Ellis,J. Pressure transients in water engineering, A guide to analysis and interpretation of behaviour
Подождите немного. Документ загружается.

3.4 Variation of a with conduit shape
In the case of other cross-sectional shapes such as square ducts for
instance, matters are complicated by deformation from the original
shape under atmospheric conditions, as pressure is increased or
decreased. Thorley and Enever (1979) provide extensive information
on propagation speeds for a range of conduit shapes. The wave speed
remains constant for a given shape of liquid-filled pipe and a specific
value of D=s. They show a general equation for polygonal ducts:
1=A dA=dp ¼ 1=EfD=s þ tan
4
ðD=sÞ
3
=15g
þ tan
2
ðD=sÞ=ð2GÞð3:5Þ
where is the angle between flats, D is the distance between the flats, s
is wall thickness and G is the shear modulus of pipe wall material. It will
be noted that as the number of flats approaches 1 the shape becomes
increasingly circular, tends to zero and the expression 1=A dA=dp
approaches D=ðsEÞ.
3.5 Influence of gas on a
Thus far the composite wave speed a has remained independent of flow
conditions. Gases are present in the majority of liquids, both as free gas
in the form of bubbles and also as dissolved gas. As pressure is increased,
the amount of gas which can be dissolved also increases, thus reducing
the initial amount of free gas. According to Knapp et al. (1970),
dissolved gas has a negligible effect upon the tensile strength of a
liquid and thus will not significantly influence the acoustic wave
speed. However, the presence of free or undissolved air or gas within
the liquid body introduces a further component to the composite
medium which does have an important influence upon speed of pressure
waves. Deformation of the liquid—gas mixture with changing pressure is
greatly influenced by the much greater compressibility d
g
=dp of the gas
with
g
representing the gas density.
If a mixture of gases is in contact with the liquid surface, Henry’s law
states that the amount of each gas that will dissolve at equilibrium is
governed by its partial pressure. This can be written as: m
g
¼ p
s
,
where m
g
is the mass of gas dissolved/unit volume of liquid, is a
temperature-dependent solubility constant and p
s
is equilibrium
partial gas pressure (bar). Under conditions just described, maximum
or saturation concentration of air in water at normal temperature and
atmospheric pressure is just under 2%, of which one-third is oxygen
32
Pressure transients in water engineering

and two-thirds is nitrogen. Although its solubility is high, the amount of
CO
2
dissolved is very low since air contains only 0.03% of this gas. The
maximum air content < saturated concentration unless special treat-
ment has been carried out.
If pressure should fall, the liquid becomes supersaturated with gas for
that pressure and so some dissolved gas is released from solution
although it usually takes several seconds for a new equilibrium to be
reached. During a short-lived transient event equilibrium may not be
achieved. Gas release rate is greater than the corresponding gas absorp-
tion rate and so there is a net amount of free gas in the form of bubbles.
This release of gas is termed ‘gaseous cavitation’. If pressure drops below
the saturation pressure of liquid vapour, a very fast release occurs
termed vaprous cavitation within a few micro-seconds.
Expressing dissolved gas mass as an equivalent volume of gas Vol
g
under atmospheric pressure and at 08C, the following relationship is
obtained:
Vol
g
=Vol
L
¼ p
s
=1:013
where Vol
L
is liquid volume, is a solubility constant in units of volu-
metric concentration ¼0.0187 for air at 208C. Void fraction
2
can be
written:
2
¼ Vol
g
=ðVol
g
þ Vol
L
Þ
provided no slippage is assumed between bubbles and flowing liquid.
The notation
2
is proportional to absolute pressure. If saturated void
fraction
2
is about 2% at atmospheric pressure p
atm
, then at
1
2
p
atm
2
¼ 1% with the other 1% having come out of solution and
become free gas. As pressure drops, free bubbles of gas expand following
the gas law, aided in their growth by gas evolving from the now super-
saturated liquid and diffusing into the bubbles. Eventually a bubble
reaches a critical diameter when the differential pressure across the
bubble wall exceeds the surface tension force. Explosive growth in
bubble size then occurs with bubbles starting to rise towards the
crown of the pipe. Since the void fraction was related to a specific pres-
sure, usually atmospheric pressure, any gas which comes out of solution
at a lower pressure will have a volume given by the gas law:
p
abs
Vol
f
¼ constant
where is the ratio of specific heats for the gas and Vol
f
is the amount of
free or undissolved gas. Then, if the amount of gas coming out of
solution is
f
and the initial amount of free gas is
1
, both being
33
Interpretation of a
measured at atmospheric pressure p
atm
, then at some pressure p
abs
the
volume fraction will be:
¼ð
1
þ
f
Þðp
atm
=p
abs
Þ
ð1=Þ
It takes a finite time for a new equilibrium condition to become estab-
lished and so the amount of gas evolving from solution
f
will not
immediately be the total amount for the pressure p
abs
. The rate at
which gas is released from solution is dependent upon a number of
factors. Dissolved gas can only appear from solution if a ‘free phase’
boundary exists. This requires the presence of interstices in conduit
walls where free gas can lodge and/or particles within the liquid
which can act as ‘hosts’ for bubbles of free gas. Crevices and small
cracks in pipe walls are found, also micro-nuclei within the flow.
Keller and Zielke (1976) carried out filtering and rinsing of test
apparatus with filter size 0.5 mm. Despite this they found an average
of 2.8 nuclei/cm
2
with particles as large as 120 mm present. It is a
practical impossibility to completely clean pipes and tunnels of dust
particles. Turbulence can also influence the rates of gas release from
solution and some observations have shown that as Reynold’s
number (Rn) increases, the rate of release also rises and the time for
bubble content to stabilise falls. For an Rn of 370 000, bubble content
stabilises after 9 s whereas if Rn ¼ 590 000, bubble content is stabilised
after 5—6 s. Since the gas release rates depend on a range of factors, it is
not surprising to find alternative approaches being used by different
modellers. For example, Fox (1977) assumed that all dissolved gas
evolved from solution with no delay and that the liquid was initially
saturated with 2% gas at atmospheric pressure. Other investigators
have adopted an exponential gas release rate. What has been noted
is that including some air produces essentially the same result and
predictions were not very sensitive to the initial free gas content,
release rate or amount of gas released.
To illustrate the influence of free gas upon behaviour of the pressure
wave consider a liquid having total void fraction at the prevailing
absolute pressure p
abs
. Let the gas density be
g
with liquid density .
If the volume of free gas is Vol
f
and volume of liquid is Vol
L
, total
volume Vol ¼ Vol
f
þ Vol
L
. Mean density of the mixture
m
is given by:
m
¼ð
g
Vol
f
þ Vol
L
Þ=Vol
or
m
¼
g
þð1 Þ
34
Pressure transients in water engineering
Compressibility of liquid is represented by:
1=K ¼ 1= d=dp
and compressibility of gas by:
1=K
g
¼ 1=
g
d
g
=dp
Compressibility of the mixture can then be represented by:
=K
g
þð1 Þ=K
Substituting in the expression for wave speed a then for a circular pipe
section:
a ¼ 1=
p
f
m
½Dc
1
=ðsEÞþ=K
g
þð1 Þ=Kg ð3:6aÞ
Essentially the same equation has appeared in a number of guises.
Martin et al. (1976) assumed the contribution of the gas fraction to
mixture density was small and so:
m
ð1 Þ
giving
a ¼ 1=
p
fð1 Þ½Dc
1
=ðsEÞþ=K
g
þð1 Þ=Kg ð3:6bÞ
Tullis et al. (1976) assumed void fraction to be small giving
1 1 so that for a thin-walled pipe free to contract in length:
a ¼ 1=
p
fðD=ðsEÞþ=K
g
þ 1=KÞg ð3:6cÞ
The compressibility of gas represented by K
g
can be replaced as follows.
Applying the gas law to a fixed mass of free gas then:
p
abs
Vol
f
¼ constant
where ¼ C
p
=C
v
is the ratio of specific heats for the gas, C
p
is specific
heat at constant pressure, and C
v
is specific heat at constant volume.
All diatomic molecules of gas display the same value of ¼ 1:4at
moderate temperatures applicable for pipeline transients. Texts on
thermodynamics will yield additional details, for example Spalding
and Cole (1963). Differentiating with respect to Vol
f
then,
dp
abs
=dVol
f
¼ constant=Vol
ð1 þÞ
f
or
dp
abs
=p
abs
¼ dVol
f
=Vol
f
Also the mass of gas is given by:
g
Vol
f
¼ constant
35
Interpretation of a
Again, differentiating with respect to Vol
f
,
d
g
=dVol
f
¼constant=Vol
2
f
¼
g
=Vol
f
and rearranging,
d
g
=
g
¼dVol
f
=Vol
f
therefore,
dp
abs
=p
abs
¼ d
g
=
g
Gas compressibility is thus represented by:
1=K
g
¼ 1=
g
d
g
=dp
abs
¼ 1=ðp
abs
Þðdp
abs
=dp
abs
Þ¼1=ðp
abs
Þ
Making these substitutions the equation for wave speed is then:
a ¼ 1=
p
f½ð1 Þ þ
g
½Dc
1
=ðsEÞþ=ðp
abs
Þ
þð1 Þ=Kg ð3:7Þ
Fox (1977) neglected the gas mass contribution to mean density,
giving,
m
ð1 Þ and assumed 1 1 in the liquid compressi-
bility term, also ¼ 1:0, thus yielding the simpler expression:
a ¼ 1=
p
fð1 Þ½D=ðsEÞþ=p
abs
þ 1=Kg ð3:8Þ
This form of the equation for a perhaps highlights the fact that wave
speed is dependent upon absolute pressure p
abs
or has become a
function of the prevailing transient flow condition where free gas is
considered as a constituent of the fluid in the pipeline. Without the
influence of gas, the wave speed remains constant and independent
of flow conditions for a specified pipeline.
Acoustic velocity a is strongly dependent on void fraction . Even a
small amount of free gas produces a dramatic fall in wave speed. At the
opposite end of the scale, as void fraction tends to 100%, the wave
speed rises towards the speed of sound in air given by:
a ¼
p
fp
abs
=
g
g
Adopting standard values at atmospheric pressure and ambient
temperature,
g
¼ 1:22506 kg=m
3
, p
atm
¼ 101:3kN=m
2
and ¼ 1:4,
then the speed of sound in air is calculated as 340 m/s.
The gas release head is considered to be around 2.4 m absolute (Fox,
1977). Once the gas has evolved from solution it is more difficult to
force it back into solution by a simple rise to atmospheric pressure
and requires a more substantial pressure increase before much of the
gas will be forced to redissolve. Fox proposed that all gas came out of
36
Pressure transients in water engineering
solution at this gas release head and that equilibrium concentration was
achieved with no delay. Other modellers have adopted an exponential
decay of rate of gas release.
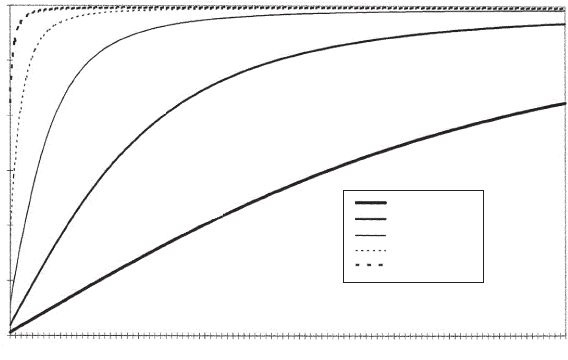
Figure 3.3 shows how wave speed changes with pressure for a range of
free gas concentrations. The gas fractions are those at atmospheric
pressure and range from 0.0001% to 1.0% by volume.
Adoption of a wave speed which varies with changing pressure
conditions within each pipeline of a network has important implications
with regard to the way in which computations are conducted. In the
presence of free gas the liquid in the pipeline system over most of the
range in free gas concentration has effectively become a fluid which
retains a density dominated by the weight of the liquid component
but with compressibility more like that of the gas. Only at the extremi-
ties of concentration range does this alter, with liquid properties
dominant when only very small amounts of gas are present, and gas
properties dominant if only quite small amounts of liquid are available.
Evolution of gas from solution and subsequent expansion of the free
gas has the effect of introducing an additional source of fluid to the
system. Suppose that upstream of a region in which free gas is present
and expanding, the flow is decelerating at some rate dV=dt. Down-
stream of the zone of gas expansion the flow will be decelerating at a
lesser rate as the flow from upstream will be augmented by liquid
released from the length of pipe containing the expanding gas in
37
0.1
0.5
0.9
1.3
1.7
2.1
2.5
2.9
3.3
3.7
4.1
4.5
4.9
5.3
5.7
6.1
6.5
6.9
7.3
7.7
8.1
8.5
8.9
9.3
9.7
10.1
Pressure (bar(abs))
Wave speed (m/s)
1200
1000
800
600
400
200
0
V
f
= 1%
V
f
= 0.1%
V
f
= 0.01%
V
f
= 0.001%
V
f
= 0.0001%
Fig. 3.3. Acoustic wave speed plotted against pressure for various void fractions
Interpretation of a
order to accommodate the increasing gas volume. The effect is to
reduce the downstream rate of deceleration and suppress to a significant
extent further pressure drop.
The equation for wave speed a in the presence of a free gas consti-
tuent has been developed on the basis of a homogeneous dispersal of
gas. Observations have revealed a large number of gas/liquid flow
regimes with no standard definition for each type of flow. Escarameia
(2005) mentions that up to 84 different terms have been in use and
illustrates some of the flow patterns for both horizontal and vertical
movement.
The present analysis has neglected ‘slip’ velocity between gas and
liquid and this can have significant implications in some circumstances.
Consider a descending section of pipeline carrying a range of bubble
sizes. The larger bubbles will tend to move more slowly — that is,
have a larger slip velocity than smaller bubbles. The smaller bubbles
will catch up and be absorbed by larger bubbles, resulting in still
larger and slower-moving bubbles. The large bubble may eventually
come to rest and start to move back upstream, collecting yet more
small bubbles and causing variations of void fraction not accommodated
in the simple theory presented. This type of behaviour can also result in
a pulsating type of flow behaviour.
A further departure arises when a gas/liquid regime such as the ‘slug’
flow approaches a local source of head loss such as a valve. The loss in
pressure, p, across the valve is given by:
p=ð
m
g Þ¼K
L
V
2
=ð2gÞ
When the mixture is predominantly liquid,
m
will be close to the
liquid density and p will be relatively large. If a ‘slug’ of gas should
arrive at the valve, the mixture density will fall and pressure drop p
will also drop proportionately, causing a pressure wave to propagate
from the valve into the pipeline. This process will occur each time a
‘slug’ of gas passes through the valve. This phenomenon is not accom-
modated by the straightforward theory.
3.6 The effect of sewage
Sewage is a generic term including the following:
. soil or sanitary sewage from households
. industrial wastewaters
. cooling water or ‘thermally’ contaminated water
38
Pressure transients in water engineering
. surface water from rainfall and storm water
. drainage water from building sites, fields and leakage from broken
pipes.
The temperature of sanitary sewage tends to be relatively uniform all
year round, usually between 108C and 218C. From 300 mg/l to 500 mg/l
of dissolved solids and 200 mg/l to 400 mg/l of suspended solids
including grease will have been added. Septic sanitary sewage may
under some circumstances produce hydrogen sulphide, which can be
corrosive to cement products such as concrete and asbestos cement
pipes. Industrial wastewater can vary considerably in temperature and
other physical characteristics and ranges from strongly acid to strongly
alkaline, requiring special materials for pipelines, pumps and fittings.
Dissolved and suspended solids may conceivably affect the density
and viscosity of the fluid. Where solid particles or larger aggregations
of matter are present in sewage this adds a further component to the
medium. Depending upon the density and compressibility of this
material carried along in the liquid there may be an effect upon the
acoustic wave speed. Generally, with both a free gas component and
solid matter present, then the average density of the medium will be:
m
¼ðVol
L
þ
g
Vol
g
þ
s
Vol
s
Þ=ðVol
L
þ Vol
g
þ Vol
s
Þ
if Vol
L
þ Vol
f
þ Vol
s
¼ Vol
t
, Vol
f
=Vol
t
¼ and Vol
s
=Vol
t
¼
s
then:
m
¼ð1
s
Þ þ
g
þ
s
s
Average compressibility becomes:
ð1
s
Þ= d=dp þ
g
=
g
d
g
=dp þ
s
=
s
d
s
=dp
¼ð1
s
Þ=K þ =K
g
þ
s
=K
s
¼ð1
s
Þ=K þ =ðp
abs
Þþ
s
=K
s
then
a ¼ 1=
p
f
m
½ð1
s
Þ=K þ =ðp
abs
Þ
þ
s
=K
s
þ Dc
1
=ðsEÞg ð3:9Þ
The manner in which K
s
changes with pressure will depend upon
constituents of the sewage. If compressibility is relatively low, like
water, then
s
=K
s
constant.
Some effluent pipelines are designed to convey sewage of a particular
type, for example from a residential area, and the solids content may be
assessed on this basis. Other lines may carry mixtures having a wider
39
Interpretation of a
range of properties from domestic and industrial wastes to stormwater
flows. Properties of the mixture will vary significantly and it is recom-
mended that investigations should include properties of stormwater
when flows may well be at a maximum. Under dry weather conditions,
primarily domestic and/or industrial waste waters may be carried. While
mean daily flows may be relatively modest, these may be conveyed in
the form of intermittent ‘plug’ flows in which discharge rates are close
to the pumps’ duty point. It may well be necessary in such circum-
stances to investigate flows involving different mixture properties.
40
Pressure transients in water engineering
4
Characteristic equations
Chapter 3 discussed how the acoustic wave speed in a pressure pipeline
system can be influenced by the properties of both the pipeline and by
any inclusions in water being conveyed. As far as sewerage systems are
concerned, a large part of the function of the water is to carry contami-
nants and impurities to treatment or disposal and it is to be expected
that both gas and some solid constituents will be present. This
chapter uses the partial differential equations together with the acoustic
wave speed a, to develop what are known as characteristic equations.
4.1 Development of characteristic equations
The force balance equation was derived in Chapter 2 as:
@V=@t þ V @V=@x þ g @H=@x þ gS ¼ 0 ð2:5Þ
and the equation balancing mass flow and storage became:
a @V=@x þ g=af@H=@t þ V @H=@xgg=aV dz=dx ¼ 0 ð2:7Þ
It is possible to proceed directly to the solution of pressure transient
flow problems using the above equations. However, many existing
modelling tools employ what are known as the ‘characteristic’ equations
and a simple derivation of these equations from the basic force and
mass balance equations (2.5) and (2.7) follows. These characteristic
equations have been used throughout this book to illustrate various
aspects of pressure transient behaviour.
Adding equations (2.5) and (2.7) balancing force and mass flow then:
@V=@t þðV þ aÞ@V=@x þ g=að@H=@t þðV þ aÞ@H=@xÞ
þ gS g=aV dz=dx ¼ 0
41
