Duan C.G., Karelin V.Y. Abrasive Erosion and Corrosion of Hydraulic machinery
Подождите немного. Документ загружается.

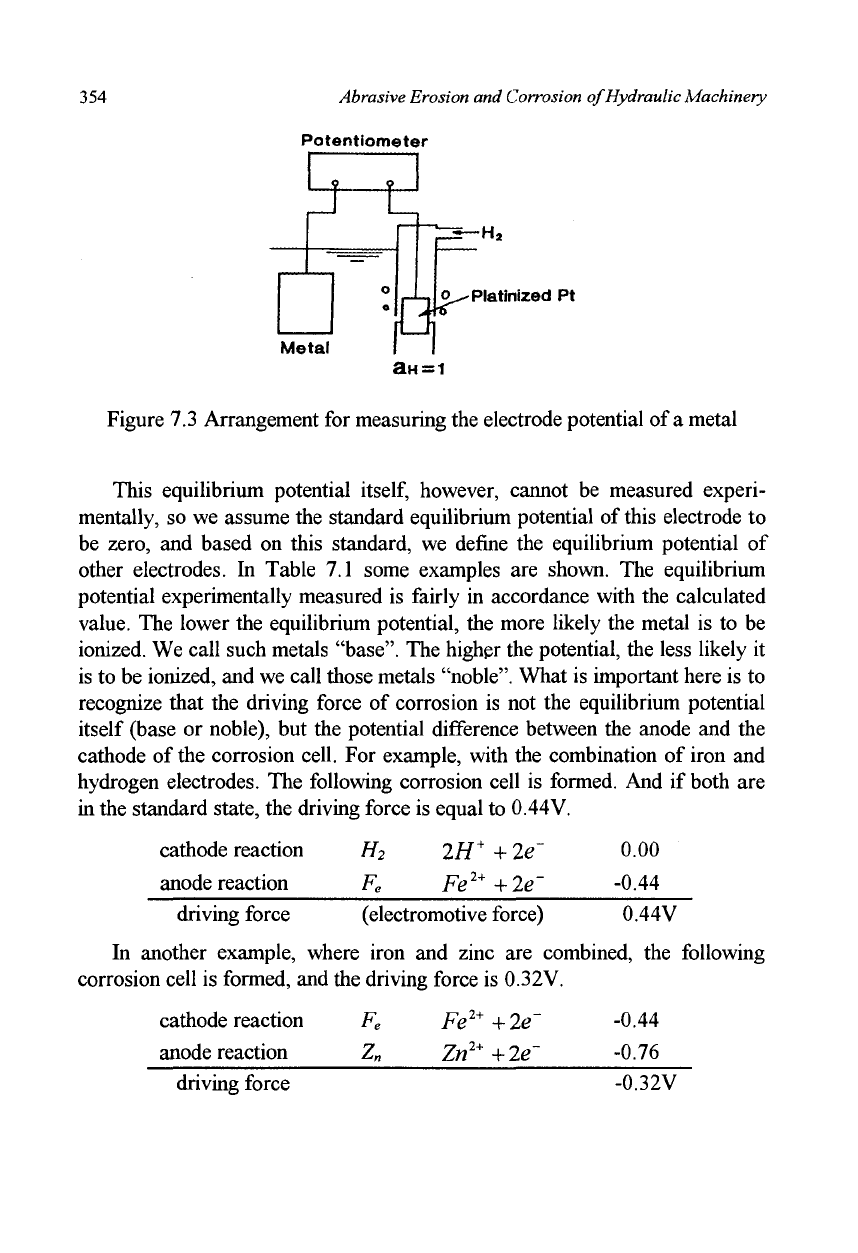
354 Abrasive Erosion and Corrosion of Hydraulic Machinery
Potentiometer
9 9
Metal
—
O
o
x
Figure 7.3 Arrangement for measuring the electrode potential of a metal
This equilibrium potential
itself,
however, cannot be measured experi-
mentally, so we assume the standard equilibrium potential of this electrode to
be zero, and based on this standard, we define the equilibrium potential of
other electrodes. In Table 7.1 some examples are shown. The equilibrium
potential experimentally measured is fairly in accordance with the calculated
value. The lower the equilibrium potential, the more likely the metal is to be
ionized. We call such metals "base". The higher the potential, the less likely it
is to be ionized, and we call those metals "noble". What is important here is to
recognize that the driving force of corrosion is not the equilibrium potential
itself (base or noble), but the potential difference between the anode and the
cathode of the corrosion cell. For example, with the combination of iron and
hydrogen electrodes. The following corrosion cell is formed. And if both are
in the standard state, the driving force is equal to 0.44V.
cathode reaction H
2
2H
+
+ 2e~ 0.00
anode reaction F
e
Fe
2+
+2e~ -0.44
driving force (electromotive force) 0.44V
In another example, where iron and zinc are combined, the following
corrosion cell is formed, and the driving force is 0.32V.
cathode reaction F
e
Fe
2+
+2e~ -0.44
anode reaction Z„ Zn
2+
+2e~ -0.76
driving force
-0.32V
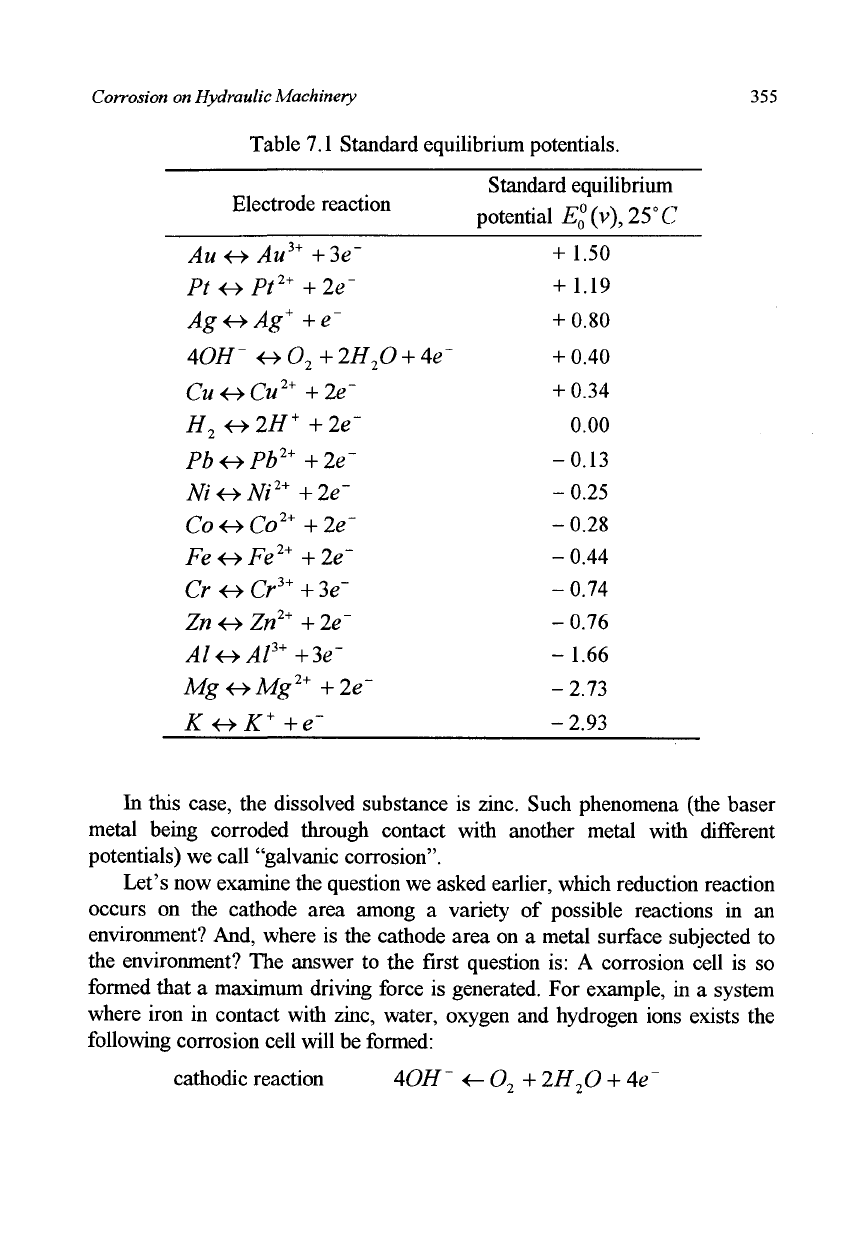
Corrosion on Hydraulic Machinery
355
Table 7.1 Standard equilibrium potentials.
Electrode reaction
Au <-> Au
i+
+3e~
Pt
<^Pt
2+
+
2e~
Ag<r>Ag
+
+e~
40H~ <H>0
2
+2H
2
0
+ 4e~
Cu<^Cu
2+
+2e~
H
2
<^2H
+
+
2e~
Pb<r>Pb
2+
+2e-
M<^iV/
2+
+2e-
Co<^Co
2+
+2e'
Fe<r>Fe
2+
+2e'
Cr<H>Cr
3+
+3e~
Zn o Zn
2+
+ 2e~
Al<^Al
3+
+3e~
Mg<r*Mg
2+
+2e~
K
<r>K
+
+e~
Standard equilibrium
potential £
0
°(v), 25°C
+ 1.50
+ 1.19
+ 0.80
+ 0.40
+ 0.34
0.00
-0.13
-0.25
-0.28
-0.44
-0.74
-0.76
-1.66
-2.73
-2.93
In this case,
the
dissolved substance
is
zinc. Such phenomena
(the
baser
metal being corroded through contact with another metal with different
potentials) we call "galvanic corrosion".
Let's now examine the question we asked earlier, which reduction reaction
occurs
on the
cathode area among
a
variety
of
possible reactions
in an
environment? And, where
is the
cathode area
on a
metal surface subjected
to
the environment?
The
answer
to the
first question
is: A
corrosion cell
is so
formed that
a
maximum driving force
is
generated.
For
example,
in a
system
where iron
in
contact with zinc, water, oxygen
and
hydrogen ions exists
the
following corrosion cell will
be
formed:
cathodic reaction
40H <- 0
2
+
2H
2
0
+ 4e
356
Abrasive Erosion and Corrosion of Hydraulic Machinery
anodic reaction Zn
—>
Zn
+
+ 1e~
The cathodic reaction occurs on the iron surface. The possibility of
hydrogen reduction reaction occurrence depends on the polarization which
will be described in the next section.
The answer to the second question is that real metals are heterogeneous.
For example, in the case of cast iron, the graphite carbon acts as cathode, the
ferrite acts as anode, and thus a corrosion cell is formed. As corrosion
proceeds, the ferrite dissolves out and a skeleton of graphite is left (This
process is called "graphite corrosion"). Mild steel consists of pearlite and
ferrite. Even in single component metals, the dissolubility is greatly influenced
by which plane of the crystal is exposed as surface. When there is either strain
of residual stress on the crystals, a difference of free energy is generated. This
results in a potential difference between the stressed area and other areas.
7.1.3 Polarization
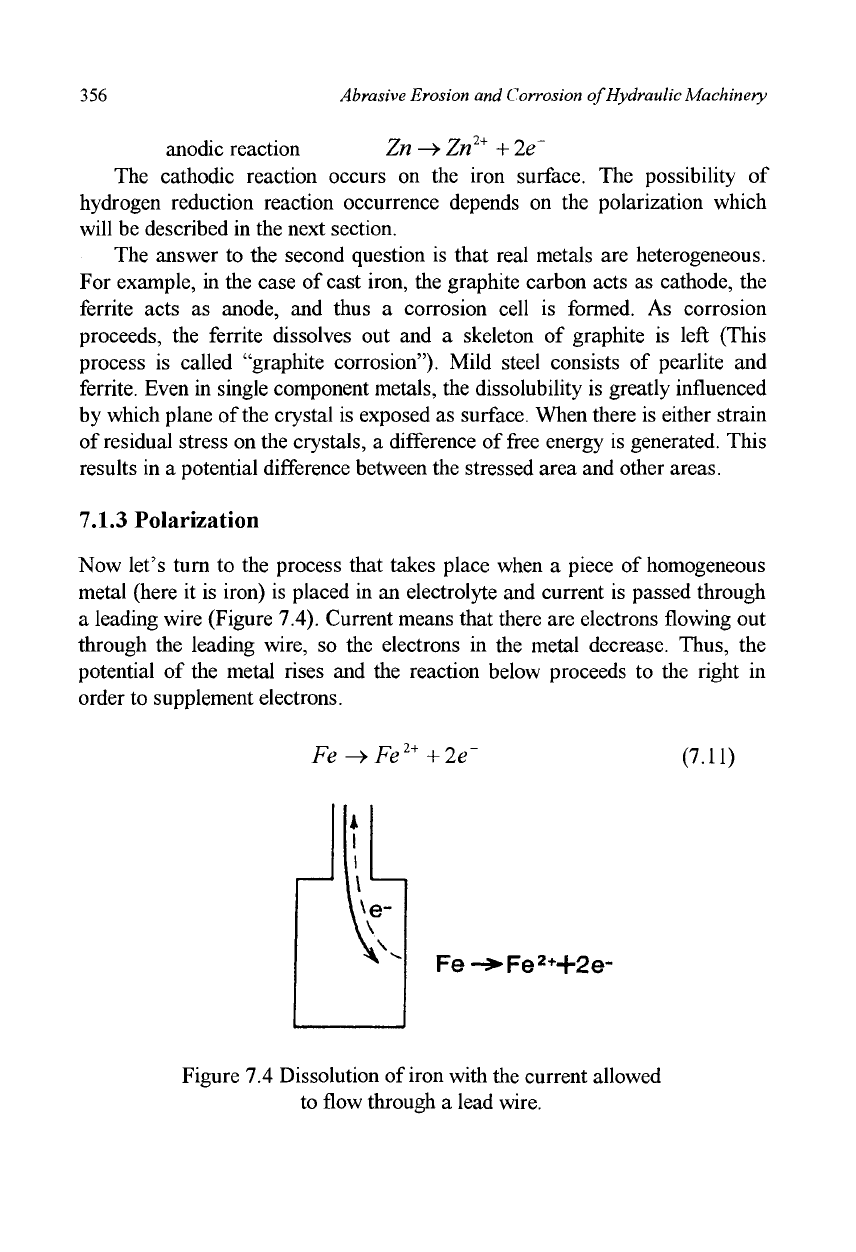
Now let's turn to the process that takes place when a piece of homogeneous
metal (here it is iron) is placed in an electrolyte and current is passed through
a leading wire (Figure 7.4). Current means that there are electrons flowing out
through the leading wire, so the electrons in the metal decrease. Thus, the
potential of the metal rises and the reaction below proceeds to the right in
order to supplement electrons.
Fe->Fe
2+
+2e~ (7.11)
i
Fe-*>Fe
2+
+2e-
Figure 7.4 Dissolution of iron with the current allowed
to flow through a lead wire.
^
Corrosion on Hydraulic Machinery 357
In other words, when current flows into the metal, the potential of that
metal rises higher and anodic current flows from the metal to the electrolyte.
On the other hand, when current flows out from the metal through the leading
wire,
electrons are flowing into the metal and the potential of the metal goes
down. The reaction above then proceeds to the left. In other words, a cathodic
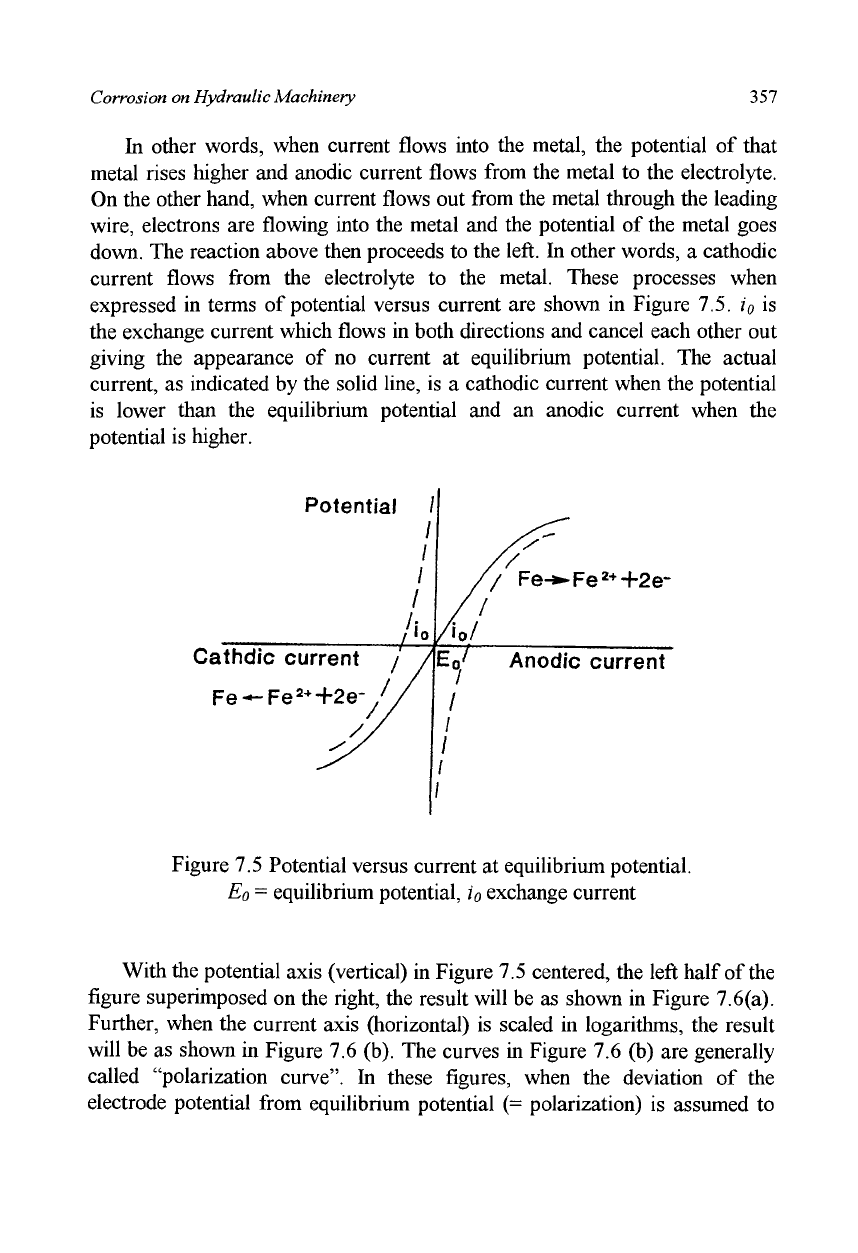
current flows from the electrolyte to the metal. These processes when
expressed in terms of potential versus current are shown in Figure 7.5. i
0
is
the exchange current which flows in both directions and cancel each other out
giving the appearance of no current at equilibrium potential. The actual
current, as indicated by the solid line, is a cathodic current when the potential
is lower than the equilibrium potential and an anodic current when the
potential is higher.
Potential
Cathdic current
Fe
—
Fe
Anodic current
Figure 7.5 Potential versus current at equilibrium potential.
E
0
= equilibrium potential,
io
exchange current
With the potential axis (vertical) in Figure 7.5 centered, the left half of the
figure superimposed on the right, the result will be as shown in Figure 7.6(a).
Further, when the current axis (horizontal) is scaled in logarithms, the result
will be as shown in Figure 7.6 (b). The curves in Figure 7.6 (b) are generally
called "polarization curve". In these figures, when the deviation of the
electrode potential from equilibrium potential (= polarization) is assumed to
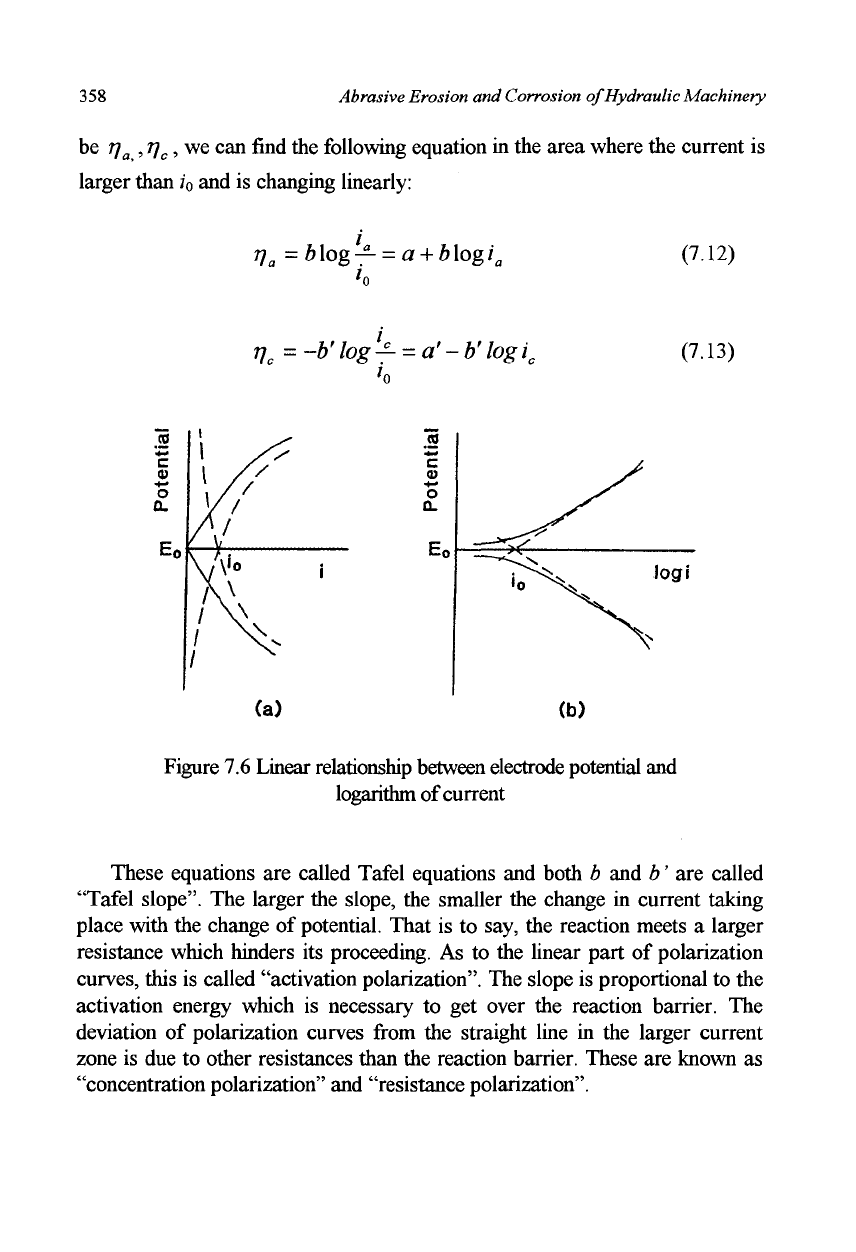
358
Abrasive Erosion and Corrosion of Hydraulic Machinery
be r)
a
,T]
C
, we can find the following equation in the area where the current is
larger than ;
0
and is changing linearly:
T]
a
= blog— = a
+
b\ogi
a
(7.12)
Vc
=~b'log^
=
a'-b'logi
c
/n
(7.13)
Figure 7.6 Linear relationship between electrode potential and
logarithm of current
These equations are called Tafel equations and both b and b' are called
"Tafel slope". The larger the slope, the smaller the change in current taking
place with the change of potential. That is to say, the reaction meets a larger
resistance which hinders its proceeding. As to the linear part of polarization
curves, this is called "activation polarization". The slope is proportional to the
activation energy which is necessary to get over the reaction barrier. The
deviation of polarization curves from the straight line in the larger current
zone is due to other resistances than the reaction barrier. These are known as
"concentration polarization" and "resistance polarization".

Corrosion on Hydraulic Machinery
359
When
the
rate
of
transformation
of
metal into
ion
exceeds
the
ion's
migration rate from anode surface to the electrolyte, ions (Fe
2+
for example)
are stored on the anode surface. The increase
in
ion concentration limits the
rate of rightward reaction of equation (7.11), making the slope of polarization
curve greater. On the other hand,
a
lack of oxygen of cations (ions charged
positive) occurs
on
the cathode surface when the reduction rate
of
cations
increases. Even when the electrolyte is flowing, in very close proximity to the
surface of
a
body there
is a
boundary layer where the flow velocity
is
quite
low and oxygen and cations
are
migration
by
diffusion. Thus, due
to the
reduction of oxygen or of cations, cathodic current cannot exceed the current
determined by the diffusion rate. In such
a
case, the polarization curve may by
expressed in the following equation:
ri
e
=-b'hga-
l
f)
(7.14)
c
where I
c
is called "limiting current".
Resistance polarization is due to the resistance, which develops when ions
move through
the
oxide layer
on the
electrode surface
or
through
the
electrolyte (current flowing).
In
ordinary
and
uniform corrosion, where
corrosion cells, and consequently the distance between anode and cathode are
small, this kind
of
polarization
is
negligible provided that the electrolyte
is
highly conductive. But
in a
liquid such
as
ion-exchanged water (de-ionized
water) with extremely few ions in it, the electrical resistance of the liquid
is
remarkably large (more than
5 x
10
6
Q
cm).
In
such
a
case the resistance
polarization is so large that corrosion cannot take place.
7.1.4 Polarization Diagram
We can summarize the above-discussed rules of corrosion with the following
statements:
i) In natural corrosion, the amount of anodic current is equal to that
of cathodic current.
ii) The difference
in
equilibrium potential between the two electrodes
is the motive force that drives the corrosion.
iii) When current flows against various resistances,
a
loss occurs
in
potential: This is polarization.
360 Abrasive Erosion and Corrosion of Hydraulic Machinery
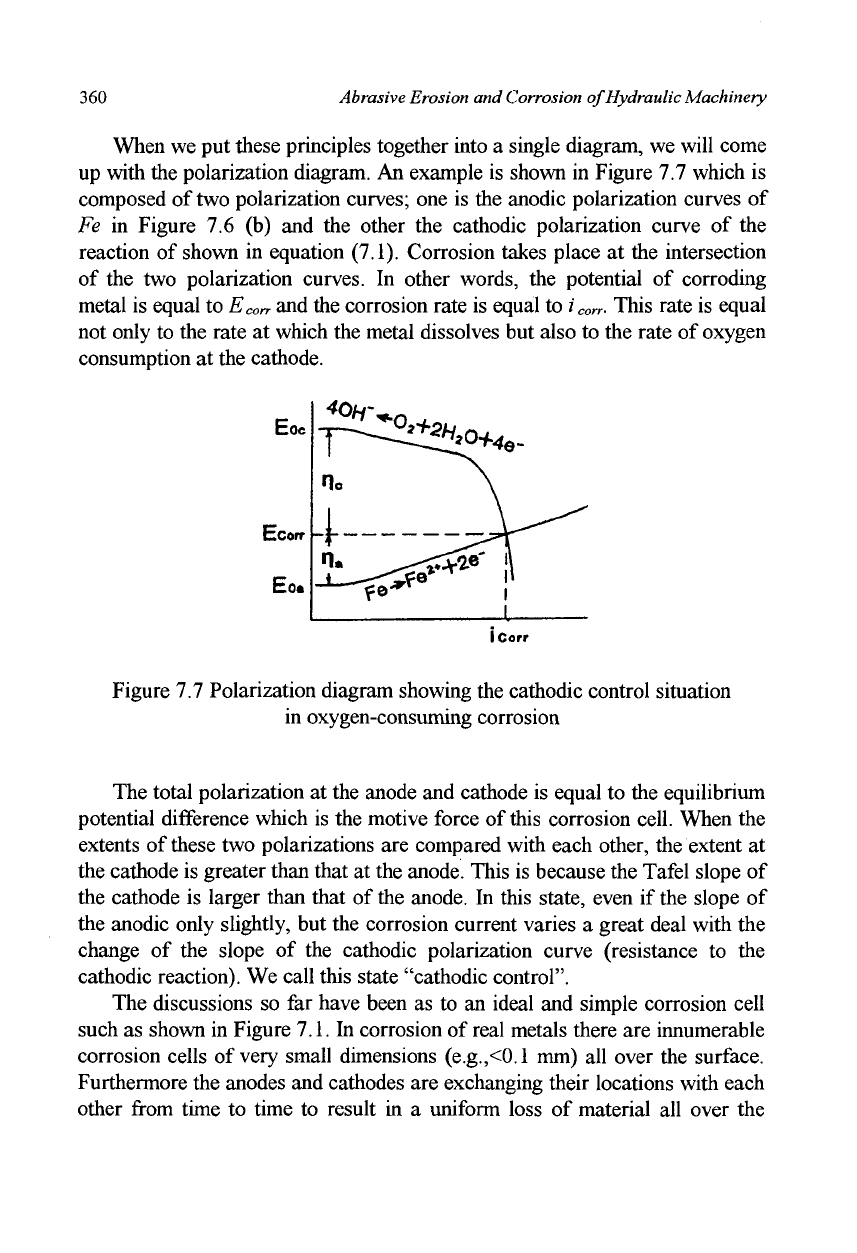
When we put these principles together into a single diagram, we will come
up with the polarization diagram. An example is shown in Figure 7.7 which is
composed of
two
polarization curves; one is the anodic polarization curves of
Fe in Figure 7.6 (b) and the other the cathodic polarization curve of the
reaction of shown in equation (7.1). Corrosion takes place at the intersection
of the two polarization curves. In other words, the potential of corroding
metal is equal to E
corr
and the corrosion rate is equal to z'
corr
. This rate is equal
not only to the rate at which the metal dissolves but also to the rate of oxygen
consumption at the cathode.
Figure 7.7 Polarization diagram showing the cathodic control situation
in oxygen-consuming corrosion
The total polarization at the anode and cathode is equal to the equilibrium
potential difference which is the motive force of
this
corrosion cell. When the
extents of these two polarizations are compared with each other, the extent at
the cathode is greater than that at the anode. This is because the Tafel slope of
the cathode is larger than that of the anode. In this state, even if the slope of
the anodic only slightly, but the corrosion current varies a great deal with the
change of the slope of the cathodic polarization curve (resistance to the
cathodic reaction). We call this state "cathodic control".
The discussions so far have been as to an ideal and simple corrosion cell
such as shown in Figure
7.1.
In corrosion of real metals there are innumerable
corrosion cells of very small dimensions (e.g.,<0.1 mm) all over the surface.
Furthermore the anodes and cathodes are exchanging their locations with each
other from time to time to result in a uniform loss of material all over the

Corrosion on Hydraulic Machinery 361
surface (general corrosion). Such corrosion cells are called "micro-cell" or
"local-action cell". In contrast with this, in some special cases, a whole part
of a machine or equipment becomes anode and the other parts cathode. As
they do not exchange location, the anode part keeps dissolving resulting in
severe damage on the anode part but the cathode not at all. We call such a
state "macro-cell corrosion". In the macro-cell corrosion, we can measure the
current flow between the anode area and cathode area by electrically isolating
the parts and then connecting them through a leading wire with an ammeter in
between. In micro-cell corrosion, however, we can not measure the current
because the areas of anode and cathode are so small and they change their
locations. A polarization curve may be impossible to measure in micro-cell
corrosion. Nevertheless, we can measure E
corr
by connecting a standard
electrode and the corroding metal to a potentiometer. The value obtained here
is the average potential of a large number of micro-cells. We can also
measure the corrosion current
i
corr
through the weight loss of corroding metal
after a giver period of time. This value is also an averaged value.Thus, at
least the point (i
corr
, E
corr
) in Figure 7.7 can be obtained for the micro-cell
corrosion. The slopes of the polarization curves will be obtained by an
approximating method which will be explained in 7.2.3.
7.2 Application of Corrosion Theories
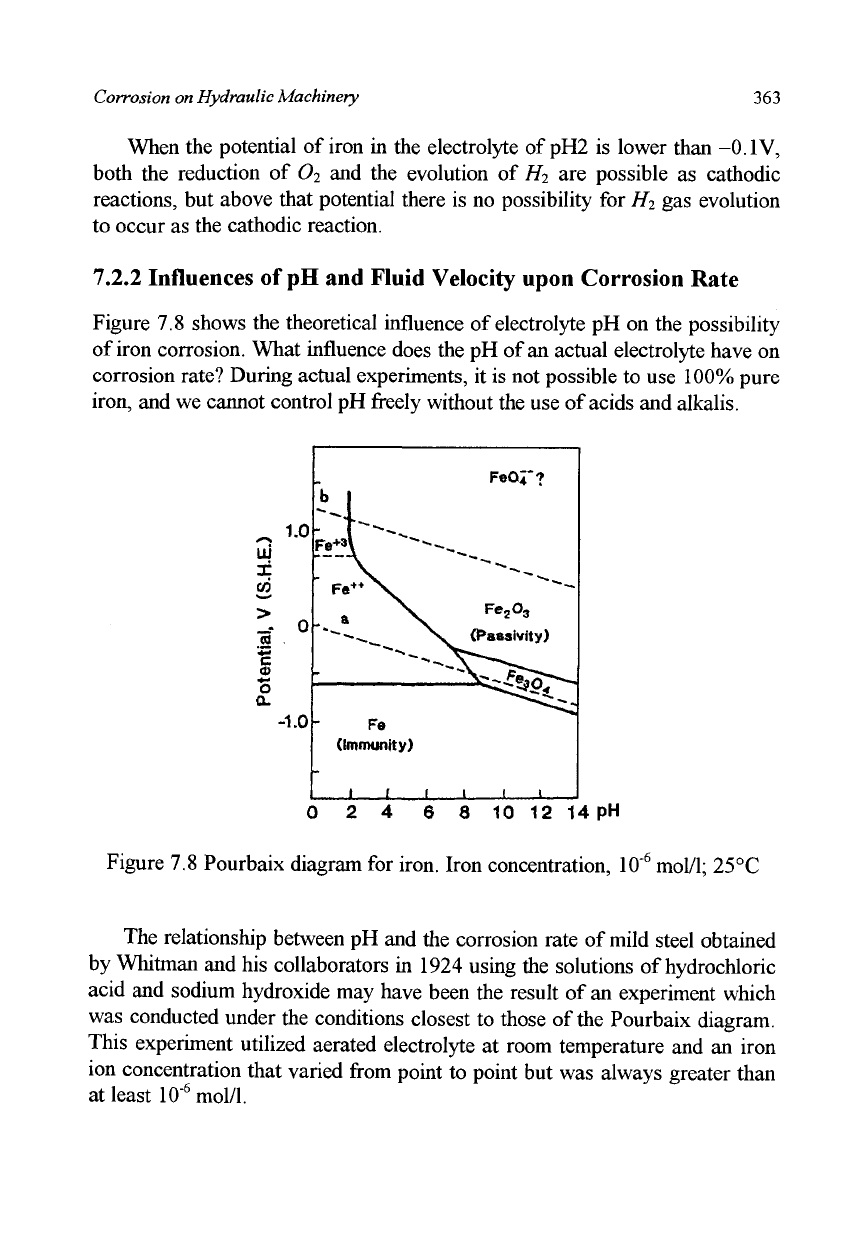
7.2.1 Pourbaix Diagram
The rate of corrosion of a metal depends, as has been explained, on the
deference in equilibrium potential of electrodes as well as on the polarization
of each electrode reaction. There are many causes of polarization, and thus,
many factors influencing it. Therefore, it is quite difficult to predict the rate at
which a given metal will corrode in a given electrolyte. However, we can
calculate the equilibrium potential of electrodes by utilizing thermodynamic
data and assuming two of three propositions (ion concentration, temperature,
etc.).
Once we know the equilibrium potential, we can predict whether the
corrosion of a metal is possible or not by comparing the potential with the
measured potential of the metal. As is known from Figure 7.5, there is no
possibility of dissolution when the potential of metal is lower than the

362 Abrasive Erosion and Corrosion of Hydraulic Machinery
equilibrium potential E
0
. The Pourbaix diagram (potential vs. pH), shown in
Figure 7.8, predicts the possibility of corrosion (not the rate of corrosion)
based on equilibrium potential using the method just described. Figure 7.8
assumes iron as the test metal, an iron ion density of 10"
6
mol/1 in the
electrolyte as the environmental condition, and 25 °C as the temperature (see
Pourbaix, 1966). Under these conditions, the equilibrium potential of the
following reaction
Fe<^Fe
2+
+2e~
can be found by the Nernst equation to be -0.62V. There is no possibility of
corrosion when the potential is lower than this value, so this condition is
called "immunity". The reason why pH is a factor for corrosion besides the
potential lies in the following reaction.
3Fe + 4H
2
0<+Fe
3
0
4
+SH
+
+8e" (7.15)
The concentration of H* (in the right side of the above equation) is related
to the equilibrium potential of
this
reaction. This equilibrium potential can be
also defined as the threshold of immunity, a line oblique to the lateral line.
Lines parallel to the vertical line have nothing to do with potential because
they participate in equilibrium irrespective of electron exchange as in the
following reaction.
3Fe
3+
+ 3H
2
0<^Fe
2
0
3
+6H
+
(7.16)
In the areas described as "passivity", oxidized substances like Fe
2
0
3
is
stable, These oxidized substances cover the metal surface forming a
protective film that reduces the rate of corrosion to nearly zero. Metal in this
condition we call passivity. In the Pourbaix diagram, the following
equilibrium potential ari also shown by the broken line.
H
2
<->2/T +2e~ (7.17)
40H~ <r>0
2
+2H
2
0
+
4e-
(7.18)
Corrosion on Hydraulic Machinery
363
When the potential of iron in the electrolyte of pH2 is lower than -0.1V,
both the reduction of 0
2
and the evolution of H
2
are possible as cathodic
reactions, but above that potential there is no possibility for H
2
gas evolution
to occur as the cathodic reaction.
7.2.2 Influences of pH and Fluid Velocity upon Corrosion Rate
Figure 7.8 shows the theoretical influence of electrolyte pH on the possibility
of iron corrosion. What influence does the pH of an actual electrolyte have on
corrosion rate? During actual experiments, it is not possible to use 100% pure
iron, and we cannot control pH freely without the use of acids and alkalis.
I
I [ ... i,. i i i 1
0 2 4 6 8 10 12 14 pH
Figure 7.8 Pourbaix diagram for iron. Iron concentration, 10
s
mol/1;
25°C
The relationship between pH and the corrosion rate of mild steel obtained
by Whitman and his collaborators in 1924 using the solutions of hydrochloric
acid and sodium hydroxide may have been the result of an experiment which
was conducted under the conditions closest to those of the Pourbaix diagram.
This experiment utilized aerated electrolyte at room temperature and an iron
ion concentration that varied from point to point but was always greater than
at least
10"
6
mol/1.
