Fahim M.A., Sahhaf T.A., Elkilani A.S. Fundamentals of Petroleum Refining
Подождите немного. Документ загружается.

Heating is necessary to prevent sulphur condensation in the catalyst bed
which can lead to catalyst fouling.
The catalytic conversion is maximized at lower temperatures, but care
must be taken to ensure operation above the dew point of sulphur.
The condensation heat is used to generate steam at the shell side of the
condenser. Before storage, the liquid sulphur stream is degassed to remove any
dissolved gases.
If the acid gas feed contains COS and/or CS
2
they are hydrolyzed in the
catalytic reactor in the following manner:
High temperature helps to hydrolyze COS and CS
2
.
COS þ H
2
O
!
H
2
S þ CO
2
ð15:24Þ
CS
2
þ 2H
2
O
!
2H
2
S þ CO
2
ð15:25Þ
The tail gas from the Claus process still containing combustible components
and sulphur compounds (H
2
S, H
2
and CO) is either burned in an incineration
unit or further desulphurized in a downstream tail gas clean-up unit (TGCU).
A typical Claus process with two catalytic stages yields 97% of the
sulphur in the input stream. Over 2.6 tons of steam will be generated for
each ton of sulphur yield.
Example E15.11
An acid gas has the following composition: 40.2 mol% H
2
S, 54.0 mol% CO
2
,
1.35 mol% CH
4
, 0.15 mol% C
2
H
6
and 4.3 mol% water. It is fed to a thermal
stage (combustion reactor) of a Claus Process Sulphur Recovery Unit (SRU) at
40
C, 179 kPa and 5000 kmol/h. The air feed to the SRU is at 118
C,
179 kPa. The product stream is fed to the waste heat boiler. The product stream
is then separated into sulphur and other product streams. The remaining product
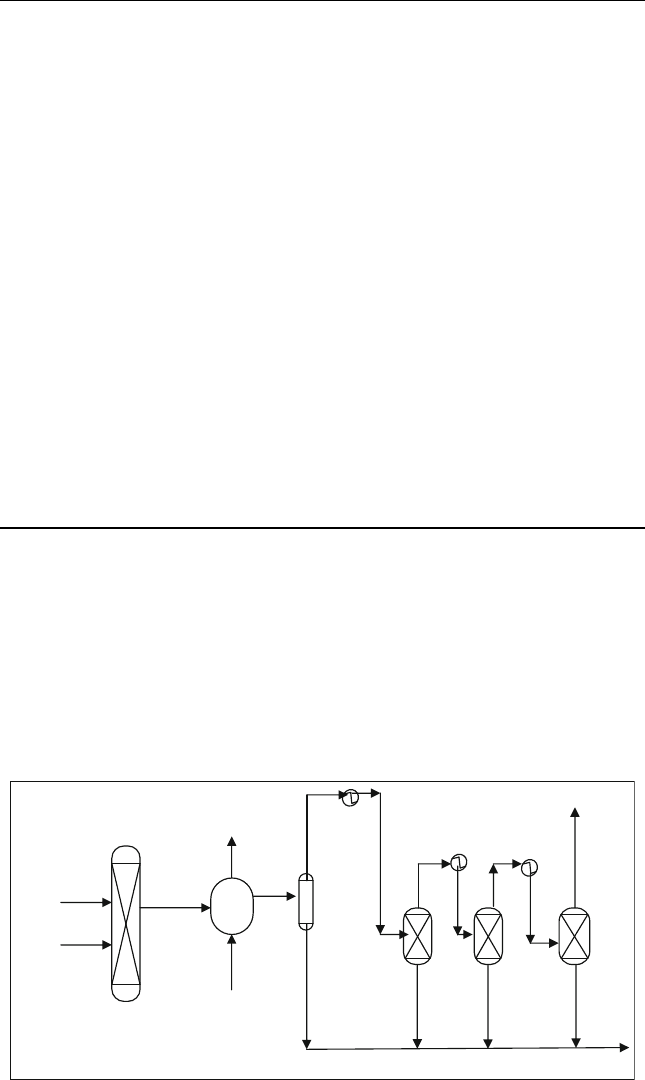
enters the catalytic step made of three reactors as shown in Figure E15.11. Each
reactor operates at 240
C; therefore, a heater is placed before each reactor.
Feed
OutR3
Air
Water
Steam
Sulphur
Separator
S
Out R1 Out R2
S/R1 S/R2 S/R3
Boiler
Combustion
Reactor
To boiler
Figure E15.11 C laus process flow chart
Acid Gas Processing and Mercaptans Removal 395

Table E15.11 Summary results (all components in kmol/h)
Feed Air To boiler Water Steam Out R
1
S/R
1
Out R
2
S/R
2
Out R
3
S/R
3
H
2
S 2010 0 1205.9 241.2 24 0
CO
2
2700 0 2700 2700 2700 2700
CH
4
67.5 0 67.5 67.5 67.5 67.5
H
2
O 215 0 1019 5505 5505 1984 1694.3 1718.4
C
2
H
6
7.5 0 7.5 7.5 7.5 7.5
SO
2
0 0 603 120.65 12.1 0
O
2
0 1005 0 0 0 0
N
2
0 3781 3781 3781 3781 3781
S 0 0 201 1447 832.2 36.17
T (
C) 40 118 833 25 100 171 171 70 212
P (kPa) 179 179 179 101 101 154 154 153 154
Total sulphur produced ¼ 2516.4 kmol/h.
All H
2
S and SO
2
are consumed.
396 Chapter 15
Simulate the process on the UNISIM software and calculate material balances.
Use conversion reactors for thermal and catalytic stages.
Solution:
Reactions (15.21) and (15.22) occur in the combustion reactor. Reaction
(15.23) occurs in the three reactors for sulphur recovery. Table E15.11 sum-
marizes the UNISIM results.
15.3.2. Tail Gas Clean Up
Incinerating the residual H
2
S after sulphur recovery produces SO
2
. There-
fore, further sulphur recovery is done for the tail gases. Typical tail gas
clean-up (TGCU) process can reduce SO
2
to 0.15 vol% and H
2
Sto
0.3 vol%. This process contains a Claus catalytic reaction followed by H
2
S
and SO
2
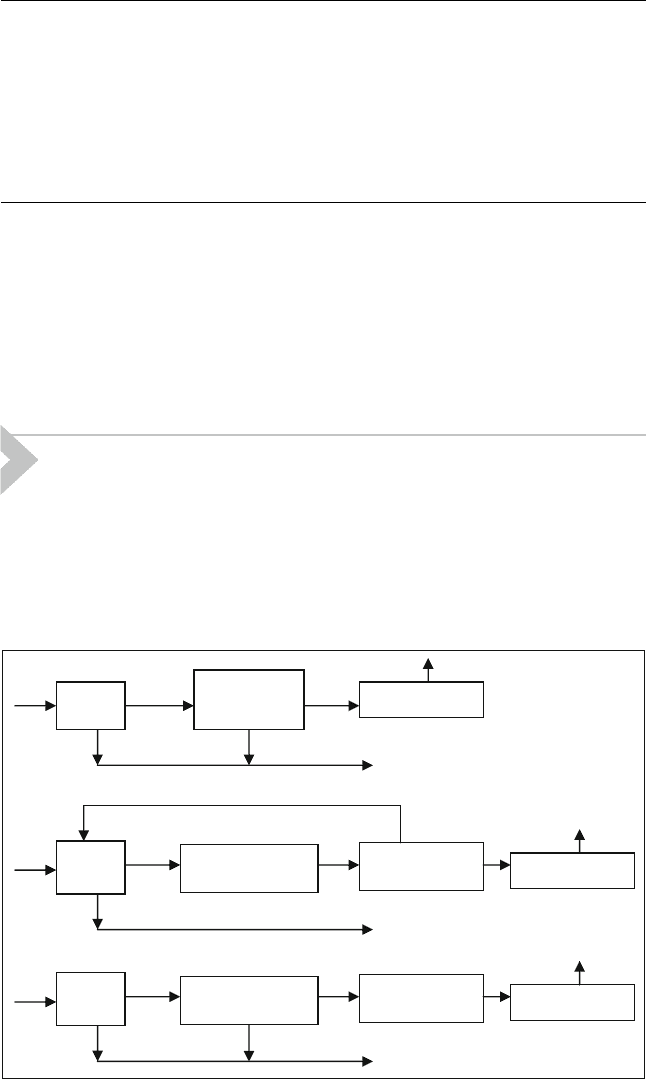
recovery section. Figure 15.8 shows a tail gas clean up scheme.
15.4. Mercaptans Removal
The predominant sulphur compounds in refinery products that usually
have an unpleasant smell are mercaptans. They are corrosive and disturb the
fuel stability due to gum formation. The principle of mercaptans removal is
oxidation. The mercaptan oxidation is called MEROX process. The role of
MEROX in the refinery is shown in Figure 15.9.
Claus
Adsorption
Incineration
Tail
Gas
Sulphur
Flue gases
Flue gases
Flue gases
Claus
Hydrogenation
Incineration
Tail
Gas
Sulphur
Solvent
Scrubbing
Claus
Hydrogenation
Incineration
Tail
Gas
Sulphur
Direct
Oxidation
Figure 15.8 TypicalTail g as clean-up scheme (Legrand and Castel,2001)
Acid Gas Processing and Mercaptans Removal 397

Gas Extraction
Merox
LPG Extraction
Merox
LSR Gasoline
Merox
Jet Fuel
Merox
LPG Extraction
Merox
Cracked Naphtha
Merox
FCC Gasoline
Merox
Alkylation
Isomerization
Gas Con.
FCC
Visbreaker
or Coker
Vacuum
Tow er
Feed
Crude
Tow er
Refinery
Gas
LPG
Gasoline
Jet Fuel
Refinery
Gas
Figure 15.9 Role of MEROX in a refinery
398 Chapter 15

MEROX sweetening involves the catalytic oxidation of mercaptans to
disulphides in the presence of oxygen and alkalinity. Air provides the
oxygen, while caustic soda provides the alkalinity. Oxygen reacts with
mercaptans through the following reaction:
4RSH þ O
2
! 2RSSR þ 2H
2
O ð15:26Þ
Removal of mercaptans by extraction starts with dissolving them in caustic
soda based on the following reaction:
RSH þ NaOH
!
NaSR þ H
2
O ð15:27Þ
The equilibrium occurs between the RSH oily phase and the RSH that
dissolves in the aqueous phase.
Extraction equilibrium is favoured by lower molecular weight mercap-
tans and lower temperatures. The rich caustic soda containing the extracted
mercaptans in the form of mercaptides is regenerated as shown in the
equation given below:
4NaSR þ O
2
þ 2H
2
O
!
2RSSR þ 4NaOH ð15:28Þ
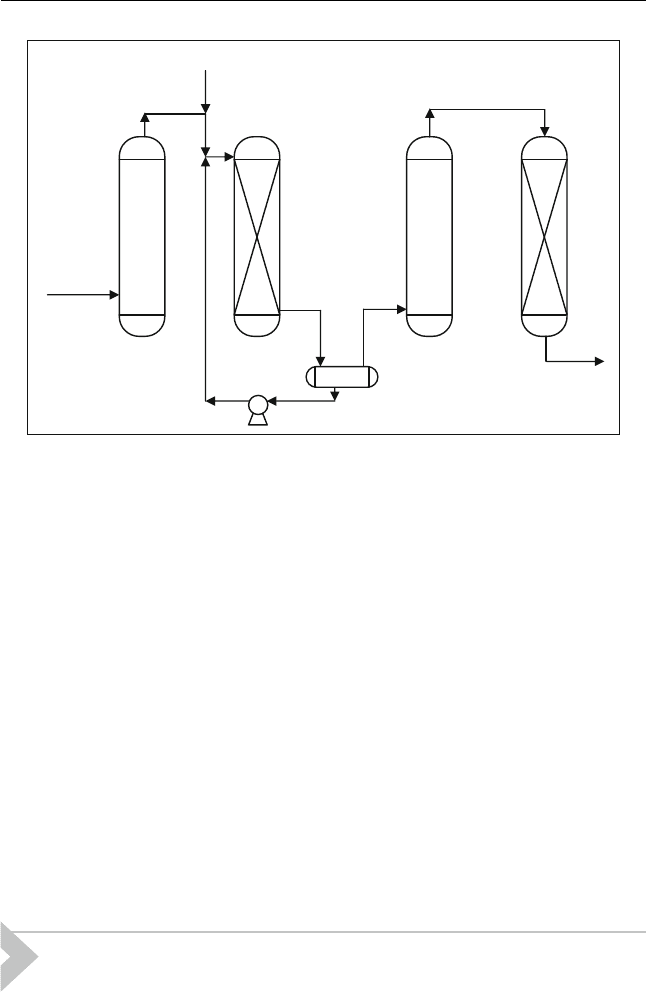
Prior to flowing to the reactor, the feedstock is passed through a caustic pre-
wash to reduce the acid. The MEROX unit consists of a fixed-bed reactor
followed by a caustic settler. Air, the source of oxygen, is injected into the
feedstock upstream of the reactor. The operating pressure is chosen to assure
that the air required for sweetening is completely dissolved at the operating
temperature. The sweetened stream exits the reactor and flows to the
reactor caustic settler.
The caustic soda settler contains a reservoir of caustic soda for use in
keeping the MEROX catalyst alkaline. The solvent product leaving the
water wash flows to a sand filter containing a simple bed of coarse sand that
is used to remove free water and a portion of the dissolved water from the
product (Figure 15.10). The regenerated caustic soda is recycled to the
MEROX reactor.
15.4.1. Gasoline MEROX
MEROX as discussed above treats mercaptans (RSH) in gasoline to meet the
desired specifications. The alkyl group (R) could be aliphatic, aromatic or
cyclic, and saturated or unsaturated.
A dilute caustic soda solution is continuously injected into the gasoline feed
prior to the addition of air. The combined stream passes through a fixed bed
of MEROX catalyst where the mercaptans are oxidized to disulphides and
the dilute caustic soda is coalesced. The sweetened hydrocarbon stream is
essentially free of entrained caustic soda and requires no further separation.
Coalesced spent caustic soda is collected and sent to the refinery’s sewer, since
it is minute in volume, low in NaOH concentration and partially neutralized.
Acid Gas Processing and Mercaptans Removal 399
15.4.2. Kerosene MEROX
The MEROX process for kerosene/jet fuel sweetening is one of the
MEROX process applications developed for the control of mercaptans.
The conventional version of this process uses air and caustic soda (NaOH)
to sweeten kerosene feedstock. Pre- and post-treatment sections are
included to ensure that jet fuel specifications are met.
Prior to flowing to the reactor, kerosene is passed through a caustic soda
pre-wash to reduce the naphthenic acids. The reactor section of the kero-
sene/jet fuel MEROX unit consists of a fixed-bed reactor followed by a
caustic settler. Air is injected into the feedstock upstream of the reactor. The
operating pressure is chosen to assure that the air required for sweetening
will be completely dissolved at the operating temperature. The kerosene
leaving the caustic soda settler passes through a water wash, which removes
trace quantities of caustic soda as well as water soluble surfactant.
Question and Problems
15.1. Absorption is to be used to recover H
2
S from a gas mixture. Readily
available pure amine will be used as the solvent. The inlet gas contains
10 mol% H
2
S and 90 mol% methane. Determine the exit gas mole
fraction if 95% of the H
2
S is recovered in the liquid phase.
Pre
Washer
MEROX
Reactor
Caustic
Settler
Water
Washer
Sand
Filter
Air
Untreated
feed
Dilute
caustic
soda
Treated
feed
Figure 15.10 Typical MEROX flowsheet
400 Chapter 15

15.2. Estimate the required absorber column diameter for the given data:
Entering gas flow is 100 kmol/h, 10 mol% CO
2
and 20 mol% H
2
S
and the balance is CH
4
, The process occurs at 66
C and atmo-
spheric pressure.
Entering liquid absorbent: 200 kmol/h pure MEA (20 wt% in
solution).
Required recovery 97% of H
2
S.
Assume a tray spacing of 24 in., working at 80% of flooding case,
and a surface tension of 70 dynes/cm.
15.3. Acid gas flowing at 100 MMSCFD at 100 psia and 100
F contains
5 mol% CO
2
and 8 mol% H
2
S, and the balance is CH
4
. Calculate the
flow rate of DEA required, then calculate the column diameter.
15.4. A tower is to be designed to absorb SO
2
from gas by using pure water at
293 K and 101.3 kPa absolute pressure. The entering gas contains
20 mol% SO
2
and the leaving gas is 5 mol%. The tower cross-sectional
area is 0.0929 m
2
,andtheheightoftransferunitH
G
is 0.825 m. Calcu-
late the SO
2
concentration in the exit liquid stream and the tower height.
Equilibrium data can be represented by y ¼ 20x.AssumeL
0
/G
0
¼ 30.
15.5. CO
2
is absorbed in a hot carbonate process at 240
F and 1000 psia. The
feed gas stream is 100 MMSCFD and contains 10 mol% CO
2
.Itis
required to release the gas stream with 2 mol% CO
2
. Assume the
circulation rate is 3.5 ft
3
/gal. Calculate the flow rate of the hot carbonate.
15.6. Perform Example E15.6 on UNISIM simulator for a feed rate of 30
MMSCFD with the same feed composition and operation conditions.
15.7. An acid gas feed flow rate is 100 MMSCFD at pressure 421 psia and at
temperature 81
F with the following composition (mol%): H
2
S ¼
5%, CO
2
¼ 15%, and the balance is the carrier gas. This feed is
introduced to a Selexol process to treat the gas to reach 50 ppm H
2
S.
Calculate the % recovery of sulphur.
15.8. An acid gas contains 30 mol% CO
2
, 40 mol% H
2
S and the balance is
the carrier gas. A Morphysorb solvent is used to treat this gas to
50 ppm H
2
S. Calculate the amount of H
2
S and CO
2
absorbed.
15.9. It is desired to capture CO
2
from a gas stream containing 10 mol% of
CO
2
via a silicone rubber membrane. The membrane thickness is
1.0 mm and has a surface area of 3000 m
2
. The applied pressure
gradient is 760 cmHg. Calculate the permeation rate.
REFERENCES
Abdel-Aal, H. K., Aggor, M., and Fahim, M. A. (2003). ‘‘Petroleum and Gas Field Proces-
sing.’’ Marcel Dekker, New York.
Chianelli, R. R., Berhault, G., Raybaud, P., Kasztelan, S., Hafner, J., and Toulhoat, H.
(2002). Periodic trends in hydrodesulfurization: In support of the Sabatier principle.
Applied. Catalysis., A 227, 83–96.
Acid Gas Processing and Mercaptans Removal 401

Geankoplis, J. C. (2003). ‘‘Transport Processes and Separation Process Principles (Includes
Unit Operations)’’, 4th ed. Prentice-Hall, Upper Saddle River, NJ.
Kohl, A., and Nielsen, R. (1997). ‘‘Gas Purification’’, 5th ed. Gulf Publishing Company,
Houston, TX.
Legrand, C. and Castel, J. (2001). ‘‘Acid gas treatment’’, In ‘‘Conversion Processes’’
Petroleum Refining Vol. 3, Leprince, P., ed., TECHNIP, France.
Topse, H., Clausen, B. S., and Massoth, F. E. (1996). ‘‘Hydrotreating Catalysis: Science
and Technology.’’ Springer-Verlag, Berlin, Germany.
UNISIM (2007) Design Suite R370. Honeywell Process Solutions, Calgary, Alberta,
Canada.
Yamaguchi, N. (2003). ‘‘Hydrodesulfurization Technologies and Costs.’’ William and Flora
Hewlett Foundation Sulfur Workshop, Mexico City, 29–30 May 2003. Trans-Energy
Research Associates, Seattle, WA, USA.
402 Chapter 15

CHAPTER SIXTEEN
Refinery Economics
16.1. Introduction
The supply and demand for crude oil and petroleum products are key
factor in determining the status of the world economy. The increase in
demand of crude oil in southeast Asia, for example, in India and China has
been largely responsible for the increase in the price of crude oil to over US$
100 per barrel in 2008. However, as the world economy started to go
through a crisis in the latter half of 2008, demand has started to slow
down, and supply has surpassed demand. This situation has resulted in a
drastic drag of crude oils to less than US$ 40 in 2009. Since the increase in
demand is due to increase in demand for petroleum products, the econom-
ics of the petroleum refining industry will undergo tremendous change. In
general, refining has historically been significantly less profitable than other
petroleum industry segments. Therefore, refiners have to be careful to
control costs to make a profit.
In this chapter, global refining capacity is discussed. Factors affecting the
refinery economics, such as costs, products prices, refinery complexity and
on-stream factors, are covered.
16.2. Refining Capacity
World refining capacity, as measured by crude distillation capacity, has
increased from just over 1 billion tons per year in 1950 to around 4 billion
tons per year or 80 million barrels per day in 1980 (1 barrel per day is
approximately 50 tons per year). Due to energy-saving measures and a
decrease in demand, the capacity decreased to around 73 million barrels
per day in the early 1990s. The worldwide refining capacity has increased in
the new millennium and it reached around 85.46 million barrels per day in
2008 as shown in Figure 16.1 (EIA, 2009). Table 16.1 lists the top refining
countries in 2008.
Fundamentals of Petroleum Refining
#
2010 Elsevier B.V.
DOI: 10.1016/ B978-0-444-52785-1.00018-8 All rights reserved.
403

16.3. Refining Costs
16.3.1. Capital Costs
The capital investment for a new refinery depends on its throughput,
complexity and location. Added to the capital cost is the lengthy time
needed for the preliminary study, obtaining the necessary permits (environ-
mental and otherwise), designing building and commissioning. The capital
cost involves the cost of the process units, utilities, security and environ-
mental facilities, storage and handling facilities, civil work, buildings and
Million barrels per day
1970
1975 1980 1985 1990 1995 2000 2005
90
85
80
75
70
65
60
55
50
45
40
Figure 16.1 World crude oil refining c apacity1970-2008
Table 16.1 Top refining countries in 2008 (EIA, 2009)
Country
Refi ning c apacity
Million barrels per day
United States 17.594
China 6.25
Russia 5.43
Japan 4.65
South Korea 2.58
Germany 2.42
Italy 2.34
Saudi Arabia 2.08
Canada 1.97
France 1.93
Brazil 1.91
United Kingdom 1.86
404 Chapter 16
