Elsevier Encyclopedia of Geology - vol I A-E
Подождите немного. Документ загружается.


Oneacre JW (1993) Subtitle D Regulations Impact on
Ground Water Monitoring. Geotechnical News 11(3):
49–52.
Oneacre JW and Figueras D (1996) Ground Water Variabil-
ity at Sanitary Landfills: Causes and Solutions, Uncer-
tainty in the Geologic Environment. Proceedings, ASCE,
Madison, WI, pp. 965–987.
Ramawsamy JN (1970) Effects of Acid and Gas Production
as Sanitary Landfills. Ph.D. Dissertation,West Virginia
University.
Rank, et al. (1992) Environmental Isotopes Study at the
Breitenqu Experimental Landfill (Lower Austria). Tracer
Hydrology. In: Hotzl and Werner (eds.) Proceedings of the
6th International Symposium on Water Tracing, Karls-
ruhe, Germany, Sept 21–26, pp. 173–177. Rotterdam:
Balkema.
Whiticar MJ and Faber D (1985) Methane Oxidation in
Sediment and Water Column Environments – Isotope
Evidence. Advances in Organic Geochemistry 10:
759–768.
ENVIRONMENTAL GEOCHEMISTRY
W E Dubbin, The Natural History Museum, London, UK
Copyright 2005, Natural History Museum. All Rights Reserved.
Introduction
Soils and sediments occupying the Earth’s surface lie
at the interface of the lithosphere, atmosphere, bio-
sphere, and hydrosphere. Within the weathered, com-
plex, and porous milieu of these Earth-surface
materials, myriad biogeochemical processes govern
the movement of both nutrients and pollutants from
the lithosphere to biota, where they are incorporated
into plant and animal tissues, or to groundwaters,
where they may be transported great distances to
streams, rivers, and oceans. This chapter describes
the most notable pollutants, both organic and inor-
ganic, and the dominant processes governing their
mobility and bioavailability in terrestrial and aquatic
environments.
Trace Elements
Trace elements are those that occur in the lithosphere at
concentrations typically less than 1g kg
1
(Table 1).
Among the trace elements are the micronutrients
(e.g., Cu, Ni, Zn), which are essential for the growth
and development of micro-organisms, plants, and
animals, and also the metalloids, which have charac-
teristics of both metals and non-metals (e.g., As, B)
(see Minerals: Arsenates). Heavy metals are defined
as those trace elements with densities >5.0 g cm
3
.
Virtually all trace elements, even the micronutrients,
exhibit toxicity to animals and plants when present at
excessive concentrations. Radionuclides are a separ-
ate but important class of inorganic contaminant that
may occur naturally (e.g.,
222
Rn,
226
Ra,
238
U) or as a
consequence of nuclear fission related to atomic
weapons testing and nuclear power generation (e.g.,
90
Sr,
137
Cs,
239
Pu).
Although trace elements occur naturally in all
terrestrial environments, anthropogenic inputs may in-
crease these concentrations considerably. The principal
anthropogenic sources of trace elements are mining and
smelting activities, fossil fuel combustion, chemical and
electronics industries, as well as the addition of fertil-
isers and biosolids arising from agricultural operations.
One notable example of severe trace metal pollution
caused by smelting activities is found near Karabash, in
the south Ural Mountains region of Russia, where for
decades smelting operations have deposited metals on
the surrounding landscape, destroying much of the
vegetation and so contributing to widespread soil
erosion (see Environmental Geology).
Toxic levels of trace elements (e.g., Cr, Ni) may also
occur naturally, as in soils derived from serpentinitic
rocks, which can lead to phytotoxicity and the conse-
quent lack of vegetation over large areas of the
landscape where the serpentine soils occur (see Clay
Minerals). Alternatively, these elevated trace element
concentrations may induce metal tolerance among
certain plant species (e.g., Thlaspi spp.) as biological
communities adapt to these metal rich environments.
Trace Element Bioavailability
and Speciation
It is well established that total trace element content
in soil or sediment is a poor indicator of toxicity.
A more reliable measure of ecotoxicity is trace elem-
ent bioavailability. A trace element is considered
bioavailable if it can be utilized by biota. Bioavail-
ability is therefore broadly equated with solubility,
although some plants and micro-organisms are
able to extract metals from solid phases normally
considered insoluble. The solubility, and hence bio-
availability, of a particular trace element is deter-
mined largely by its solid-phase speciation and
mode of surface complexation. The main parameters
ENVIRONMENTAL GEOCHEMISTRY 21

Oneacre JW (1993) Subtitle D Regulations Impact on
Ground Water Monitoring. Geotechnical News 11(3):
49–52.
Oneacre JW and Figueras D (1996) Ground Water Variabil-
ity at Sanitary Landfills: Causes and Solutions, Uncer-
tainty in the Geologic Environment. Proceedings, ASCE,
Madison, WI, pp. 965–987.
Ramawsamy JN (1970) Effects of Acid and Gas Production
as Sanitary Landfills. Ph.D. Dissertation,West Virginia
University.
Rank, et al. (1992) Environmental Isotopes Study at the
Breitenqu Experimental Landfill (Lower Austria). Tracer
Hydrology. In: Hotzl and Werner (eds.) Proceedings of the
6th International Symposium on Water Tracing, Karls-
ruhe, Germany, Sept 21–26, pp. 173–177. Rotterdam:
Balkema.
Whiticar MJ and Faber D (1985) Methane Oxidation in
Sediment and Water Column Environments – Isotope
Evidence. Advances in Organic Geochemistry 10:
759–768.
ENVIRONMENTAL GEOCHEMISTRY
W E Dubbin, The Natural History Museum, London, UK
Copyright 2005, Natural History Museum. All Rights Reserved.
Introduction
Soils and sediments occupying the Earth’s surface lie
at the interface of the lithosphere, atmosphere, bio-
sphere, and hydrosphere. Within the weathered, com-
plex, and porous milieu of these Earth-surface
materials, myriad biogeochemical processes govern
the movement of both nutrients and pollutants from
the lithosphere to biota, where they are incorporated
into plant and animal tissues, or to groundwaters,
where they may be transported great distances to
streams, rivers, and oceans. This chapter describes
the most notable pollutants, both organic and inor-
ganic, and the dominant processes governing their
mobility and bioavailability in terrestrial and aquatic
environments.
Trace Elements
Trace elements are those that occur in the lithosphere at
concentrations typically less than 1 g kg
1
(Table 1).
Among the trace elements are the micronutrients
(e.g., Cu, Ni, Zn), which are essential for the growth
and development of micro-organisms, plants, and
animals, and also the metalloids, which have charac-
teristics of both metals and non-metals (e.g., As, B)
(see Minerals: Arsenates). Heavy metals are defined
as those trace elements with densities >5.0 g cm
3
.
Virtually all trace elements, even the micronutrients,
exhibit toxicity to animals and plants when present at
excessive concentrations. Radionuclides are a separ-
ate but important class of inorganic contaminant that
may occur naturally (e.g.,
222
Rn,
226
Ra,
238
U) or as a
consequence of nuclear fission related to atomic
weapons testing and nuclear power generation (e.g.,
90
Sr,
137
Cs,
239
Pu).
Although trace elements occur naturally in all
terrestrial environments, anthropogenic inputs may in-
crease these concentrations considerably. The principal
anthropogenic sources of trace elements are mining and
smelting activities, fossil fuel combustion, chemical and
electronics industries, as well as the addition of fertil-
isers and biosolids arising from agricultural operations.
One notable example of severe trace metal pollution
caused by smelting activities is found near Karabash, in
the south Ural Mountains region of Russia, where for
decades smelting operations have deposited metals on
the surrounding landscape, destroying much of the
vegetation and so contributing to widespread soil
erosion (see Environmental Geology).
Toxic levels of trace elements (e.g., Cr, Ni) may also
occur naturally, as in soils derived from serpentinitic
rocks, which can lead to phytotoxicity and the conse-
quent lack of vegetation over large areas of the
landscape where the serpentine soils occur (see Clay
Minerals). Alternatively, these elevated trace element
concentrations may induce metal tolerance among
certain plant species (e.g., Thlaspi spp.) as biological
communities adapt to these metal rich environments.
Trace Element Bioavailability
and Speciation
It is well established that total trace element content
in soil or sediment is a poor indicator of toxicity.
A more reliable measure of ecotoxicity is trace elem-
ent bioavailability. A trace element is considered
bioavailable if it can be utilized by biota. Bioavail-
ability is therefore broadly equated with solubility,
although some plants and micro-organisms are
able to extract metals from solid phases normally
considered insoluble. The solubility, and hence bio-
availability, of a particular trace element is deter-
mined largely by its solid-phase speciation and
mode of surface complexation. The main parameters
ENVIRONMENTAL GEOCHEMISTRY 21

governing trace element speciation are pH and pe, the
so-called ‘master variables’, as well as the total con-
centration of the ion in solution. These variables
determine whether the ion in question will undergo
hydrolysis, precipitation, redox reactions, or any
number of complexation reactions at surfaces or in
aqueous solution. Generally, metal cations are most
soluble and available at low pH, principally pH <5,
where hydrolysis is minimal and sorption to layer
silicates and metal oxides is limited. Conversely, oxy-
anions such as chromate (see Minerals: Chromates)
(HCrO
4
) and selenite (HSeO
3
) show greatest solubil-
ity at high pH, where electrostatic repulsion with
negatively charged colloids minimizes surface com-
plexation.
Redox-sensitive elements such as As, Cr, Fe, and
Mn are subject to oxidation state changes under
Earth surface conditions, with important impli-
cations for solubility and toxicity. Of the two As
oxidation states predominating under Earth surface
conditions (i.e., As(III) and As(V) ), As(III) is more
problematic because of its greater mobility and
bioavailability. Given the ubiquity of manganese
oxides (e.g., d-MnO
2
) in soils and sediments, these
minerals have been proposed as key agents in
the natural attenuation of As(III) contamination, as
the oxidation of As(III) to As(V) by Mn(IV) is both
rapid and thermodynamically favourable. The human
health implications of As bioavailability is illustrated
most clearly on the deltaic plains of Bangladesh
and West Bengal, where groundwater As concentra-
tions may exceed 400 mgl
1
as a consequence of bio-
logically mediated reductive dissolution of Fe(III)
oxides, leading to the release to solution, in bioa-
vailable form, of the previously sorbed As. Manga-
nese(IV) oxides can also mediate the oxidation of
Cr, from Cr(III) to Cr(VI). However, this redox reac-
tion is undesirable because, unlike As, the oxidized
form of Cr, Cr(VI), is the form of Cr most mobile
and toxic.
Table 1 Abundance, speciation, and toxicity of trace elements in soil and sediment
Element
Median soil content
a
(mg kg
1
) Dominant solution species
b
Function; Toxicity
Ag 0.05 Ag
þ
, AgCl None known; Plant and animal toxin
As 6 As(OH)
3
, AsO
3
3
,H
2
AsO
4
, HAsO
4
2
None known; Plant and animal toxin
B20 H
3
BO
3
,H
2
BO
3
Plant nutrient; Phytotoxin
Be 0.3 Be
2þ
, Be(OH)
3
, Be(OH)
4
2
None known; Plant and animal toxin
Bi 0.2 Bi
3þ
None known; Plant and animal toxin
Cd 0.35 Cd
2þ
, CdSO
4
, CdCl
, CdHCO
3
þ
None known; Animal toxin
Cl 100 Cl
Plant nutrient; Phytotoxin
Co 8 Co
2þ
, CoSO
4
, Co(OH)
2
Plant and animal nutrient; Plant and animal
toxin
Cr 70 Cr(OH)
2
þ
, Cr(OH)
4
, HCrO
4
Animal nutrient; Cr(VI) is a plant and animal
toxin
Cs 4 Cs
þ
None known; None known
Cu 30 Cu
2
þ
, CuCl
, CuCO
3
, CuHCO
3
þ
, Cu(OH)
þ
Plant and animal nutrient; Plant and animal
toxin
Hg 0.06 Hg
2þ
, HgCl
2
,CH
3
Hg
þ
, Hg(OH)
2
None known; Plant and animal toxin
Mo 1.2 H
2
MoO
4
, HMoO
4
, MoO
4
2
Plant and animal nutrient; Plant and animal
toxin
Ni 50 Ni
2þ
, NiSO
4
, NiHCO
3
þ
, NiCO
3
Plant and animal nutrient; Plant and animal
toxin
Pb 19 Pb
2þ
, PbSO
4
, PbHCO
3
þ
, PbCO
3
, PbOH
þ
None known; Plant and animal toxin
Rb 67 Rb
þ
None known; Phytotoxin
Sb 1 Sb(OH)
2
þ
, Sb(OH)
3
, Sb(OH)
4
, Sb(OH)
6
None known; Plant and animal toxin
Se 0.4 HSeO
3
, SeO
3
2
, SeO
4
2
Animal nutrient; Plant and animal toxin
Sn 4 Sn
4þ
None known; Plant and animal toxin
Tl 0.2 Tl
þ
None known; Plant and animal toxin
U 2.7 UO
2(s)
,UO
2
2þ
,UO
2
CO
3
,UO
2
(CO
3
)
3
4
, (UO
2
)
3
(OH)
7
None known; Animal toxin
V90 VO
2þ
,VO
2
þ
,VO
2
(OH)
2
,VO
3
(OH)
2
Plant and animal nutrient; Plant and animal
toxin
W 1.5 WO
4
2
None known; None known
Zn 70 Zn
2þ
, ZnSO
4
, ZnHCO
3
þ
, ZnCO
3
, Zn(OH)
þ
Plant and animal nutrient; Plant and animal
toxin
a
Bowen (1979).
b
Hayes and Traina (1998).
22 ENVIRONMENTAL GEOCHEMISTRY

Organic Contaminants
The environmental geochemistry of organic contam-
inants primarily concerns the sources, movement, and
fate of petroleum hydrocarbons and their by-
products, as well as the halogenated hydrocarbons,
the group to which many pesticides belong. Petroleum
hydrocarbons and associated compounds such as the
oxygenates (e.g., methyl t-butyl ether (MTBE)) con-
stitute a significant environmental risk by virtue of
their widespread occurrence, mobility, and ecotoxi-
city (see Geochemical Exploration). Following release
of hydrocarbons to the environment by multiple path-
ways, the low molecular weight volatile fraction
(<C
15
), often containing the carcinogenic benzene
and polycyclic aromatic hydrocarbons, is largely lost
to the atmosphere through volatilisation, leaving a
relatively small but environmentally important por-
tion of the light hydrocarbon pool to react with soil or
sediment, or to enter groundwater. The less soluble,
more chemically inert higher molecular weight hydro-
carbons (>C
14
) are potentially more disruptive to
ecosystems, as illustrated by the spill in 1989 of
crude oil from the Exxon Valdez oil tanker, which
contaminated nearly 1750 km of Alaskan shoreline.
Halogenated hydrocarbons have both natural and
anthropogenic origins, and many belong to the class
of contaminant known as persistent organic pollutants
(POPs), recalcitrant organic compounds that bioac-
cumulate and exhibit animal toxicity. POPs are domin-
ated by the chlorinated hydrocarbons, which include
many of the pesticides, such as aldrin, atrazine, ch-
lordane, DDT, heptachlor, and the polychlorinated
biphenyls. Despite the relative recalcitrance of POPs,
their degradation can be mediated both by abiotic pro-
cesses (e.g., oxidation by d-MnO
2
)aswellasbythe
native soil microflora, particularly the fungi, which
employ hydrolytic, reductive, or oxidative reactions
to induce molecular dehalogenation. The hydroxy-
lated compound so produced is thus rendered more
susceptible to the degradation reactions of other soil
microbiota.
The tendency of certain POPs to migrate from
tropical and temperate climates to the colder polar
regions has been the subject of study and debate
for decades. A model of redistribution described
as ‘global distillation’, involving POP evaporation
followed by transport and condensation in colder
regions, has received wide acceptance. Fractionation
of POPs, during redistribution to higher latitudes, is
driven by differential migration rates arising from
variable POP vapour pressures and partition coeffi-
cients, with POP transport occurring in distinct jumps
which are closely coupled to diurnal and seasonal
temperature cycles.
Acidification of Terrestrial and
Aquatic Environments
An important aspect of environmental geochemistry
is acid deposition and the related acidification of
Earth surface environments through both anthropo-
genic and natural processes. The burning of fossil
fuels releases SO
2
and NO
x
compounds which com-
bine with atmospheric water to yield H
2
SO
4
and
HNO
3
that may be carried great distances before
deposition as rain, mist, fog, or snow. Deposition of
these acidic materials impacts negatively on soils,
vegetation, and water bodies, particularly lakes
which are poorly buffered and whose aquatic organ-
isms are therefore at risk of increased soluble Al
concentrations following a significant decrease in
lake pH. Monuments and buildings constructed of
limestone and marble, and which frequently represent
much of our cultural heritage, are also at risk from
acid deposition through dissolution of their constitu-
ent carbonate minerals (see Minerals: Carbonates).
The effects of acid deposition are not entirely nega-
tive, however, as the additions of N and S to soils are
beneficial, and these added nutrients frequently com-
prise a significant portion of the available soil N and
S in highly industrialized regions.
An aspect of acidification of growing environmen-
tal importance concerns the oxidation of mining
waste rich in reduced S, principally in the form of
pyrite (FeS
2
)(see Environmental Geology, Minerals:
Sulphides). Oxidation of this S can yield vast amounts
of H
2
SO
4
, giving rise to highly acidic waters, known
as acid mine drainage (AMD) waters, containing
toxic levels of soluble metals (Figure 1). The environ-
mental significance of these acidic, metal-rich waters
was emphasised in dramatic fashion with the col-
lapse in 1998 of the Aznalco
´
llar mine tailings dam
in southwest Spain. Failure of the dam led to the
release of 1.3 million cubic metres of AMD waters,
laden with Ag, As, Cd, Cu, Pb, and Zn, and the
subsequent flooding of nearly 4,600 hectares of land
with this toxic effluent.
Environmental Restoration
Decontamination of terrestrial environments is often
costly and time-consuming, owing to the frequent
occurrence of multiple pollutants, as well as the
complexity of the contaminated matrices (e.g., soil
or sediment). Strategies employed to remediate con-
taminated environments involve both in situ and ex
situ techniques (Table 2), with the former generally
receiving wider acceptance because of greater efficacy
and lower implementation costs. In situ remediation
may simply involve introducing a liming material
ENVIRONMENTAL GEOCHEMISTRY 23

(e.g., CaO, Ca(OH)
2
, CaCO
3
) to raise pH and
thus decrease metal bioavailability through sorption
and precipitation. The addition of smectites or zeo-
lites (see Minerals: Zeolites) serves to remove
pollutants by means of ion exchange reactions,
whereas apatite addition induces the formation of
sparingly soluble metal phosphate precipitates.
Phytoremediation encompasses a variety of in situ
strategies involving the use of plants to remove or
render environmental pollutants harmless. The most
common of these strategies is phyto-extraction,
which involves growing plants capable of hyperaccu-
mulating the contaminant of interest, followed by
plant harvest to remove both plant and contaminant
from the site. Among the hundreds of hyperaccumu-
lators recently identified are the brake fern (Pteris
vittata), an effective As accumulator, and also the
basket willow (Salix viminalis), which effectively se-
questers both Zn and Cd. Biodegradation is a related
in situ remediation strategy that utilises native micro-
bial populations to degrade multiple organic contam-
inants. This microbial degradation is optimal within
aerobic rhizosphere communities at circumneutral
pH, particularly in the presence of abundant nutri-
ents, which may be augmented with external sources
to enhance microbial activity.
The more costly ex situ techniques are generally
applied only to the most high-value contaminated
sites. The simplest of the ex situ remediation strat-
egies involves direct excavation of the contaminated
material followed by landfill disposal. Soil washing
(see Soils: Modern) is more complex, involving ex-
cavation followed by mechanical screening to obtain
coarse (>50 mm) and fine (<50 mm) fractions which
are subsequently treated with a series of surfactants
and chelates to remove both organic and inorganic
contaminants, including radionuclides. The aim of
soil washing is to concentrate the pollutants within
the fine fraction, which is then treated with a solidifi-
cation/stabilizing agent (e.g., Portland cement, lime,
fly ash) prior to landfill disposal.
Figure 1 Acid mine drainage waters near the Kristineberg
Cu and Zn mine, Sweden (photo courtesy L. Lovgren, Umea
University).
Table 2 Summary of in situ and ex situ environmental restoration techniques
Method Contaminant Processes Limitations
In situ
Lime addition Metals, radionuclides pH increase causing sorption,
precipitation
Ineffective for oxyanions
Smectite, zeolite, apatite
addition
Metals, radionuclides Ion exchange, sorption, precipitation Selective, short term remediation
Phytoremediation Metals, organics Phytoaccumulation, phytodegradation Unsuitable for highly
contaminated sites
Biodegradation Organics Microbial degradation Long-term remediation
Volatilization Volatile organics Evaporative loss of volatile pollutants Contaminant must be volatile
Ex situ
Excavation and disposal All Removal and disposal Costly, risk of pollutant dispersal
Soil washing Metals, organics,
radionuclides
Excavation, leaching of contaminants Costly
Solidification/stabilization Metals, radionuclides Addition of stabilising agent Volatile organics not immobilized
24 ENVIRONMENTAL GEOCHEMISTRY

See Also
Analytical Methods: Geochemical Analysis (Including X-
Ray). Clay Minerals. Environmental Geology. Geo-
chemical Exploration. Minerals: Arsenates; Carbon-
ates; Chromates; Sulphides; Zeolites. Soils: Modern.
Weathering.
Further Reading
Adriaens P, Gruden C, and McCormick ML (2004) Biogeo-
chemistry of halogenated hydrocarbons. In: Lollar BS
(ed.) Environmental Geochemistry, vol. 9, pp. 511–539.
Treatise on Geochemistry Holland HD and Turekian KK
(eds.). Oxford: Elsevier-Pergamon.
Adriano DC (1986) Trace Elements in the Terrestrial Envir-
onment. New York: Springer-Verlag.
Alexander M (1999) Biodegradation and Bioremediation,
2nd edn. London: Academic Press.
Alloway BJ (1995) Heavy Metals in Soils, 2nd edn. London:
Blackie Academic and Professional.
Blowes RJ, Ptacek CJ, Jambor JL, and Weisener CG (2004)
The geochemistry of acid mine drainage. In: Lollar BS
(ed.) Environmental Geochemistry, vol. 9, pp. 149–204.
Treatise on Geochemistry Holland HD and Turekian KK
(eds.). Oxford: Elsevier-Pergamon.
Bowen HJM (1979) Environmental Chemistry of the Elem-
ents. London: Academic Press.
Cozzarelli IM and Baehr AL (2004) Volatile fuel hydrocar-
bons and MTBE in the environment. In: Lollar BS (ed.)
Environmental Geochemistry, vol. 9, pp. 435–474. Trea-
tise on Geochemistry Holland HD and Turekian KK
(eds.). Oxford: Elsevier-Pergamon.
Hayes KF and Traina SJ (1998) Metal ion speciation and its
significance in ecosystem health. In: Huang PM (ed.) Soil
Chemistry and Ecosystem Health, pp. 45–84. Madison,
USA: Soil Science Society of America.
Schwarzenbach RP, Gschwend PM, and Imboden DM
(1993) Environmental Organic Chemistry. New York:
Wiley.
Sposito G (1996) The Environmental Chemistry of Alumi-
num, 2nd edn. London: CRC Press.
a0005 ENVIRONMENTAL GEOLOGY
P Doyle, University College London, London, UK
ß 2005, Elsevier Ltd. All Rights Reserved.
Introduction
Environmental geology has grown in stature as a dis-
cipline over the past 40 years, as considerations of
economic geology have moved away from the simple
exploitation of the late nineteenth and early twentieth
centuries. Today, environmental geology is a broad
area of geological endeavour and a major industry in
its own right.
Defining Environmental Geology
Environmental geology may be defined as the
interaction of humans with their – fundamentally
geological – environment. The environment can be
considered to consist of both the constituents of the
Earth itself (rocks, sediments, and fluids) and its surface
and the processes that operate to change it through
time.
Environmental geology is a subset of environmental
science, which is the study of the interaction of
humans with all aspects of their environment – phys-
ical, atmospheric, and biological – and is linked dir-
ectly with engineering geology (see Engineering
Geology: Overview). This definition clearly indicates
that it is the introduction of the human element to the
equation that defines the concept of the environmen-
tal sciences – and, therefore, environmental geology –
and it is a consideration of both the debits (impacts)
and credits (benefits) of our existence. Environmental
science is a way of managing our existence so as to
maximize human success while minimizing the nega-
tive aspects. At the heart of environmental geology, as
with all environmental sciences, is the concept of sus-
tainable management – working with natural systems
to sustain development but not at an unacceptable
environmental cost.
Scope of Environmental Geology
It has been estimated that around 50% of the Earth’s
population live in urban centres, centres that cover just
1% of the Earth’s surface. Given that environmental
geology is the interaction of humans with their environ-
ment and given that the majority of humans live in
cities, it follows that environmental geology can be
considered as primarily an urban issue, with the most
challenging problems occurring within the immediate
hinterland of urban centres.
Environmental geology has been defined as an
urban concept, a ‘machine’ that balances inputs, out-
puts and maintenance, most if not all of which have a
geological component (Figure 1). The inputs include
water (derived locally or from more distant locations,
but feeding into the city), raw materials (in the form of
ENVIRONMENTAL GEOLOGY 25
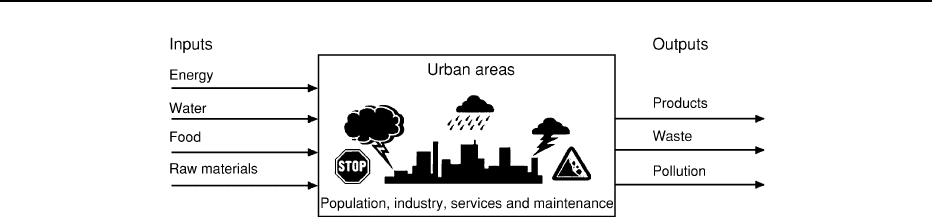
mineral resources for industry and construction), food
resources (both locally produced and transported),
and energy (the result in many cases of mineral re-
sources such as coal, gas, and uranium). Outputs from
the ‘machine’ include products from industry, wastes
(in the form of worn-out materials, by-products of
industry, and day-to-day wastes from domestic, indus-
trial, and commercial sources), and pollution from
poor waste-management strategies. The urban ma-
chine needs constant maintenance to replace its infra-
structure and foundations, and to protect it from
natural hazards.
The demands of the machine drive our demands on
the environment, with the need for exploration and
exploitation of mineral, water, and soil resources to
provide the inputs; the need to combat environmental
stress and natural hazards in protecting the workings
of the machine, and the requirement to deal with the
outputs, particularly wastes and their potential to
pollute if not handled with appropriate sensitivity.
All these aspects (and more) fall within the broad
scope of environmental geology, and they can be
distilled into the following themes.
1. Geology of resource management, including ex-
ploration and exploitation of resources (e.g. fuels,
industrial minerals, and water) and the mitigation
and limitation of associated adverse environmental
impacts.
2. Geology of the built environment, particularly
the constraints that ground conditions place on
development.
3. Geology of waste management, specifically the
disposal of wastes in the physical environment.
4. Geology of natural hazards.
Geology of Resource Management
Resources may be defined in the geological context as
naturally occurring solids, liquids, or gases that are
known or thought to exist in or on the Earth’s crust in
concentrations that make extraction economically
feasible either at present or at some time in the future.
This definition includes those geological materials
that can be extracted with currently available tech-
nology (reserves) as well as those that are thought
to exist but that will require further technological
development to remove them.
In the short term, resource-management options
focus on reserves, and environmental considerations
have more power than at any other time in the past
to reduce available reserves by altering the economics
of their exploitation. Long-term planning must, of
course, be based on the probability of new resources
being discovered. The size of the available reserves
relative to the total resources can be altered by several
factors, such as commodity price, exploration, increas-
ing the extent of the known resource, technological
developments, and changes in regulation.
Economic Mineral Resources
Economic mineral resources are varied and can be
defined in their broadest sense as any geological ma-
terial that is of commercial value to society. This broad
definition includes such diverse materials as fuels (e.g.
coal, gas, uranium), construction materials, industrial
minerals, metals, and precious minerals. Extraction of
these materials varies from bulk extraction to special-
ist mining, and, although the basic concepts of explor-
ation and exploitation may have many parallels, the
environmental impacts posed by each one may well
differ strongly.
Environmental Impacts of Mineral Extraction
The environmental impacts of mineral extraction vary
according to the type of mineral and the extent of its
deposit, with impacts varying throughout the working
life of a mine or quarry, and with the issues often
continuing long after the deposits worked are no
longer economically viable. Typical issues are aspects
of mine operation, mining subsidence, tackling mine
wastes, and quarry or mine restoration.
There are a wide range of issues associated with
mining. Quarries are troubled by blast noise and
vibration, which can lead to increased rock-fall and
Figure 1 The urban machine. (Reproduced with permission from Bennett MR and Doyle P (1997) Environmental Geology: Geology and
the Human Environment.
Chichester: John Wiley.)
26 ENVIRONMENTAL GEOLOGY
landslip activity. In deep mines, the emission of me-
thane and other gases is a problem, while wastewaters
may be charged with iron and may be acidified by the
breakdown of pyrite and other sulphides. Acid mine
drainage is a growing problem in many disused metal
mines and has adverse affects on groundwater supplies
and water bodies, such as estuaries, rivers, and lakes.
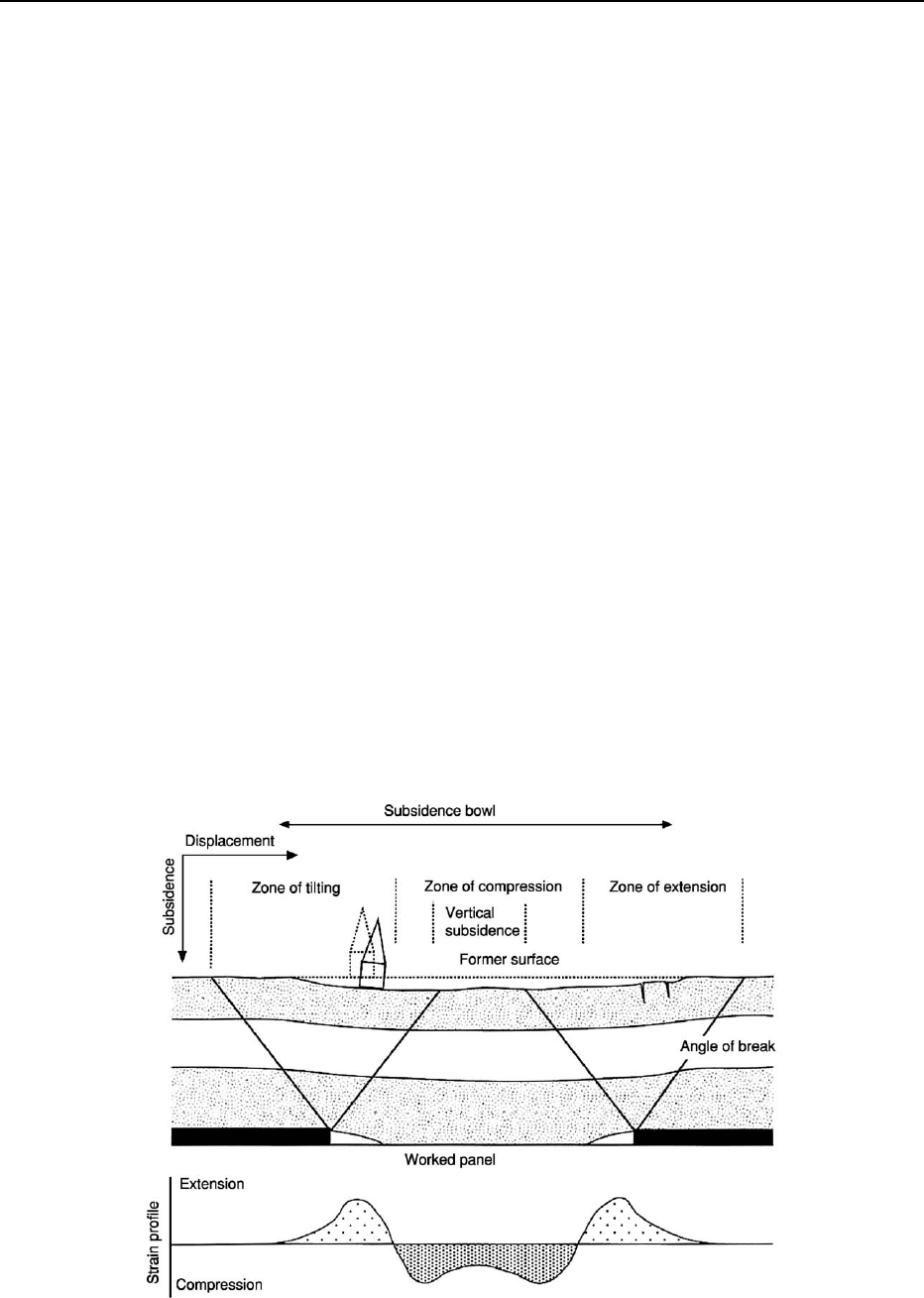
Mining subsidence is a result of the removal of geo-
logical materials underground, creating a void space,
which subsequently collapses (Figure 2). Similar situ-
ations occur when voids are created for purposes other
than mineral extraction. Longwall mining is associ-
ated with deep coal extraction and generally involves
the planned subsidence of a relatively large area.
Pillar-and-stall mining, which dates back centuries,
can lead to the differential collapse of the pillars,
giving rise to graben-like failure structures. In other
cases, removal of deep salt deposits through the
process known as brining – the pumping out of
brine-charged waters – can lead to severe subsidence.
Large-scale subsidence is often difficult to manage,
and the possibility of constructing buildings over an
unknown area of mining activity is a very live issue,
even where the mining is known to date back to an-
tiquity, as in Rome. Raft foundations, grouting, deep
piling, and excavation and backfill are all possibilities,
depending on the depth and extent of the problem.
Mine wastes are produced at two stages during
the mineral-extraction process: during mining, when
waste rock or spoil is produced; and during further
processing of the materials extracted, which creates a
further set of mineral wastes, generally much finer
than simple spoil and referred to as tailings. Spoil is
of variable grain size and generally accumulates in
open tips close to the mine workings. Tailings pro-
duced by mineral processing tend to be much richer
in minerals and more uniform and of a finer grain
size owing to milling. In some cases these materials
have a high toxicity, as hazardous chemicals are used
in the separation of mineral particles. Tailings are also
more likely to be distributed by weathering and
erosion, raising the consequent possibility of hazard-
ous air-borne particulate matter. The long-term sta-
bility of mine wastes is also of concern, as with
the South Welsh Aberfan disaster in 1966, where
111 000 m
3
of debris moved down a 13
slope,
enveloping a school with tragic loss of life.
Dealing with waste tips is a matter of concern for
environmental geologists, and the solution depends
very much on the nature of the material. In the UK,
tax on aggregate extraction led to the reuse of some
tax-exempt spoil, particularly slate and shale, as poor-
quality aggregate. Other mineral spoil may be reused
for rock fill or inert landfill or even in dealing with
mine subsidence and quarry-restoration projects. Tail-
ings, on the other hand, given the potentially high level
of toxicity, have to be treated with caution. Wet tail-
ings have to be ponded, treated, and removed, while
dry tailings have to be carefully monitored so that
water passing through the wastes does not lead to
Figure 2 Subsidence associated with longwall coal mining.
ENVIRONMENTAL GEOLOGY 27
groundwater pollution. Restoration and stabilization
of wastes is essential, but this can be a tall order when
vegetation that could be useful in binding surfaces is
inhibited by the toxicity of the waste material itself.
Quarry and mine restoration is a major task. For
mines, it is important first of all to ensure the safety of
the underground workings themselves and to monitor
such issues as the build-up of gases and acid mine
drainage. In other cases, the mine workings may
present a hazard in the form of future subsidence.
Pit-head machinery and processing works, which may
have used chemical processes requiring specialist atten-
tion, will also be an issue. Finally, the mine wastes
themselves must be dealt with. For quarries, restoration
issues reflect the nature of the materials quarried, with
production-blasted hard-rock quarry faces represent-
ing an unsafe and unstable option. Restoration depends
very much on the projected end use of the quarry; if left
as a void, then faces will require engineering attention.
This is particularly so given that many quarries are now
being used to house industry or commercial concerns.
In other cases, the void will be filled, most often as
landfill.
Water Resources
Water is a vital geological resource that sustains life,
yet most of the Earth’s water is unavailable, with
98% being in the oceans and 1.6% in ice-sheets,
leaving only 0.4% for drinking. Suitable drinking-
water resources are distributed unevenly across the
globe, and this, together with the predictions of
environmental change, means that the search for
drinking-water supplies is a major preoccupation of
environmental geologists. Water-resource manage-
ment is essentially a combination of three factors:
acquisition, redistribution, and the treatment and
disposal of wastewaters. The potential to pollute
existing supplies through inadequate waste-disposal
strategies is also a major issue, and this will be dealt
with later when considering waste management.
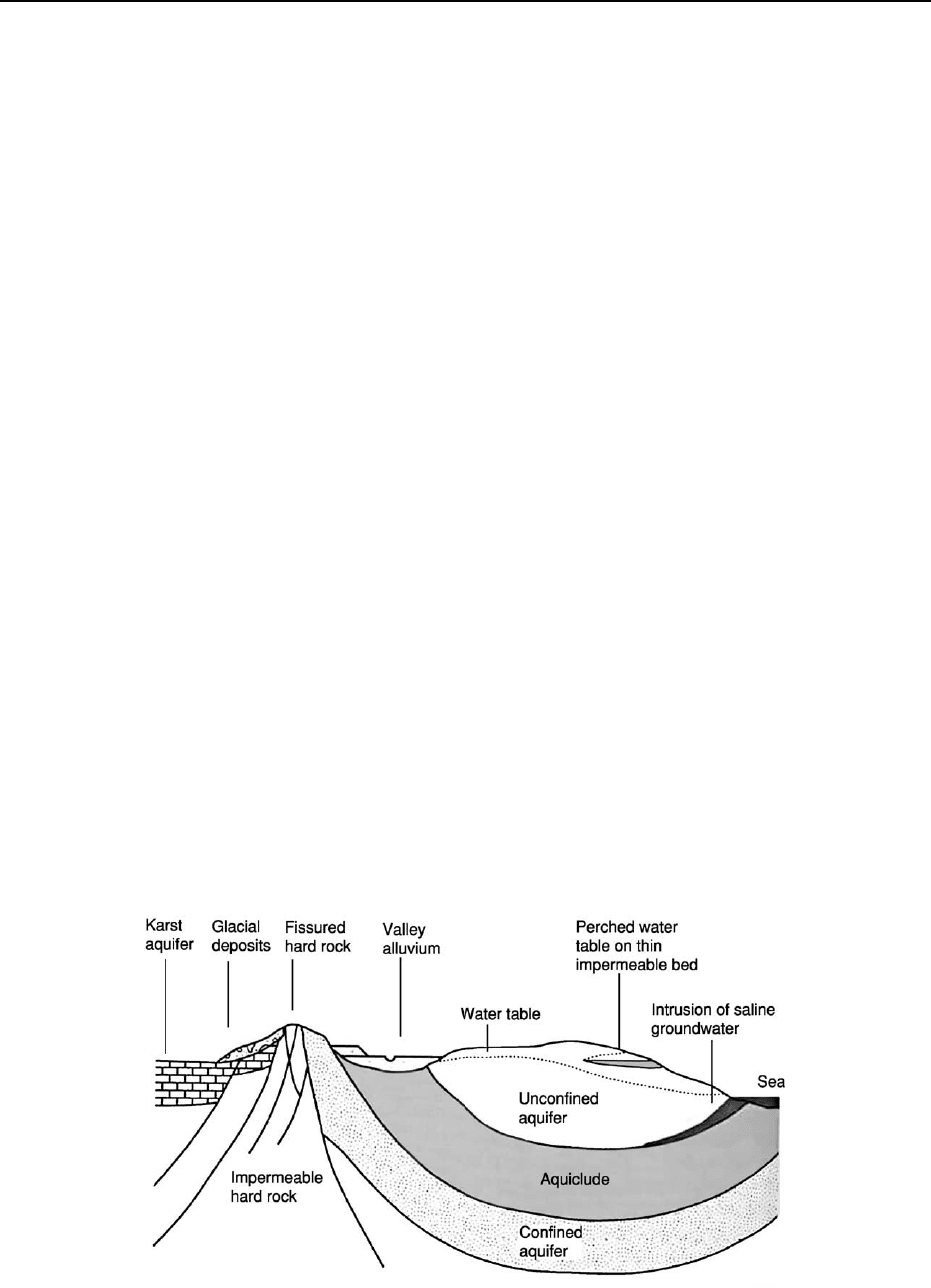
Acquisition of water is primarily a process of ex-
traction from groundwaters, which are controlled by
rainfall and the porosity and permeability of the rocks
present in a given area (Figure 3). These can be highly
variable, and, even within a small area such as the
UK, the percentage of water derived from this source
is variable. Where groundwater is not an option, reli-
ance on surface waters – rivers, lakes, and reservoirs –
becomes much more significant, with desalination of
seawater also possible.
The redistribution of supplies is an enormous under-
taking and has lead to major international disputes
when some countries have sought to dam rivers to the
detriment of others downstream. Such international
tensions are unlikely to recede in the future and could
lead to ‘water wars’ if diplomacy breaks down. The
creation of dams to contain waters and generate hydro-
electric power has been an attractive but often highly
controversial approach in some water-poor countries.
However, inadequate site assessment has led to the fail-
ure of some dam sites, with leakage of waters or failure
of the dam structure itself, so the high-cost deployment
of such schemes requires a complete understanding and
assessment of the issues involved.
Soil Resources
Together with water, soils are a fundamental geological
resource, allowing us to grow sufficient crops to supply
the food required to sustain the planet’s growing popu-
lation. The distribution and formation of soils across
Figure 3 Schematic diagram of the main water-bearing units. (Reproduced with permission from Bennett MR and Doyle P (1997)
Environmental Geology: Geology and the Human Environment. Chichester: John Wiley.)
28 ENVIRONMENTAL GEOLOGY
the world is contingent on climate, rainfall, biological
activity, topography, and the underlying geology. Soils
vary considerably, with an infinite variety – there are
at least 19 000 different types in the USA alone – and
widely differing classification schemes across the world
(see Soils: Modern). The potential to erode and denude
soils depends on the intensity of use, the ground cover,
and so on. Soil conservation is therefore a major
issue and can vary with land-use changes, new
technologies, and colonization.
In the UK, raising the awareness of soils and the need
to conserve them has led to the construction of five
basic principles: that soils are an essential part of life
support; that soils should enjoy the same level of pro-
tection as water and air; that integrated environmental
management should include soils; that contaminated
soils should be remediated; and that contamination
should be avoided. These issues obviously have a global
relevance.
Aesthetic and Scientific Resources
Conservation of aesthetic and scientific resources in-
volves the conservation of areas of landscape and/or
specific geological sites – known as geodiversity – for
future generations (see Geological Conservation). The
concept is based on four basic convictions: that they
should be conserved for their own sake; that they form
the basis for exploitation; that they form the basis for
research and training; and that they have aesthetic and/
or cultural value. This approach is growing in stature
across the world, leading to a wider awareness of the
value of geological features.
Geology of the Built Environment
The built environment – the towns and cities in which
the vast majority of people live – is the central pillar
of the urban machine, and, in many cases, the cause
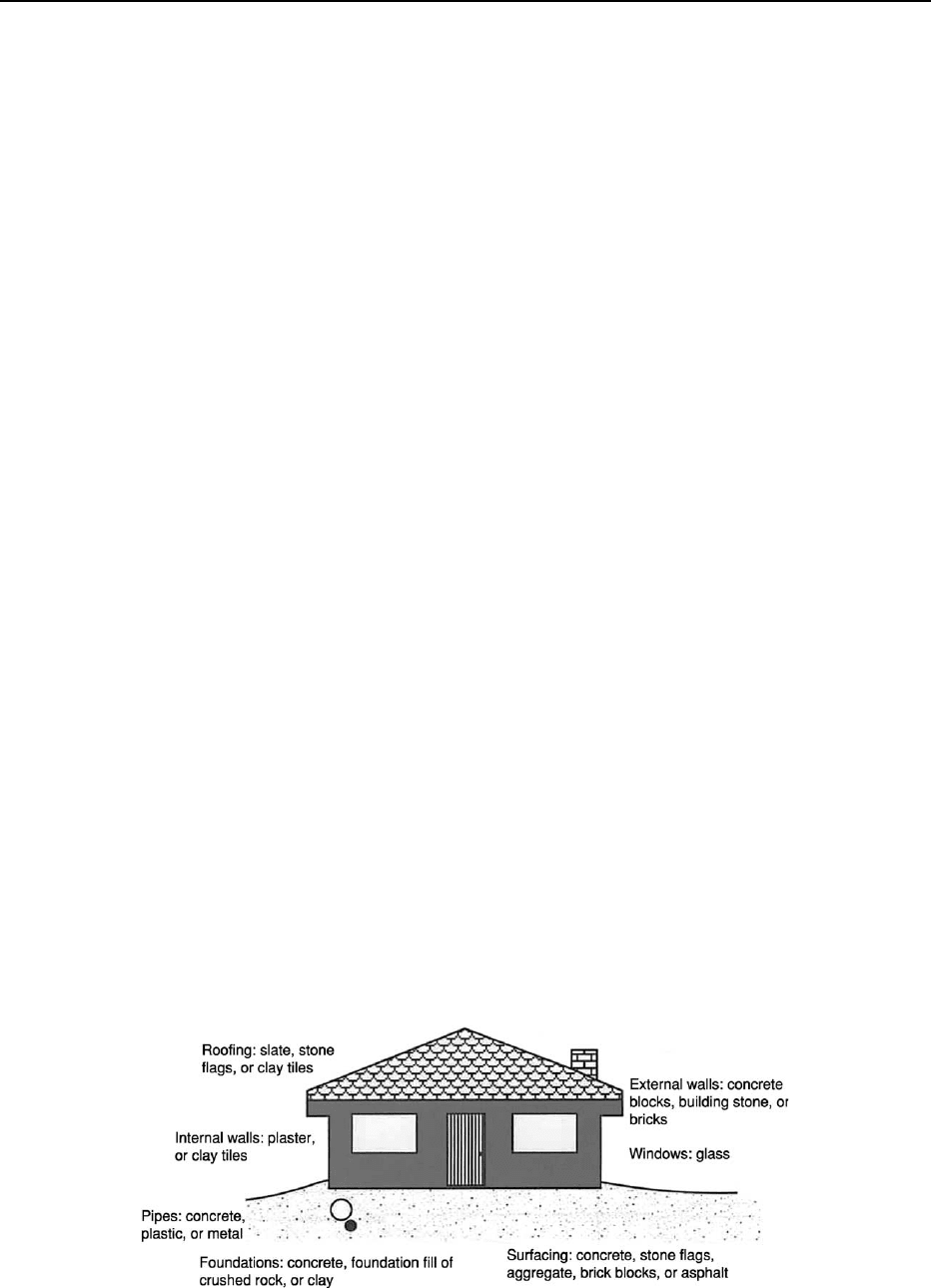
of many environmental issues. Built from geological
resources – geomaterials – towns and cities are in
constant need of renovation, renewal, and develop-
ment, with the consequent need for greater explor-
ation for aggregates, stone, and other materials
(Figure 4). Engineering geology is an important factor
in the construction of the built environment, particu-
larly in the provision of sound foundations. Many of
the older cities of the world are founded on ancient
settlement sites, and this in turn creates its own issues
of long-term stability.
Geomaterials
Construction materials, known as geomaterials, are
quarried and mined from a wide variety of settings.
The pressure to discover new resources is increasing,
as the pressure to house the world’s burgeoning popu-
lation also increases. Stone and clay for adobe bricks
have been the main building resources of the world
since antiquity, and concrete has a long and venerable
history. These materials are still in demand, and
quarrying for them creates much the same environ-
mental issues as any other type of extractive industry
(see Aggregates).
Another important role for environmental geolo-
gists is in assessing the appropriateness of geomater-
ials for the job for which they are intended. This
might include, for example: a durable, structurally
strong, easily worked, and attractive stone for con-
struction; large relatively uniform blocks of stone that
can take a high degree of pounding without breaking
down for use as armourstone blocks in coastal de-
fences; or the provision of aggregates for roads that
do not polish easily and can take a fair amount of
crushing from large vehicles. Durability of stone and
the effects of stone cleaning are major issues in some
of the major cities of the world, needing constant
monitoring (see Building Stone).
Figure 4 The main geomaterials and their uses. (Reproduced with permission from Bennett MR and Doyle P (1997) Environmental
Geology: Geology and the Human Environment.
Chichester: John Wiley.)
ENVIRONMENTAL GEOLOGY 29
