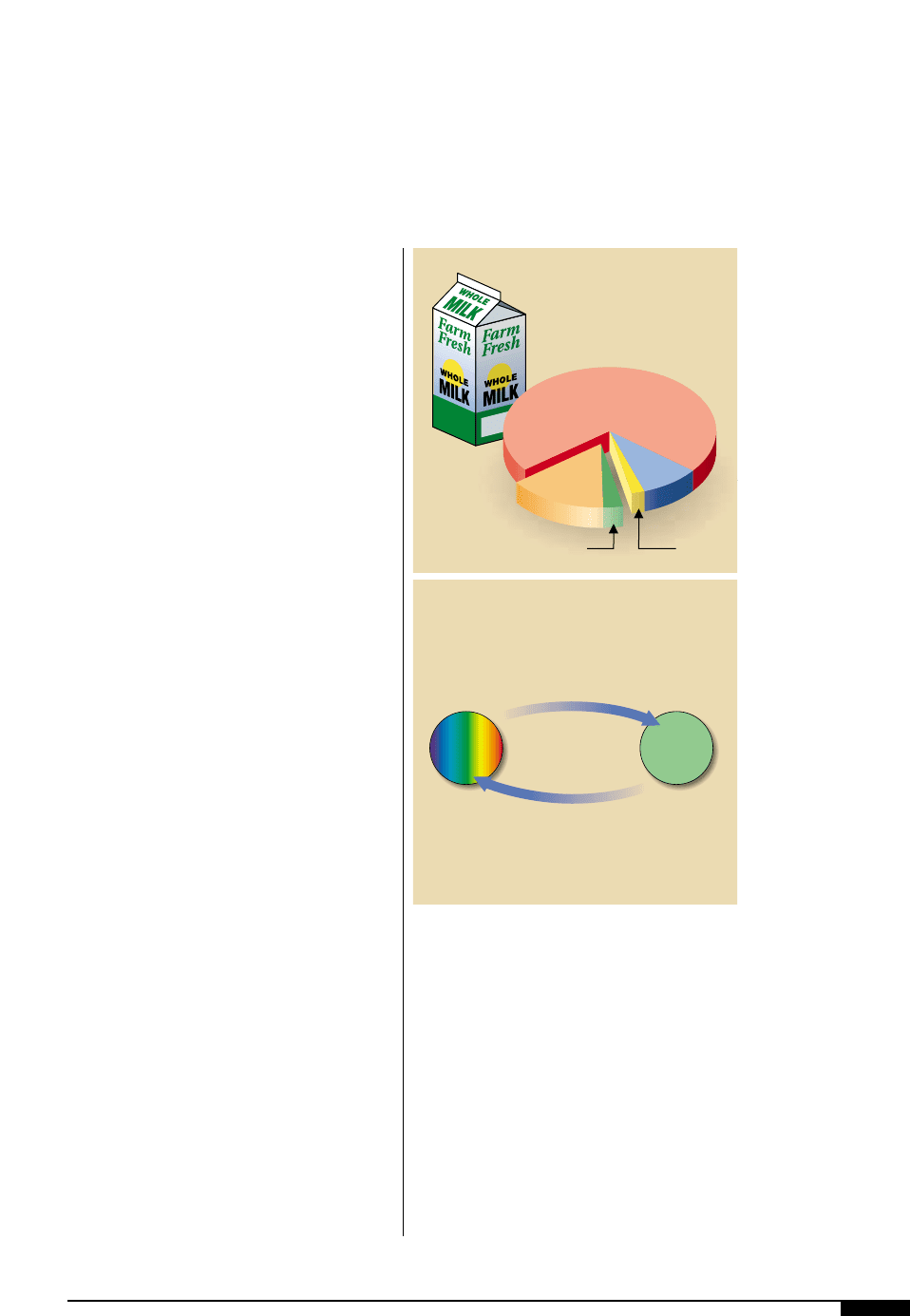
Ink is a small part of the total cost of the
printed product. For example, cost figures
for liquid packaging converters show that
ink is only 2% of the total (Figure
3(
). It is
apparent that ink which is defective or mis-
managed can cause considerable waste in
the converting operation. Problems due to
incorrect ink use can reveal themselves
immediately, or in some cases further down
the value-added chain, where the cost of the
problem is multiplied.
The purpose of any ink-blending opera-
tion is to deliver the right amount of the right
ink to the right press at the right time. This is
a simplistic view of a fairly complex process
and easier said than done (Figure
4)
).
Waste due to bad ink can have many caus-
es. Some typical causes of production waste
due to ink-related issues are;
• off-shade;
• wrong ink delivered to the press;
• too little or too much ink delivered to
the press; or
• the ink is not at press-ready viscosity.
Board: 71%
Other: 15%
Labor:
9%
Ink: 2%Manufacturing Fixed: 3%
Manufacturing costs
82% of total,
Ink 2% of total
3(
P
r
e
s
s
D
e
p
a
r
t
m
e
n
t
a
s
S
u
p
p
l
i
e
r
I
n
k
r
o
o
m
a
s
S
u
p
p
l
i
e
r
Documented Press Returns
Ink Feedback
Process Changes
Inkroom
Press
Dept.
Consistent Ink
Troubleshooting
Color Standards
Ink Stats
4)

48 FLEXOGRAPHY: PRINCIPLES & PRACTICES
Another common cause of press down-
time, due to ink-related waste issues, is that
the press department has not been instruct-
ed on how to use the ink system in question.
For example, the job may meet all appear-
ance standards yet fail an adhesion test
because the press department was not
aware of the web temperature requirements
to pin on the ink. Thus the ink prepress prod-
uct is not just ink, it is also information, ser-
vice and training. The inkroom must regard
the press department as the customer for
product and service. At the same time, the
press department, as a consumer, has
responsibilities to the inkroom. The inkroom
must receive timely process change infor-
mation, correctly marked press returns and
feedback of ink performance on press.
PREPRESS PROCESS
Ink prepress covers the steps and proce-
dures that take place between receiving the
ink from the ink supplier and delivery of ink
to the press (Figure
4!
). These boundaries
are typical, but not absolute, in terms of what
is necessary for a successful operation. For
instance, some ink departments are respon-
sible for managing ink into the press pumps,
while others may actually do some ink for-
mulation and ink assembly normally associ-
ated with the ink supplier. The main objec-
tives are to understand where the boundaries
have been drawn in the converter operation,
manage the input products from the ink sup-
plier(s), and optimize outputs of products
and services to the press department. Once
the boundaries have been drawn, communi-
cated and understood, ink prepress opera-
tions can be designed to meet all needs.
THE INKROOM DESIGN
A typical inkroom occupies about 1,000
square feet. The drawing shows how an
inkroom this size could be organized
(Figure
4@
). There are two areas: an
office/laboratory area and an ink-handling
area. In the laboratory area, color matching,
proofing, quality control, diagnostic testing
and ink management information system
(MIS) functions are performed. This room
would be connected electronically with the
ink supplier and perhaps with the converter
MIS department and shop floor data system.
Major equipment in the ink lab includes
the following:
• proofer;
• weighing scales;
• color measurement device; and
• computer with ink management software
and modem
Major equipment for ink production would
include the following:
• ink-dispensing system;
4!
Ink prepress covers the
steps and procedures
that take place between
receiving the ink from
the ink supplier and
delivery of ink to the
press.
4@
A typical ink room lay-
out occupies approxi-
mately 1,000 square
feet.
Receive
Ink
Estimate
Usage
Generate
Batch
Ticket
Color Standard
and
Standard Formula
Make
Batch
Stage
Ink
Part 1
End Use
Part 2
Ink
Formulation
Part 3
Ink
Prepress
Part 4
Ink on
Press
4!
Press “A”
Press “B”
Press “C”
Returned
Inks
Blended Inks
in Inventory
Inks Staged
for Press
Dispenser
Light
Booth
Ink Lab
Pressroom
Inkroom
4@

• air mixer;
• drum dolly; and
• hand truck.
It should be noted that the ink-dispensing
system can be as simple as drums on racks
with appropriate valves or as complex as a
computer-controlled dispenser.
INKROOM SYSTEMS
Regardless of the print, end-use and the
types of presses being served, the inkroom
must have procedures in place that define
and control key areas.
Safety
This is always the number-one priority in
any manufacturing operation; but it is partic-
ularly important in the inkroom, due to the
nature of the materials being handled. Proper
handling of chemicals is essential. Formal
training programs for the operators are man-
dated by legislation and must be document-
ed. If flammable materials are present, a spe-
cial emphasis should be placed on maintain-
ing explosion-proof systems, grounding of
containers and arranging exhaust in areas
where open containers are present.
The safety of the inkroom is very important
and must be audited on a regular basis. The
result will make for a safer workplace.
Housekeeping, personal safety equipment,
personal hygiene and adherence to safe work
practices are key factors to be reviewed in an
ink operation.
Color Standard
There should be a process in place that
generates, approves and stores color stan-
dards for use in the inkroom, as well as in
the actual printing operation. This can save a
lot of discussion, time and money, as well as
preserve the printer’s reputation with cus-
tomers. A good system should provide a cur-
rent standard, signed and dated. There
should be at least three samples available:
one on file in the inkroom for color match-
ing, one for pressroom use, and one in a
master file that is used only if the other two
are in question. All standards should be up-
dated at least once a year. At the very least,
they should be inspected and, if still accept-
able, a new date applied.
Proofing System
The heart of any ink-blending operation is
a correlated proofing system that can pre-
dict the ink strength and shade that will be
obtained on the press. Only by having a good
proofing system can the inkroom be confi-
dent in its ability to deliver “press ready”
inks to the printing operation. This will save
hours of press downtime and help eliminate
color variation complaints. The method
should be one that can be used by everyone
in the inkroom with consistent results. This
system will also allow for better matching on
press return ink.
Where a printing operation has a mix of
different presses and inking systems, differ-
ent proofing methods may be required to
ensure that there is a correlation between
the proof from the inkroom and the print
from the press.
Inventory Control
Ink should be used on a first-in, first-out
basis, due to the shelf-life limitations of any
chemical mixture. The issue here is simply
using the oldest ink first. The ink supplier
should have dates on all containers to facili-
tate this process. If there is no date of manu-
facture on the container, get one from the sup-
plier. Some suppliers utilize date-coding sys-
tems that may not be immediately apparent.
There should also be a system of control
that always allows ink to be available while
maintaining the lowest possible inventory. A
basic min-max system can work well with a
weekly inventory and facilitate ordering in
time to fill the pipeline. An area that is often
INK 49

50 FLEXOGRAPHY: PRINCIPLES & PRACTICES
overlooked is press returns. Press returns
should be identified and weighed, and the
containers kept sealed to improve the prob-
ability of reuse. Since waste inks become a
regulated hazardous waste, control of press
returns will save the time and cost of dis-
posing of these materials.
Usage Records
Accurate records play a central part in the
control of inventories. They will also help to
reduce waste, since most colors are
matched for a specific job and anything not
used is returned to inventory with the hope
that it can be used later. A basic system con-
sists of recording the weight of ink made, the
weight returned, and the run size. This will
provide a record for each job, allowing for a
more accurate estimate of ink requirements
for future runs. More sophisticated comput-
erized systems are available to track this
information. The objective is the same –
have the right amount of ink on press and
only return the minimum amount of ink to
the inkroom. This will reduce cost, waste
and on-hand inventory.
Information Systems
A great deal of information is usually man-
aged by the inkroom. Some examples are:
Ink Systems. Ink formulations must be
matched to specific substrates based upon
their compatibility and end-use properties.
Ink Additives. Anything added to the ink must
be identified and information provided for
their proper use. Misusing additives can
result in downstream problems.
Press Performance. On press, color data and
corrective actions are important to track.
This is valuable feedback that can save the
department from solving the same problems
over and over.
Scheduling. The ink department should have
a good system that links the ink operation
with the press schedule and press setup,
thereby coordinating the timing of ink needs
on the press and quick reaction to changes
in schedule.
Equipment. There is less equipment in an
inkroom than the pressroom, but it is just as
important and must always be working
properly. Scales must be accurate and in
good working order. The mixer must run
smoothly and be sized to handle the batch-
size requirements of the operation. Hoists
and other lifting equipment must have the
rated capacity to handle all containers pre-
sent, and they must move easily without
restriction. Manual material handling equip-
ment should also be inspected on a regular
basis to ensure they are in good working
condition. Color-control equipment (i.e.,
color booths, spectrophotometers, etc.)
must be calibrated and maintained to ensure
optimum performance.
COLOR MANAGEMENT
Color communicates. Color sells. Color is
the sizzle that drives the sale of virtually
every consumer product in the world. It
evokes a wide range of emotions that draw
the buyer to the product. Color management
professionals know that color is a crucial
part of the selling process because it is such
an important part of the “buying decision.” If
color is used effectively in the manufactur-
ing and marketing of an item, potential buy-
ers will perceive added value in that product.
To use color effectively in a flexo package
design requires an understanding of the
entire process, from initial design to final
package (Figure
4#
). Many elements and
professionals are involved, each relying on
interaction with the other. Print buyers and
designers start the process, taking into
account consumer preferences. Colors must
be specified and communicated, from the
visual appeal of the final package to the
capabilities and tolerances of the flexo-
graphic printing process. In a package
design, color and all other elements must be

within the capability of the production
process.
At each step of production, output from
the previous step becomes the input to the
next process. Colors are communicated
among several different individuals who
may render and reproduce the colors on
many different devices. For final production,
a contract proof, viewed under standard
conditions, will show a close approximation
of the final printed product. Once the client
has agreed on the contract proof, the ink
department is responsible for supplying ink
and color standards that allow the agreed
upon color to be obtained; impression after
impression and press run after press run. To
assure consistent reproduction, color mea-
surement and control is essential. This sec-
tion will touch on this subject in three parts:
color theory, color measurement, and color
matching. Color theory and color manage-
ment application are explained more fully in
the Process Color Printing volume.
COLOR THEORY
Color results from the interaction between
light, an object and the viewer (Figure
4$
).
The human observer, or viewer, sees this
modified light and perceives it as a distinct
color. All three elements, light, object and
viewer, must be present for color as we know
it to exist.
Light Source and Color
If an object is viewed using other than a
white-light source, the perceived color will
take on the hue of the illumination. While
INK 51
4#
Colors must be
communicated and
specified, taking into
account the capabilities
and tolerances of the
flexographic printing
process. In a package
design, color and all
other elements must be
within the capability of
the production process.
BOB’S
Print
Buyer
Graphic
Designer
Structural
Designer
Pre-Press
Suppliers
Printer/
Converter
Consumer
4#
