Duan C.G., Karelin V.Y. Abrasive Erosion and Corrosion of Hydraulic machinery
Подождите немного. Документ загружается.

374 Abrasive Erosion and Corrosion of Hydraulic Machinery
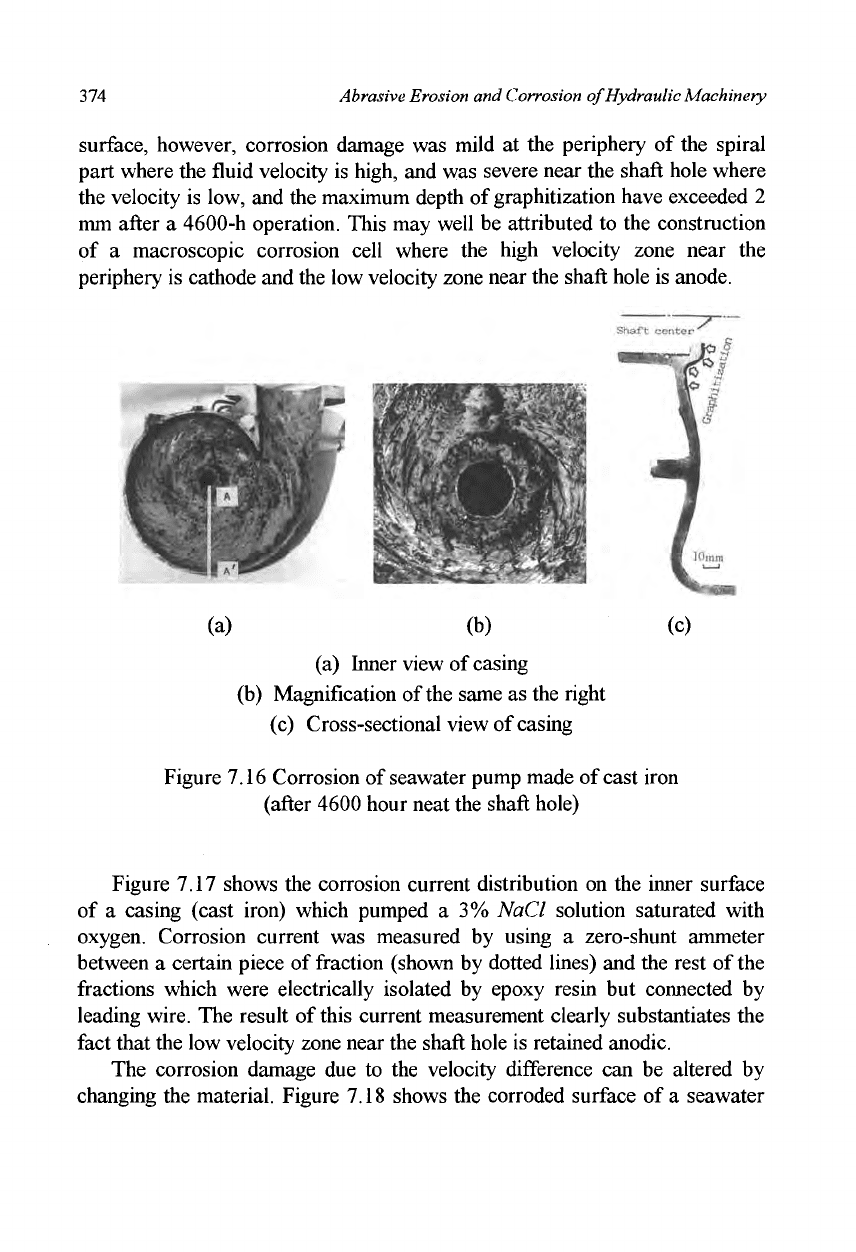
surface, however, corrosion damage was mild at the periphery of the spiral
part where the fluid velocity is high, and was severe near the shaft hole where
the velocity is low, and the maximum depth of graphitization have exceeded 2
mm after a 4600-h operation. This may well be attributed to the construction
of a macroscopic corrosion cell where the high velocity zone near the
periphery is cathode and the low velocity zone near the shaft hole is anode.
(a) (b) (c)
(a) Inner view of casing
(b) Magnification of the same as the right
(c) Cross-sectional view of casing
Figure 7.16 Corrosion of seawater pump made of cast iron
(after 4600 hour neat the shaft hole)
Figure 7.17 shows the corrosion current distribution on the inner surface
of a casing (cast iron) which pumped a 3% NaCl solution saturated with
oxygen. Corrosion current was measured by using a zero-shunt ammeter
between a certain piece of fraction (shown by dotted lines) and the rest of the
fractions which were electrically isolated by epoxy resin but connected by
leading wire. The result of this current measurement clearly substantiates the
fact that the low velocity zone near the shaft hole is retained anodic.
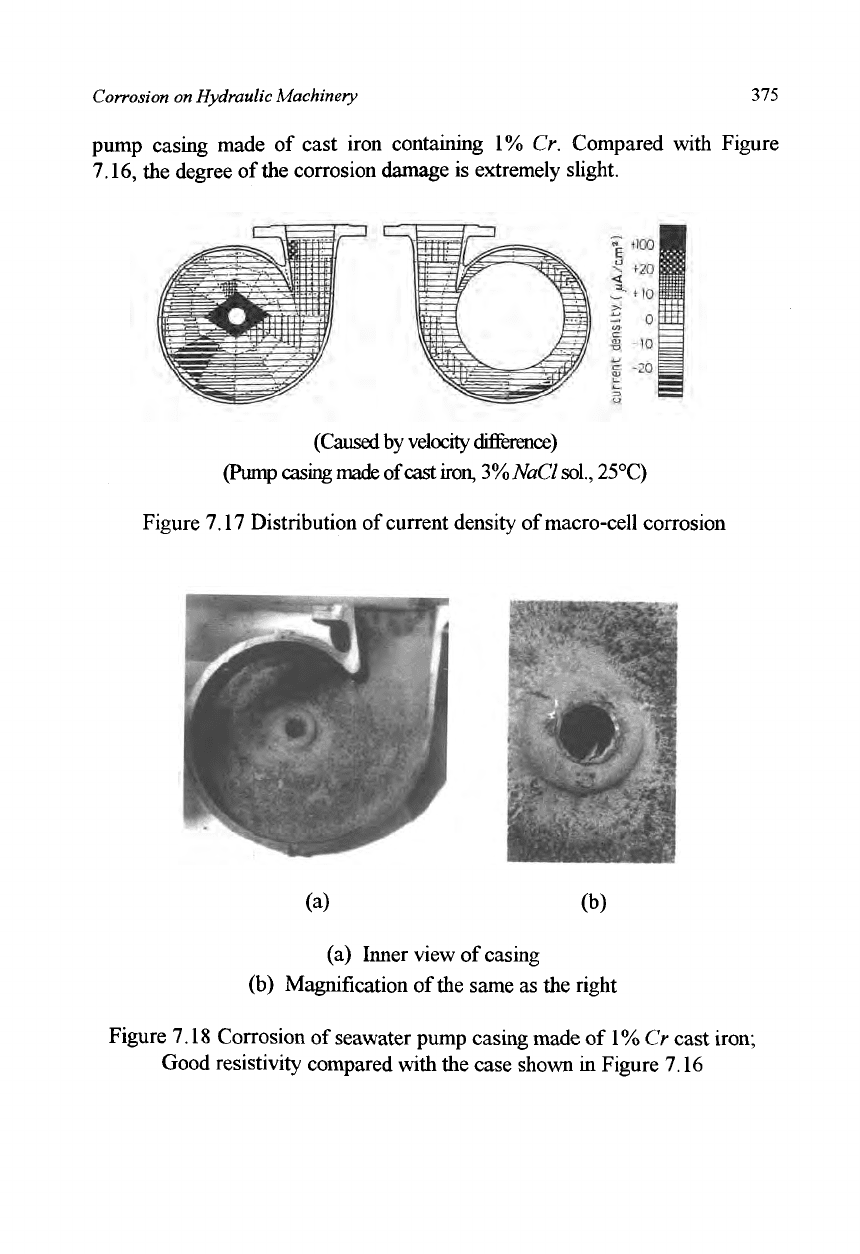
The corrosion damage due to the velocity difference can be altered by
changing the material. Figure 7.18 shows the corroded surface of a seawater
Corrosion on Hydraulic Machinery
375
pump casing made of cast iron containing 1% Cr. Compared with Figure
7.16, the degree of the corrosion damage is extremely slight.
(Caused
by velocity
difference)
(Pump
casing made
of cast
iron,
3%NaCl
sol.,
25°C)
Figure 7.17 Distribution of current density of macro-cell corrosion
(a) (b)
(a) Inner view of casing
(b) Magnification of the same as the right
Figure 7.18 Corrosion of seawater pump casing made of 1% Cr cast iron;
Good resistivity compared with the case shown in Figure 7.16
376
Abrasive Erosion and Corrosion
of
Hydraulic Machinery
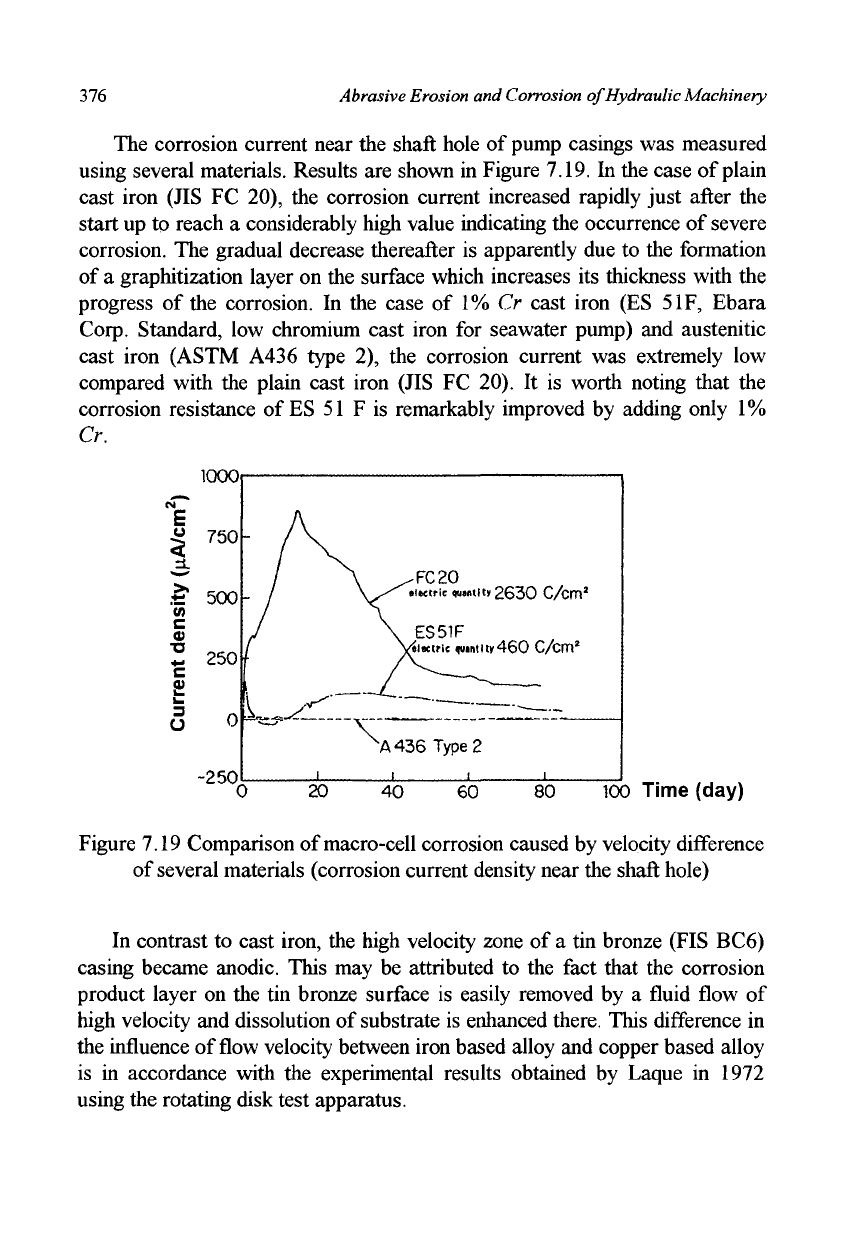
The corrosion current near the shaft hole of pump casings was measured
using several materials. Results are shown in Figure 7.19. In the case of plain
cast iron (JIS FC 20), the corrosion current increased rapidly just after the
start up to reach a considerably high value indicating the occurrence of severe
corrosion. The gradual decrease thereafter is apparently due to the formation
of a graphitization layer on the surface which increases its thickness with the
progress of the corrosion. In the case of 1% Cr cast iron (ES
5
IF, Ebara
Corp.
Standard, low chromium cast iron for seawater pump) and austenitic
cast iron (ASTM A436 type 2), the corrosion current was extremely low
compared with the plain cast iron (JIS FC 20). It is worth noting that the
corrosion resistance of ES 51 F is remarkably improved by adding only
1
%
Cr.
1000
E
o
£ 500
>.
♦■»
'55
0)
■o
O
750-
Z 250
0
-250.
.
L
..^
i
/
.-FC20
tteetric quantfty
\ ES51F
V^laetrlc
quint
It
\\436
Type
2
i i ...
2630 C/cm
2
,460 C/cm
2
—-
i
20
40
60
80
100 Time (day)
Figure 7.19 Comparison of macro-cell corrosion caused by velocity difference
of several materials (corrosion current density near the shaft hole)
In contrast to cast iron, the high velocity zone of a tin bronze (FIS BC6)
casing became anodic. This may be attributed to the fact that the corrosion
product layer on die tin bronze surface is easily removed by a fluid flow of
high velocity and dissolution of substrate is enhanced there. This difference in
the influence of flow velocity between iron based alloy and copper based alloy
is in accordance with the experimental results obtained by Laque in 1972
using the rotating disk test apparatus.

Corrosion on Hydraulic Machinery 377
7.3.2 Corrosion Promoted by Mixed Use of Different Materials
(Galvanic Corrosion)
Initially, use of stainless steel in large-sized seawater pumps was confined to
some limited parts only. The remaining parts of the pump were of plain cast
iron which acted as a sacrificial anode to protect the stainless steel parts from
corrosion. This led people to the unfortunate misunderstanding that stainless
steel was a perfect material for use in seawater, which further accelerated the
use of stainless steel in seawater pumps. The increase of the surface area of
stainless steel (cathode) and the decrease of the surface area of cast iron
(anode) increased the rate of corrosion on the cast iron parts, which made cast
iron lose its reputation as a construction material. Pump designers and users
made another mistake in introducing tar-epoxy coating in order to reduce the
corrosion damage. If they had had right knowledge of corrosion theory, they
would have coated the stainless steel parts in order to reduce the cathode area.
They chose, however, to do the opposite; the cast iron was coated and the
stainless steel remained uncoated. What was worse, those portions of
the
cast
iron parts which were in direct contact with stainless steel, e.g., fringe surface
or faucet joint surfaces, remained uncoated in order to maintain precise
measurements. As a result, corrosion currents concentrated on the uncoated
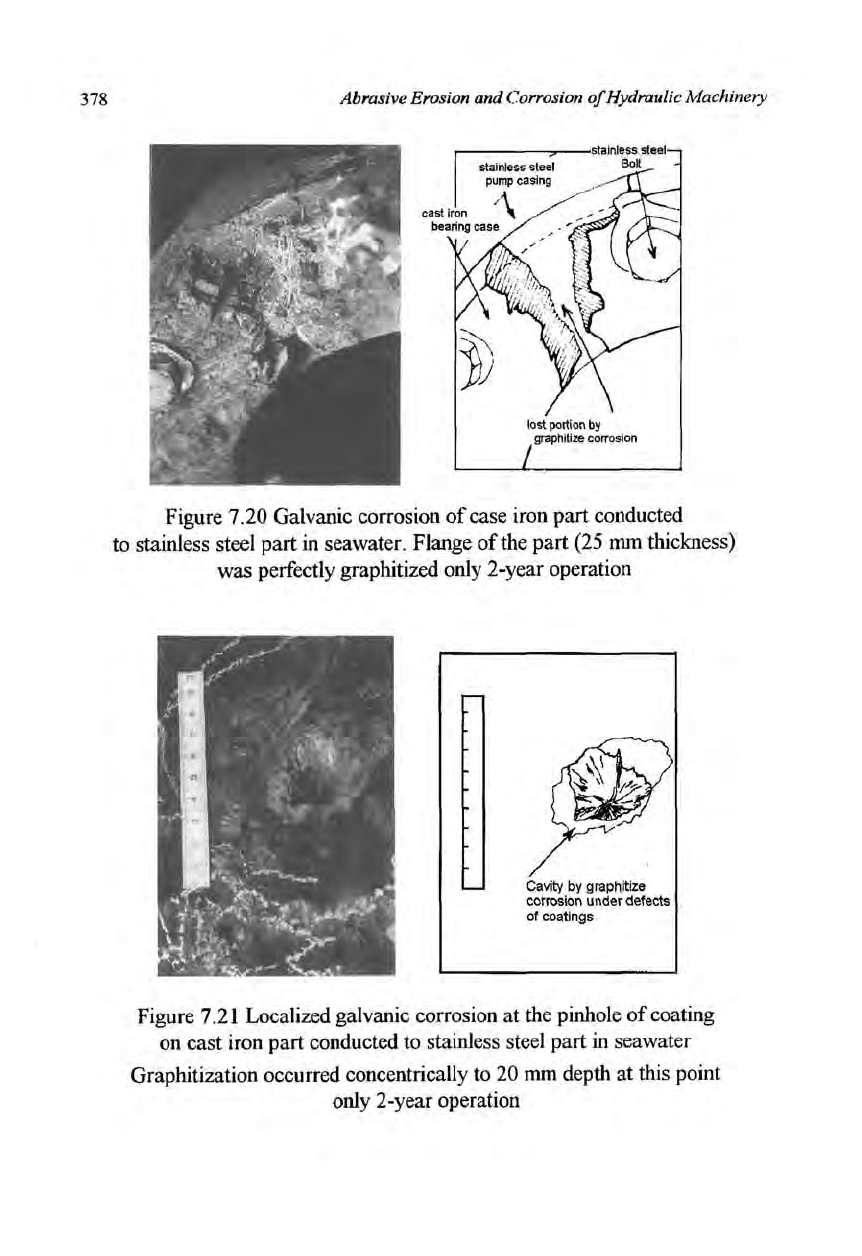
surface causing decisive damage there in quite a short period (Figure 7.20).
Pinholes on the coating layer also brought about severe damage (Figure 7.21).
Recently, people have gradually obtained a correct understanding of the
problem and have begun to devise adequate means for its prevention. Figure
7.22 shows an example of such countermeasures; an isolating phenol resin
plate as well as isolating bolts and nuts used between the stainless steel parts
and plain cast iron parts.
The possibility of galvanic corrosion caused by the coupling of materials
other than stainless steel and cast iron has of course to be examined before
any combination is put into use. Various kinds of seawater corrosion tests
were conducted from 1977 for five years at several power plants located
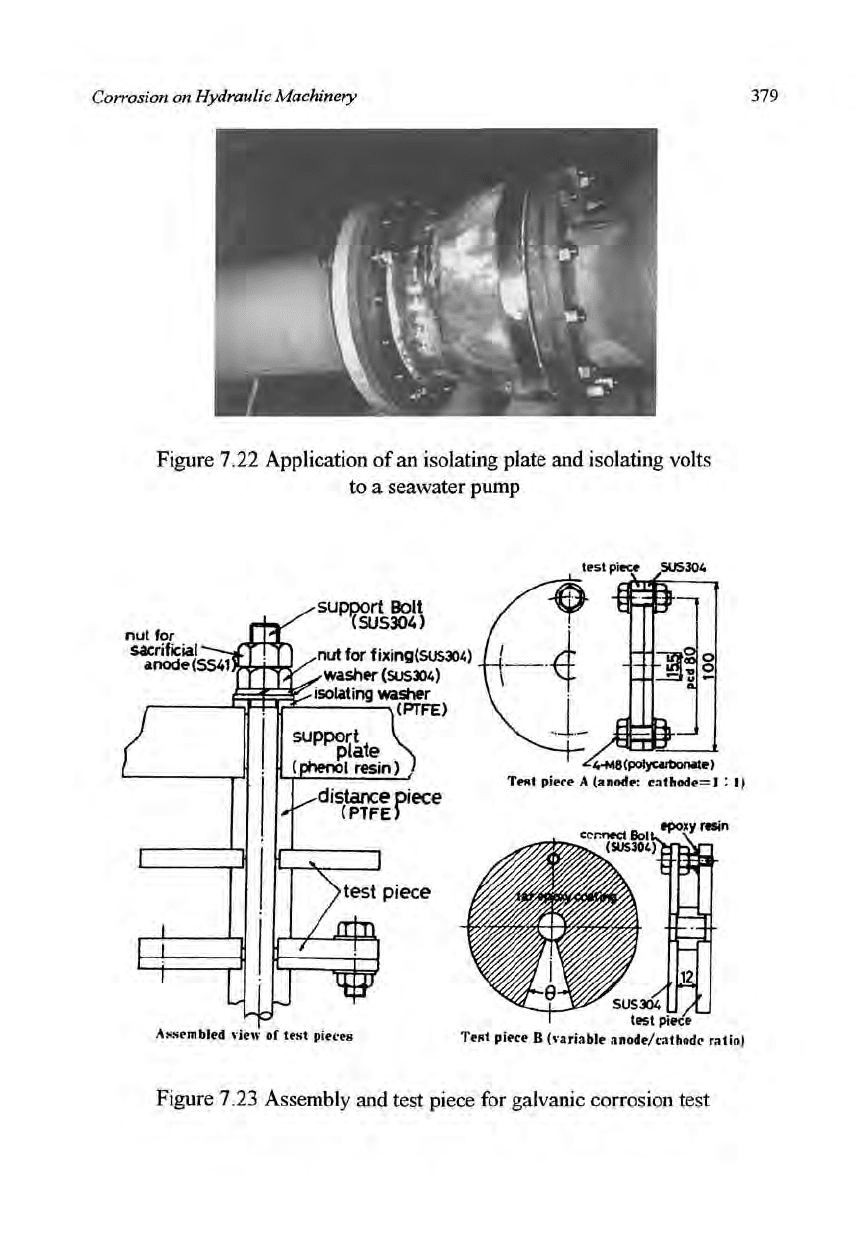
along the coast Japan. Figure 7.23 shows the assembly and test piece for one
of the above mentioned galvanic corrosion tests; in test piece A, the
anode/cathode ratio was 1/1, and was variable in test piece B through the
regulating of
angle
0.
378
Abrasive Erosion and Corrosion of Hydraulic Machinery
Figure 7.20 Galvanic corrosion of case iron part conducted
to stainless steel part in seawater. Flange of the part (25 mm thickness)
was perfectly graphitized only 2-year operation
Cavity by graphitize
corrosion under defects
of coatings
Figure 7.21 Localized galvanic corrosion at the pinhole of coating
on cast iron part conducted to stainless steel part in seawater
Graphitization occurred concentrically to 20 mm depth at this point
only 2-year operation
Corrosion on Hydraulic Machinery
379
Figure 7.22 Application of an isolating plate and isolating volts
to
a
seawater pump
lest piece SUS304
nut for
sacrificial •
anode(SS4l2
support Bolt
(SUS304)
nut for f ixing(SUS304)
J/
^washer (sussw)
isolating washer
v(PTFE)
(phenol resin) /
distance piece
(PTFE)
test piece
'4-M8 (polycarbonate)
Test piece
A
(anode: cnthode=l
: I)
•poxy resin
Assembled view
of
test pieces
SUS3<xU/L
test piece
Test piece
B
(variable anode/cathode ratio)
Figure 7.23 Assembly and test piece for galvanic corrosion test
380
Abrasive Erosion and Corrosion of Hydraulic Machinery
In the case of 1/1 ratio coupling of tin bronze (JIS BC 6) and stainless
steel (JIS SUS 304), the former was anodic and its corrosion rate was
increased to 1-6 times that of it's isolated condition. The corrosion rate ranged
from 0.07 to 0.45 mm/year. In the coupling of aluminum bronze (JIS A1BC 2
or A1BC3) and stainless steel (JIS SUS 304), the bronze was anodic and its
corrosion rate was increased to 1 -3.7 times that of it's isolated condition.
The corrosion rate was 0.02 ~ 0.2 mm/year. A decrease in anode/cathode
ratio brought about some crevice corrosion on the stainless at contact surface.
By the coupling of austenitic cast iron and plain cast iron, the austenitic
cast iron acted as cathode to promote corrosion in the plain cast iron (see the
first column of Table 7.2). On the other hand, the austenitic cast iron was
seriously damaged when it was coupled with stainless steel as shown in the
last column of Table 7.2.
Table 7.2 Galvanic corrosion in natural seawater at several points
throughout Japan (Anode/Cathode =1)
Geographical
location
Tomakomai
Sendai
Fukushima
Kashima
Chiba-1
Chiba-2
Naooya
Hlmeji
Fukuyama
Tokuyama
Kokura
5 ita-1
0 ita-2
Naoasaki
FC20/A436 type2
0.87 mm/Y
0.65
0.38
0.45
0.83
0.83
0.29
0.38
0.44
1.37
0.42
0.46
1.15
0.74
Galvanic
couples
FC20/SUS304
1.12 mm/Y
0.76
0.49
0.56
0.79
0.80
0.29
0.38
0.41
1.43
0.41
0.43
1.18
0.99
A436 type2/SUS304
0.95 mm/Y
0.50
0.35
0.35
0.48
0.49
0.17
0.19
0.18
0.63
0.21
0.14
0.39
0.27
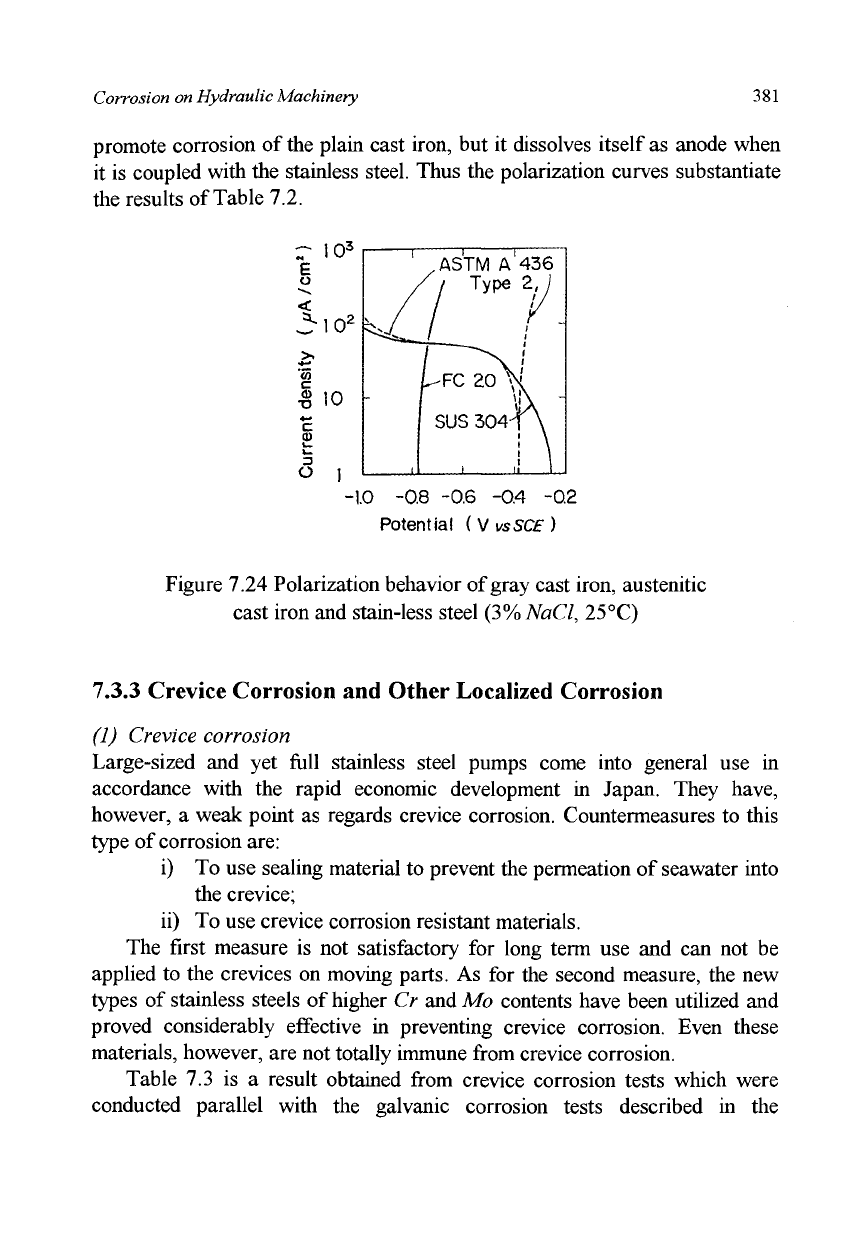
Polarization curves for these materials in a
3%
NaCl solution are given in
Figure 7.24 which indicates that the austenitic cast iron acts as cathode to
Corrosion on Hydraulic Machinery
381
promote corrosion of the plain cast iron, but it dissolves itself as anode when
it is coupled with the stainless steel. Thus the polarization curves substantiate
the results of Table 7.2.
-1.0 -0.8 -0.6 -0.4 -0.2
Potential ( V
vsSCE
)
Figure 7.24 Polarization behavior of gray cast iron, austenitic
cast iron and stain-less steel (3%NaCl, 25°C)
7.3.3 Crevice Corrosion and Other Localized Corrosion
(I) Crevice corrosion
Large-sized and yet full stainless steel pumps come into general use in
accordance with the rapid economic development in Japan. They have,
however, a weak point as regards crevice corrosion. Countermeasures to this
type of corrosion are:
i) To use sealing material to prevent the permeation of seawater into
the crevice;
ii) To use crevice corrosion resistant materials.
The first measure is not satisfactory for long term use and can not be
applied to the crevices on moving parts. As for the second measure, the new
types of stainless steels of higher Cr and Mo contents have been utilized and
proved considerably effective in preventing crevice corrosion. Even these
materials, however, are not totally immune from crevice corrosion.
Table 7.3 is a result obtained from crevice corrosion tests which were
conducted parallel with the galvanic corrosion tests described in the
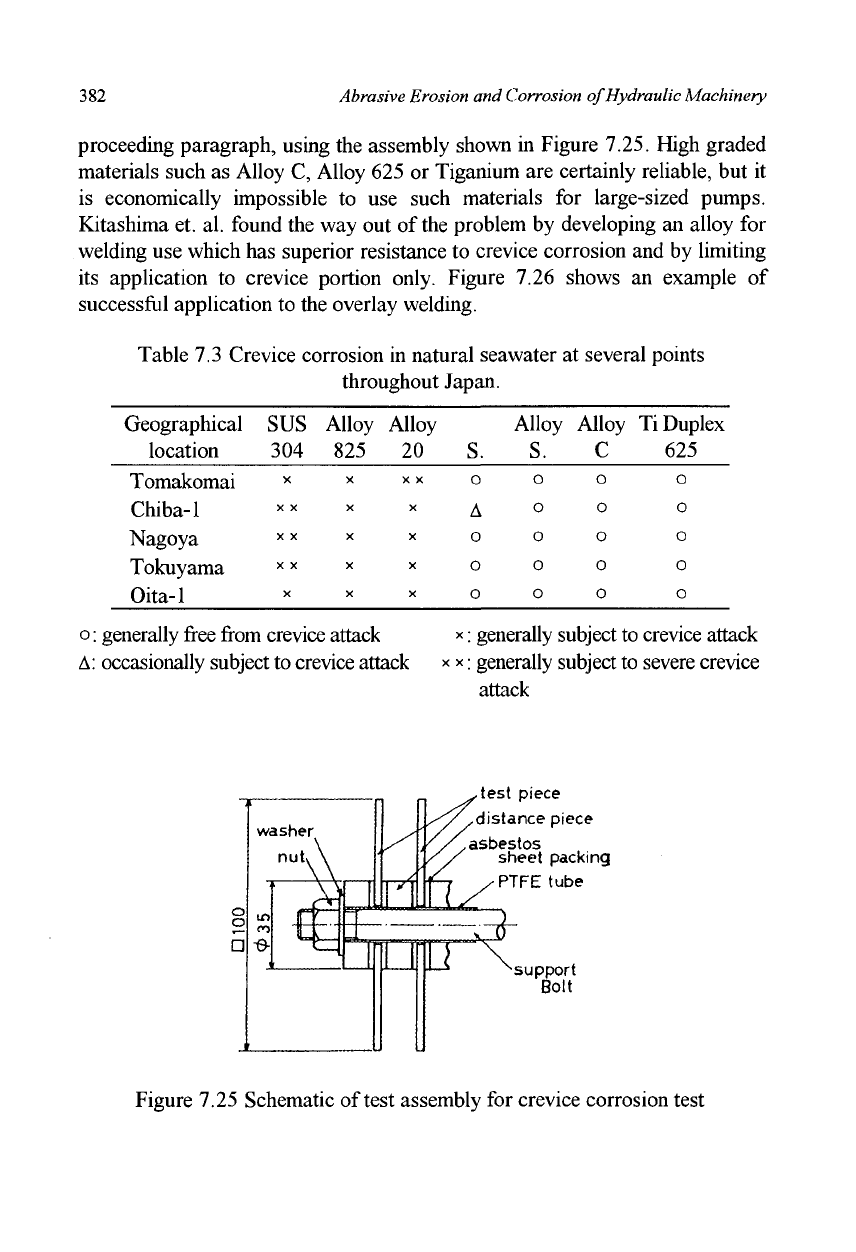
382
Abrasive Erosion and Corrosion of Hydraulic Machinery
proceeding paragraph, using the assembly shown in Figure 7.25. High graded
materials such as Alloy C, Alloy 625 or Tiganium are certainly reliable, but it
is economically impossible to use such materials for large-sized pumps.
Kitashima et. al. found the way out of
the
problem by developing an alloy for
welding use which has superior resistance to crevice corrosion and by limiting
its application to crevice portion only. Figure 7.26 shows an example of
successful application to the overlay welding.
Table 7.3 Crevice corrosion in natural seawater at several points
throughout Japan.
Geographical
location
Tomakomai
Chiba-1
Nagoya
Tokuyama
Oita-1
sus
304
X
X X
X X
X X
X
Alloy Alloy
825
X
X
X
X
X
20
X X
X
X
X
X
s.
o
A
o
O
O
Alloy Alloy
S.
o
o
o
o
o
C
o
o
o
o
o
Ti Duplex
625
o
o
o
o
o
o: generally free from crevice attack
A: occasionally subject to crevice attack
x: generally subject to crevice attack
x: generally subject to severe crevice
attack
test piece
distance piece
asbestos
sheet packing
PTFE tube
support
Bolt
Figure 7.25 Schematic of test assembly for crevice corrosion test

Corrosion on Hydraulic Machinery 383
(a) Flange surface of column pipe (base material; SUS316L)
(b) Flange surface of intermediate bearing support (base material; SCS 14
solution heat treatment after overlay).
Figure 7.26 Parts of seawater pump weld overlaid with corrosion resistant
alloy (Ni 30%
Cr-10%
Mo alloy) after
1
year operation.
(2) Corrosion of weld metal:
A similar material to the base metal is usually used for plugging welding of
the vent hole or for repairing welding of the defects in castings. Unexpected
damage can occur even in these weldings. Figure 7.27 (a) shows an example
of severe corrosion damage to the deposit metal at the plugging welding. The
base metal was type 304-cast stainless steel (JIS SCS 13) and the
environment was a dense saline solution of 70 ~ 90°Ccontaining solid
particles. Investigations revealed that the microstructure of the deposit metal
was normal and there was no sign of sensitization as shown in Figure 7.27
(b).
A little difference, however, was found in chemical composition between
the base metal and the deposit metal; deference
in
Mo content should be noted
in Table 7.4 which shows the chemical composition of the metals, i.e., the Mo
content of the deposit metal (0.006%) is lower than that of the base metal
(0.67%).
The contents of other elements were quite normal for type 304
stainless steel, specifically; levels of Ni and Cr were rather higher than in the
base metal.
Test specimens with an exposure surface of 2 cm
2
were prepared from
each metal and dipped into a pseudo-environmental liquid (without solid
particles) in order to measure the potential as well as the galvanic current
