Fahim M.A., Sahhaf T.A., Elkilani A.S. Fundamentals of Petroleum Refining
Подождите немного. Документ загружается.

Material balance: G
0
y
1
1 y
1
þ L
0
x
2
1 x
2
¼ G
0
y
2
1 y
2
þ L
0
x
1
1 x
1
;
0:5
0:1
1 0:1
þ 30
0
1 0
¼ 0:5
0:01
1 0:01
þ 30
x
1
1 x
1
:
Solving for x
1
¼ 0.001512, this leads to y
1
¼ 20 0:001512
ðy y
Þ
av
¼
ðy
1
y
1
Þðy
2
y
2
Þ
ln
y
1
y
1
y
2
y
2
¼
ð0:1 0:001512 20Þð0:02 0:0Þ
ln
0:1 0:001512 20
0:02 0 :0
¼ 0:0358;
N
G
¼
y
1
y
2
ðy y
Þ
av
¼
0:1 0:02
0:0358
¼ 2:23 ;
Z ¼ H
G
N
G
¼ 0:825 2:23 ¼ 1:84 m:
Example E15.5
A solvent recover y plant consists of a tray column absorber and a plate column
stripper. Ninety percent of the solute in the gas stream is recovered in the
absorption column. The concentration of solute in the inlet gas is 6 mol%. The
solvent entering the top of the absorber contains 1 mol% of solute. Superheated
steam is used to strip the solvent out of the solute. The stripped solvent stream is
recycled back to the absorber unit. The solute-free solvent rate to solute-free gas
rate ratio equals 2.0. Figure E15.5 shows the process flow diagram.
The equilibrium data are as follows:
In the absorber y ¼ 0:25x;
In the stripper steam y ¼ 3:89x:
Calculate:
(1) The theoretical number of stages in the absorber.
(2) The minimum amount of steam used in the stripper.
Absorber
Stripper
Solvent x
2
= 0.01
steam
L
1
x
1
G
2
y
2
L
2
x
2
G
1
y
1
= 0.06
Figure E15 .5 Schematic diagram of absorber^stripper system
Acid Gas Processing and Mercaptans Removal 385
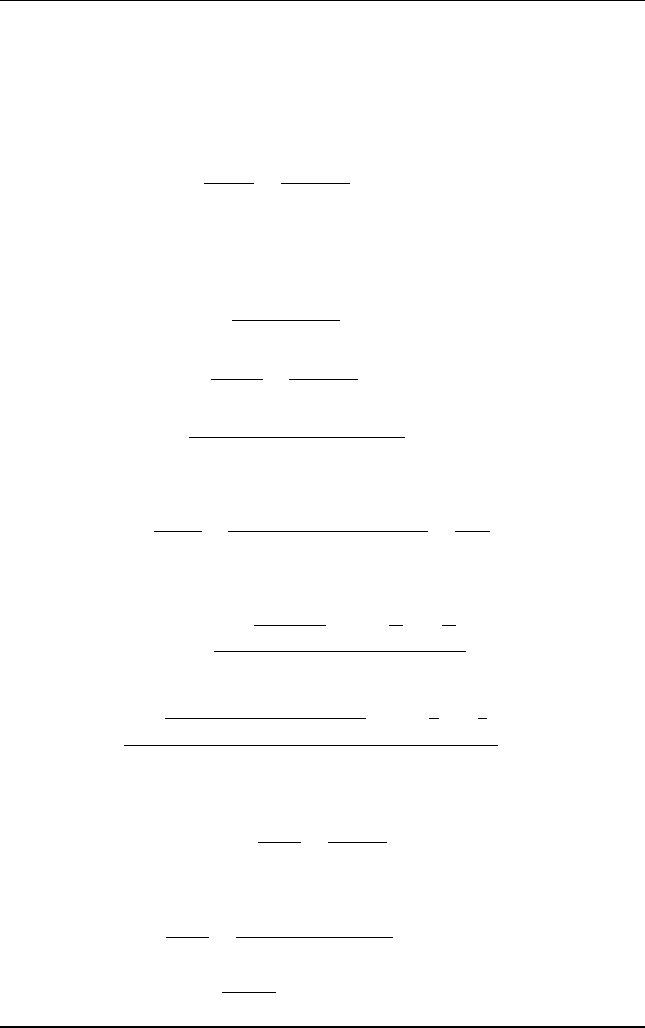
Solution:
(1) Given L
0
/G
0
¼ 2.0,
if L
0
¼ 2:0 mol=h;
and G
0
¼ 1:0 mol=h;
G
1
¼
G
0
1 y
1
¼
1
1 0:06
¼ 1:0638 mol=h:
Solute in gas to absorber ¼ 0.06 (1.0638) ¼ 0.06383 mol/h,
Solute absorbed ¼ 0.9 (0.06383) ¼ 0.05745 mol /h,
Solute in G
2
¼ 0.1 (0.06383) ¼ 0.006383 mol/h,
y
2
¼
0:006383
1 þ 0:006383
¼ 0:0063427;
L
2
¼
L
0
1 x
2
¼
2
1 0:01
¼ 2:02 mol = h;
x
1
¼
0:05745 þ 0:01 2:02
0:05745 þ 0:01 2:02 þ 2
¼ 0:03737:
Absorption factor A:
A ¼
L
0
=G
0
m
¼
slope of operating line
slope of equilibrium line
¼
2
0:25
¼ 8:
Number of theoretical trays in the absorber N (Geankoplis, 2003)is
N ¼
ln
y
1
mx
2
y
2
mx
2
1
1
A
þ
1
A
lnðAÞ
;
N ¼
ln
0:06 0:25 0:01
0:0063427 0:25 0:01
1
1
8
þ
1
8
lnð8Þ
¼ 1:24:
(2) For the stripper unit,
L
0
G
0
min
¼
y
1
y
s
x
1
x
2
Since the stream is solute-free y
s
¼ 0, then
L
0
G
0
min
¼
0:03737 3:89 0
0:03737 0:01
¼ 5:3113;
G
min
¼
2
5:3113
¼ 0:3765 mol=h of steam:
386 Chapter 15
Example E15.6
Twenty-five MMSCFD (1245 kmol/h) gas stream of the composi tion shown in
Table E15.6.1 enters a DEA treatment process at 30
C and 6895 kPa.
The DEA flow rate is 1874 kmol/h with 28 mol% DEA in solution. Per-
form material balance using UNISIM software (UNISIM, 2007).
Solution:
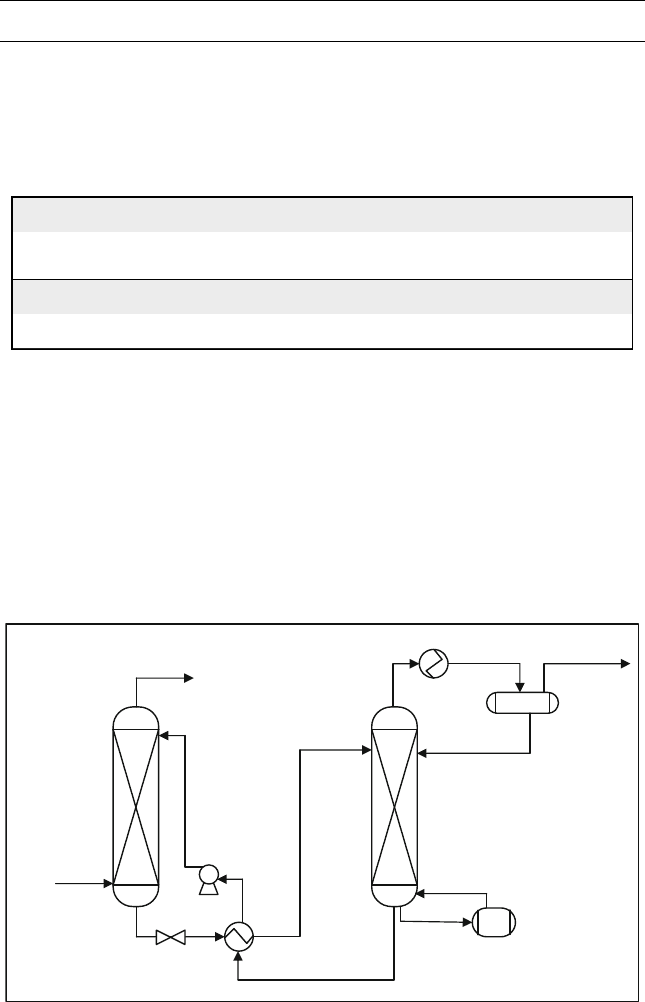
The gas stream is first introduced to a separator to knock out water and heavy
hydrocarbons as liquid stream. Then the remaining gas enters the DEA absorber
as shown in Figure E15.6. The rich solvent stream goes to a distillation column
to separate hydrocarbons from the solvent and recycle it back to the absorber.
Table E15.6.2 shows the UNISIM results.
Table E15.6.1 Feed composition
Component N
2
CO
2
H
2
SH
2
OC
1
C
2
C
3
Mole % 0.16 4.15 1.70 0.17 87.6 3.9 0.92
Component iC
4
nC
4
iC
5
nC
5
nC
6
nC
7
Mole % 0.25 0.28 0.13 0.11 0.15 0.48
Sweet gas
To Absorber
Absorber
Stripper
Regenerated
Solvent
Rich
Solvent
Cooler
Acid Gas
Amine
Re-boiler
Figure E15.6 DEAtreatment process
Acid Gas Processing and Mercaptans Removal 387

15.2.1.2. Carbonate Process
In this process, hot potassium carbonate (K
2
CO
3
) is used to remove both
CO
2
and H
2
S. It can also remove COS. The following reactions take place
(Abdel-Aal et al., 2003):
K
2
CO
3
þ CO
2
þ H
2
O
!
2KHCO
3
ð15:17Þ
K
2
CO
3
þ H
2
S
!
KHS þ KHCO
3
ð15:18Þ
It can be observed that high CO
2
partial pressure, in the range of 2–6 bar
(30–90 psi) and temperature between 110–116
C (230–240
F), are required
to keep KHCO
3
KHS in solution. Therefore, this process cannot be used for
streams that contain H
2
S only because KHS is very hard to regenerate unless a
considerable amount of KHCO
3
is present. Precipitation of KHCO
3
can be
hindered by keeping the carbonate concentration around 35%.
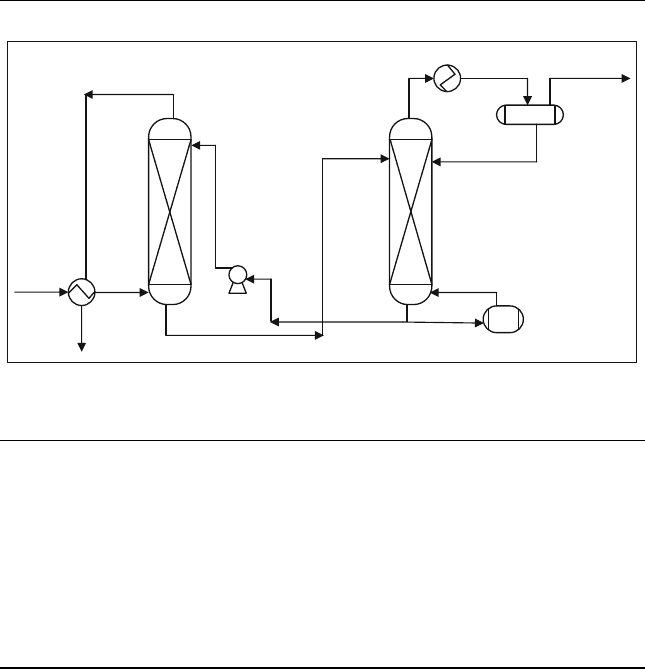
The hot carbonate process, shown in Figure 15.4, includes the absorber
and the stripper as in the amine process. The sweet gas coming from the top
of the absorber is used to heat the sour gas feed.
Table E15.6.2 UNISIM results for DEA process treatment
Stream
To
absorber Sweet g as
Rich
solvent
Regenerated
solvent
Acid
gas
N
2
(mol%) 0.16 0.17 0.0 0.0 0
CO
2
(mol%) 4.15 0.219 2.58 0.1 65.2
H
2
S (mol%) 1.7 0 1.08 0.0 28.33
H
2
O (mol%) 0.17 0.123 90.3 93.6 6.25
C
1
(mol%) 87.6 92.8 0.0 0.0 0.22
C
2
(mol%) 3.9 4.2 0.0 0.0 0
C
3
(mol%) 0.92 0.98 0.0 0.0 0
iC
4
(mol%) 0.25 0.27 0.0 0.0 0
nC
4
(mol%) 0.28 0.30 0.0 0.0 0
iC
5
(mol%) 0.13 0.14 0.0 0.0 0
nC
5
(mol%) 0.11 0.12 0.0 0.0 0
nC
6
(mol%) 0.15 0.51 0.0 0.0 0
nC
7
(mol%) 0.48 0.12 0.0 0.0 0
DEA (mol%) 0.0 0.0 6.04 6.3 0
Flow (kmol/h) 1233 1162 1948 1874 74.2
T (
C) 30 35 60 124 49
P (kPa) 6895 6860 620 217 189.6
388 Chapter 15
Example E15.7
CO
2
is absorbed in a hot carbonate process at 240
F and 1000 psia. The feed gas
stream flow rate is 50 MMSCFD and contains 5 mol% CO
2
.Itisrequiredto
release the gas stream with 2 mol% CO
2
. Assuming a circulation rate of 3.5 ft
3
/gal,
calculate the flow rate of hot carbonate.
Solution:
Volume of CO
2
inlet ¼ 50 10
6
/(24 60)(0.05) ¼ 1735 ft
3
/min,
Amount of hot carbonate ¼ (1735 ft
3
/min)/(gal/3.5 ft
3
) ¼ 504 gpm.
15.2.2. Physical Solvents
Physical solvents allow the absorption of acid compounds without any
chemical reaction. Table 15.3 lists the most commonly used physical sol-
vents in acid gas treatment. The difference in H
2
S and CO
2
physical
solubility gives the solvents their selectivity. Organic solvents are used in
these processes to absorb H
2
S more than CO
2
at high pressures and low
temperatures. Regeneration is carried out by releasing the pressure. Henry’s
law can be applied here:
P
i
¼ y
i
P ¼ Hx
i
: ð15:19Þ
The acid gas composition (x
i
), absorbed in a liquid solvent, is related to the
gas mole fraction (y
i
) by Henry’s constant (H ).
Clean gas
Acid
Gas
Clean
gas
Absorber
Stripper
Lean
Solvent
Rich Solvent
Cooler
AG
Steam
Boiler
Figure 15.4 Hot carbonate process
Acid Gas Processing and Mercaptans Removal 389
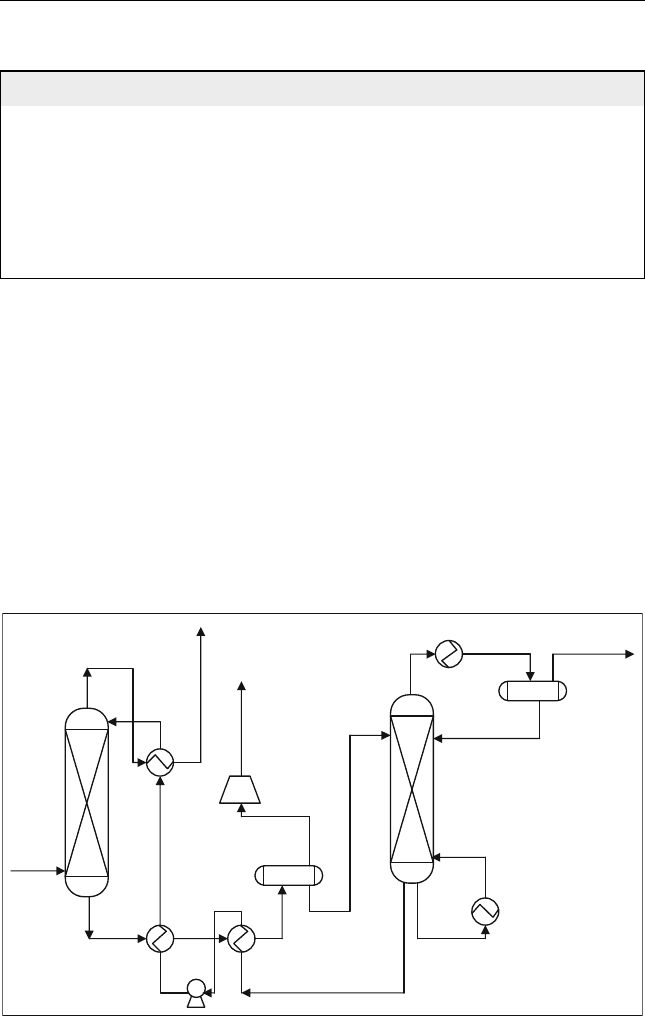
15.2.2.1. Selexol Process
Selexol is a physical solvent, unlike amine-based acid gas removal solvents
that rely on a chemical reaction with the acid gases. It is dimethyl ether of
polyethylene glycol. Since no chemical reactions are involved, Selexol
usually requires less energy than the amine-based processes. It has high
selectivity for H
2
S over CO
2
that equals to 9–10. A typical process flow
chart is shown in Figure 15.5.
Table 15.3 Physical solvent properties (Abdel-Aal et al., 2003)
Selexol Sulphi nol Fluor
Solvent Diethylene dimethyl
ether
Sulpholane Propylene
carbonate
Molecular weight 134 120 102
Solubility (cm
3
gas/cm
3
solvent)
H
2
S 25.5 13.3
CO
2
3.6 3.3
COS 9.8 6.0
Acid
Gas
Absorber
Stripper
Lean
Solvent
Rich Solvent
Cooler
AG
Stripper
Reboiler
Clean
Gas
To COS
H2 unit
Flash Drum
Figure 15.5 Selexol process
390 Chapter 15

Example E15.8
A feed gas at a flow rate of 200 MMSCFD, 421 psia and 81
F with the
following composition (mol%): H
2
S ¼ 1.7%, CO
2
¼ 10%, is introduced to a
Selexol process to treat the gas to reach 40 ppm H
2
S. Calculate the percentage
recovery of sulphur.
Solution:
H
2
S free gas ¼ (200 10
6
/379/24)(1 0.017) ¼ 21,614 lbmol/h,
Amount of H
2
S in the inlet gas ¼ (200 10
6
/379/24)(0.017) ¼373.8 lbmol/h,
H
2
S in the exit clean gas ¼ (40/10
6
)(21,614) ¼ 0.8645 lbmol/h,
% recovery of H
2
S ¼ (373.8 0.8645)/373.8 100 ¼ 99.78% of H
2
S.
15.2.2.2. Morphysorb Process
The morphysorb process uses a mixture of N-formyl morpholine and N-
acetyl morpholine, for the removal of acid gases (Kohl and Nielsen, 1997).
This solvent is selective for the removal of H
2
S, CO
2
, COS, CS
2
and
mercaptans. The main advantages of this process are:
Higher solvent loading and hence a lower circulation rate.
Lower absorption of hydrocarbons.
Low corrosion and low environmental hazard.
A simplified flow chart for the Morphysorb process is shown in Figure 15.6.
To Sulphur
Recovery
Sour
Gas
Absorber
Acid Gas Flash Drums
Lean
Morphysorb
Solvent
pump
Clean Gas
Figure 15.6 Morphysorb process
Acid Gas Processing and Mercaptans Removal 391
Example E15.9
An acid gas stream contains 20 mol% CO
2
, 10 mol% H
2
S and the balance is the
carrier gas. A morphysorb solvent is used to treat this gas to 100 ppm H
2
S. On
the basis of 100 mol, calculate the amount of H
2
S and CO
2
absorbed.
The selectivity of Morphysorb solvent ¼ H
2
S/CO
2
¼ 9/1.
Solution:
Assuming that initially no CO
2
is absorbed, the H
2
S free gas is 70 mol.
H
2
S in clean gas ¼ (100 10
6
)(70) ¼ 0.007 mol H
2
S.
H
2
S absorbed ¼ 10 0.007 ¼ 9.993 mol.
Then for selectivity ¼ 9, the amount of CO
2
absorbed ¼ 1.11 mol.
CO
2
in the exit gas ¼ 20 1.11 ¼ 18.89 mol.
Exit gas ¼ 70 þ 0.007 þ 18.89 ¼ 88.96 mol.
H
2
S in clean gas ¼ (100 10
6
)(88.96) ¼ 0.008896 mol H
2
S.
H
2
S absorbed ¼ 10 0.0089 ¼ 9.9911 mol.
Then for selectivity ¼ 9, the amount of CO
2
absorbed ¼ 1.11 mol.
15.2.3. Membrane Absorption
Selective permeation for gases occurs depending on the solubility at the
surface contact between the gas and the membrane. The rate of permeation
of the gas depends on the partial pressure gradient as follows (Abdel-Aal
et al., 2003):
q
A
¼
1
t
ðPMA
m
DP
A
Þ; ð15:20Þ
where PM is the gas permeability, A
m
is the membrane surface area, t is the
membrane thickness and DP
A
is the partial pressure of gas A across the
membrane.
The acid gas basically diffuses through the membrane if high pressure is
maintained to ensure a high permeation rate. A membrane such as the Spiral
Wound, has a high selectivity for H
2
S and CO
2
over methane and other
gases. For example, it has a permeation rate of 10 and 6 for H
2
S and CO
2
,
respectively, while for methane, it is only 0.2.
Example E15.10
It is desired to capture CO
2
from a gas stream containing 10 mol% of CO
2
via a
silicone rubber membrane. The membrane thickness is 1.0 mm and surface area
is 3000 m
2
. Calculate the required pressure difference to get a permeation rate of
6cm
3
/s.
The gas permeability for CO
2
in silicone rubber is 0.27 10
6
cm
3
(STP)
cm/(s cm
2
/cmHg).
392 Chapter 15
Solution:
q
A
¼
1
t
ðPMA
m
DP
A
Þ¼6cm
3
=s ¼
1
0:1
ð0:27 10
6
ð3000ÞDP
A
Þ;
DP
A
¼ 740 cmHg:
15.3. Sulphur Recovery
Acid gas streams from hydrodesulphurization containing H
2
Saresentto
sulphur recovery unit (Claus unit). Furthermore, sulphur removal is carried out
by tail gas clean up schemes. The purpose of removing the sulphur is to reduce
the sulphur dioxide (SO
2
) emissions in order to meet environmental guidelines.
15.3.1. Claus Process
The Claus process is the most significant elemental sulphur recovery process
from gaseous hydrogen sulphide. Gases with an H
2
S content of over 25%
are suitable for the recovery of sulphur in the Claus process. Hydrogen
sulphide produced, for example, in the hydrodesulphurization of refinery
products is converted to sulphur in Claus plants (Yamaguchi, 2003; Topse
et al., 1996 and Chianelli, 2002). The main reaction is
2H
2
S þ O
2
! 2S þ 2H
2
O DH ¼186:6kJ=mol: ð15:21Þ
The Claus technology can be divided into two process stages (Figure 15.7):
thermal and catalytic. In the thermal stage, hydrogen sulphide partially oxidized
at temperatures above 850
C (1562
F) in the combustion chamber. This
causes elemental sulphur to precipitate in the downstream process gas cooler.
If more oxygen is added, the following reaction occurs:
2H
2
S þ 3O
2
! 2SO
2
þ 2H
2
O DH ¼518 kJ=mol: ð15:22Þ
Air to the acid gas is controlled such that in total, 1/3 of all hydrogen
sulphide (H
2
S) is converted to SO
2
.
Sulphur produced in the process is obtained in the thermal process stage.
The main portion of the hot gas from the combustion chamber is cooled
down. This caused the sulphur formed in the reaction step to condense.
A small portion of the process gas goes to the catalytic stage. This gas
contains 20–30% of the sulphur content in the feed stream. Activated
alumina or titanium dioxide is used. The H
2
S reacts with the SO
2
and
results in gaseous, elemental sulphur. This is called the Claus reaction:
2H
2
S þ SO
2
! 3S þ 2H
2
O DH ¼41:8kJ=molðSulphur vapourÞ
ð15:23Þ
Acid Gas Processing and Mercaptans Removal 393

Acid Gas
to
combustion
Air Water
Steam
Sulphur
Tail Gas
Reactors
Separator
Figure 15.7 Claus process
394 Chapter 15
