Drake G.W.F. (editor) Handbook of Atomic, Molecular, and Optical Physics
Подождите немного. Документ загружается.


1343
Surface Physic
89. Surface Physics
This chapter describes various applications of
atomic and molecular physics to phenomena that
occur at surfaces. Particular attention is placed
on the application of electron- and photon-atom
scattering processes to obtain surface specific
structural and spectroscopic information.
The study of surfaces and interfaces touches
on many fields of pure and applied science.
In particular there are applications in the
fields of semiconductor processing, thin film
growth, catalysis, corrosion and fundamen-
tal physics in two-dimensions. A number of
recent texts cover surface physics in gen-
eral [89.1–4] as well as specific areas such as
experimental techniques [89.5], surface electron
spectroscopies [89.6, 7], and the application of
synchrotron radiation to surface science [89.8].
Also a number of book series that deal with ar-
eas of particular interest are published at regular
intervals, such as surface chemistry [89.9], sur-
face vibrations [89.10] and stimulated desorption
processes [89.11].
89.1 Low Energy Electrons and Surface Science1343
89.2 Electron–Atom Interactions ..................1344
89.2.1 Elastic Scattering:
Low Energy Electron
Diffraction (LEED).......................1344
89.2.2 Inelastic Scattering:
Electron Energy Loss
Spectroscopy ............................1345
89.2.3 Auger Electron Spectroscopy .......1345
89.3 Photon–Atom Interactions ...................1346
89.3.1 Ultraviolet Photoelectron
Spectroscopy (UPS).....................1346
89.3.2 Inverse Photoemission
Spectroscopy (IPES) ....................1347
89.3.3 X-Ray Photoelectron
Spectroscopy (XPS) .....................1348
89.3.4 X-Ray Absorption Methods .........1348
89.4 Atom–Surface Interactions ...................1351
89.4.1 Physisorption ...........................1351
89.4.2 Chemisorption ..........................1352
89.5 Recent Developments...........................1352
References ..................................................1353
89.1 Low Energy Electrons and Surface Science
To obtain information specific to the first few layers of
atoms at the surface of a solid, techniques must be de-
vised that discriminate between signals from the surface
and from the bulk of the material. If a solid has dimen-
sions of ≈ 1cm
3
, then only approximately one atom in
10
7
is located at the surface. This makes the application
of bulk techniques to the study of surfaces (e.g. X-ray
diffraction [89.12]) problematic.
There are several experimental approaches to achiev-
ing surface sensitivity. If the probe used is an atom or
molecule that can be scattered from or desorbed from
the surface, then these species can be analyzed by tech-
niques such as mass spectrometry or resonant ionization.
A more recent innovation has been the use of scanning
probe microscopies [89.13] (e.g., the scanning tunnel-
ing microscope and its variants) which exploit a surface
sensitivity such as the surface valence electron density.
However the most common surface analytical tech-
niques use the intrinsic surface sensitivity of low energy
(10–2000 eV) electrons.
The surface specificity of low energy electrons arises
from the very short inelastic mean free path (MFP)for
these electrons in a solid. This property can be seen in
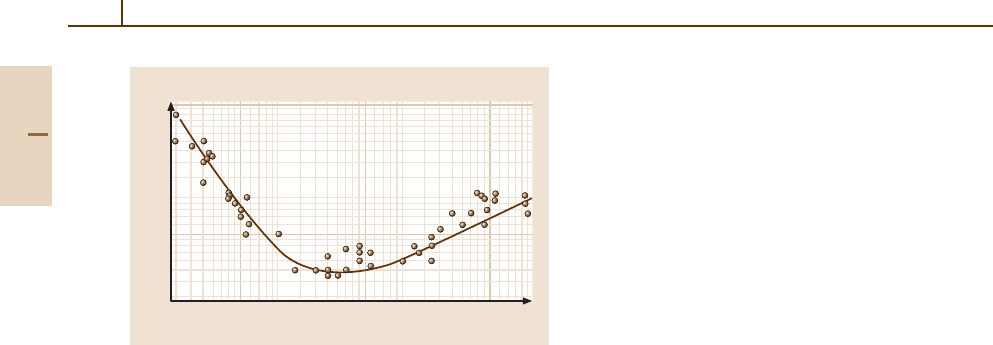
Fig. 89.1 which plots measured values of the inelastic
MFP for a number of materials and electron energies
between 1 and 2000 eV. The curve is called “univer-
sal” because the same general trend of short inelastic
MFP is observed for nearly all materials [89.14]. The
dominant energy loss mechanisms are valence band
excitations (plasmons and electronic excitations), and
since most materials have similar valence electron den-
sities, the resultant inelastic scattering MFP is to a good
approximation, material independent.
The very short inelastic MFPs for low energy elec-
trons has the result that any that escape from the solid
without having undergone inelastic scattering can only
Part G 89
1344 Part G Aplications
100
7
6
5
4
3
2
10
7
6
5
4
3
10 100 1000
3456 2 3456 2 3456 2
Mean free path λ(Å)
Electron energy (eV)
Fig. 89.1 The variation of the inelastic mean free path
with electron kinetic energy (“universal curve”). Based on
Briggs and Seah [89.14]
have originated very close to the surface, usually
within a few atomic layer spacings. Many of the sur-
face analysis techniques described in this chapter use
this surface sensitivity to obtain surface structural and
spectroscopic information. The same techniques are
easily applicable to conducting and semiconducting
materials, and can also be applied to insulating ma-
terials, if the charging effects are dealt with in some
manner.
89.2 Electron–Atom Interactions
89.2.1 Elastic Scattering:
Low Energy Electron Diffraction
(LEED)
The elastic scattering of low energy (20–500 eV) elec-
trons at surfaces is historically important in physics as
the experiments of Davisson and Germer provided early
experimental evidence of the wave nature of electrons.
After these initial experiments, the technique was largely
unused until the advent of cleaner ultra-high vacuumsys-
tems and surface preparation techniques in the 1960’s.
LEED is one of the most important and widely used
surface characterization techniques due to its surface
sensitivity and wide utility [89.5].
Diffraction from a two-dimensional net of scatterers
results in a two-dimensional array of reciprocal lattice
“rods” oriented normal to the surface. All kinematically
allowed rods intersect the Ewald sphere at all energies
— unlike the case in bulk X-ray diffraction. The short
inelastic MFP for scattering low energy electrons yields
the surface sensitivity of LEED for a crystal surface. The
kinematic theory used in X-ray diffraction is not directly
applicable to LEED since the low energy electrons can
undergo several elastic collisions in the surface region.
The elastic MFPs for low energy electrons are compara-
ble in magnitude to the inelastic mean free paths shown
in Fig. 89.1. This “multiple scattering” does not affect
the positions of the diffraction beams, but does alter their
intensities due to interference effects.
Kinematic analysis is sufficient to determine the
diffraction beam positions for a proposed structure, and
this is the most common use for LEED. A diffraction
pattern is often sufficient to determine the surface pe-
riodicity and unit mesh size. However determining the
surface crystal basis from the intensities of the diffracted
LEED beams requires moderately sophisticated calcu-
lations to be performed for proposed structures.
Quantitative LEED generally compares measured
“I(V) curves” (the intensity I of a particular diffrac-
tion beam as a function of electron energy measured
in volts) with a calculated I(V) profile for a proposed
surface crystal structure. These calculations determine
the propagating electron wave functions Ψ
LEED
that
take into account electron-ion core scattering cross
sections and phase shifts, available multiple scatter-
ing pathways, inelastic scattering cross sections and
Debye-Waller effects. The structural parameters are var-
ied systematically until agreement can be reached with
the experimental I(V) curves [89.15, 16]. These calcu-
lations can yield surface atom positions to better than
0.1 Å vertically and 0.2 Å horizontally. Over 1000 sur-
face structures have been determined using LEED and
associated techniques [89.17].
Clean surfaces often have a different crystallography
than simple termination of the bulk crystal structure. Due
to the absence of neighbors above the surface, the surface
atoms can undergo both relaxation (change in interlayer
spacing) and reconstruction (changes in periodicity and
bonding) [89.2]. While relaxation and reconstruction do
occur on all types of surfaces, the reconstructions found
on semiconductors are most striking. The strong co-
valent bonds that are broken when a semiconductor is
cleaved give rise to high energy “dangling bonds”. The
surface energy is minimized by having the surface re-
construct to reduce the total number of these bonds. The
resultant structure is formed through a balance between
eliminating as many dangling bonds as possible and the
resultant stress caused in other bonds due to the dis-
Part G 89.2
Surface Physics 89.2 Electron–Atom Interactions 1345
placements of the surface atoms. One example of this
is the clean Si(111) surface, in which 49 surface atoms
form a new periodic arrangement to make up the recon-
structed Si(111)–(7×7) unit cell [89.2]. The number of
dangling bonds is thereby reduced from 49 to 19.
89.2.2 Inelastic Scattering:
Electron Energy Loss Spectroscopy
Low energy surface excitations such as phonons, plas-
mons and electron-hole pair excitations can be studied
using inelastic electron scattering. High resolution elec-
tron energy loss spectroscopy (HREELS) [89.18]uses
incident electron beam energies of 1–300 eV with
energy resolutions of 1–10 meV. Inelastic scattering
phenomenon are divided into three types: dipole, impact
and resonant scattering. Surface vibrations are also stud-
ied using infra-red absorption spectroscopy and inelastic
atomic beam scattering techniques [89.19].
Dipole Scattering
In dipole scattering HREELS, the incident electron
(1 < E
i
< 20 eV) undergoes a long range Coulomb in-
teraction with dipole fields associated with a dynamic
dipole moment. The main characteristic of dipole scat-
tering is that the inelastically scattered electrons have
an angular distribution that is strongly peaked (width
∆θ ≈
ω/E
i
) close to the specular direction (θ
i
= θ
f
).
In this limit there is no momentum exchange with the
surface vibrational mode (i. e., k
= 0 in the bandstruc-
ture), so dipole scattering HREELS is similar to infrared
spectroscopy in that only ir active vibrational modes at
the zone center can be studied. At the surface of a con-
ductor, dielectric screening guarantees that only ir active
modes having a component perpendicular to the surface
are visible — modes having dipole moments parallel to
the surface are screened by the surface and so cannot
be excited by dipole scattering. This “quasi-selection
rule” allows the site symmetry for some systems to be
decided [89.18]. Dipole scattering is most commonly
used in the study of vibrations of molecules on sur-
faces, which often have dipole-active vibrational modes
that are both intrinsic and caused by adsorption (i. e.,
frustrated translations and rotations).
Impact Scattering
In impact scattering, the incident electron samples the
short-range interatomic potential, and is not restricted
to dipole-active vibrational modes [89.18]. Higher elec-
tron energies are used (30 < E
i
< 300 eV) since the cross
section for impact scattering generally increases with en-
ergy. The inelastically scattered electrons are distributed
at all angles, and so there can be exchange of parallel mo-
mentum (k
) with the surface, allowing the mapping of
surface excitation bandstructure
ω(k
). The cross sec-
tions for impact scattering HREELS can be calculated
using an approach similar to LEED, but now consider-
ing the normal displacements of atoms from equilibrium
positions. The scattering potential V(r, [R]) (where [R]
is the set of position vectors of the N atoms in the sur-
face region) can be expanded in terms of the normal
displacements µ
i
from equilibrium according to
V
r, [R]
= V
r, [R
0
]
+
N
i=1
∇
µ
i
V
r, [R]
[R
0
]
· µ
i
+··· .
(89.1)
The first term is responsible for elastic scattering
(LEED) and the second term is the dominant term for
impact scattering. The inelastic cross section for mode i
is then [89.20]
dσ
dΩ
=|Ψ
∗
LEED
(k
f
)|
∂V
∂µ
i
|Ψ
LEED
(k
i
)|
2
(89.2)
using electron wave functions calculated using the same
formalism as LEED. The symmetry properties of this
matrix element can be used to determine the polariza-
tion direction of surface vibrational modes by searching
for systematic absences in the inelastic intensity in par-
ticular high symmetry direction of the surface [89.20].
Impact scattering studies are most commonly made in
the study of surface phonon bandstructure and the vi-
brational modes of adsorbed molecules that are not
dipole-active.
Resonant Scattering
Resonant electron scattering at surfaces [89.21, 22]is
usually applied to the study of adsorbed molecules, and
has much in common with resonant scattering from gas-
phase atoms and molecules (see Chapt. 47). In resonant
scattering, the incident electron combines with a target
molecule to form a short-lived molecular ion, which
subsequently decays and can leave the molecule vi-
brationally or electronically excited. The cross sections
for this process have a typical profile. For example, in
a shape resonance the formation of the temporary nega-
tive ion intermediate corresponds to adding the incident
electron to a particular unoccupied orbital of the tar-
get molecule. The study of the angular dependence of
resonance scattering cross sections [89.22] can yield in-
formation on the orientation of molecules on surfaces,
Part G 89.2
1346 Part G Aplications
since the electron capture and emission cross sections
are fixed by the molecular orientation and the resonance
symmetry.
89.2.3 Auger Electron Spectroscopy
Auger electron spectroscopy (AES) is one of the most
widely used surface science techniques due to its chem-
ical and surface sensitivity [89.5,14,23]. Core holes are
created in near surface atoms using a high energy elec-
tron beam (2–5 keV, 1–100 µA) or less commonly, an
X-ray source. For these low binding energy core holes,
the Auger decay mode is highly probable (Sect. 61.2).
Although the Auger energy and lineshape can give spec-
troscopic information, in surface science this is seldom
used – rather it is the chemical fingerprint of the atoms
which is of interest.
Auger electron spectroscopy is a surface sensi-
tive technique by virtue of the low kinetic energy
(50–1000 eV) of the emitted Auger electrons. Auger
electrons from atoms more than a few Ångstroms below
the surface are inelastically scattered and so not detected
by the energy selective detector. The kinetic energy of
the Auger electrons is
T
e
= E
A
− E
B
− E
C
− U (89.3)
where E
A
is the binding energy of the initial core elec-
tron and E
B
, E
C
are the binding energies of the other
electrons (one or both are valence levels) involved in
the Auger process. Energy shifts and relaxation are
accounted in the term U which includes hole-hole inter-
actions and atomic and solid state (dielectric) screening
of the holes, and hence can be sensitive to the local
chemical environment.
Auger spectra are typically obtained in derivative
mode [i. e., N
dN(E)/ dE] to separate the Auger tran-
sitions from the secondary electron background, and
comparison is made to reference spectra [89.24]. The
raw chemical sensitivity of AES is very high, and sur-
face concentrations of ≈1% of many common chemical
species can be detected. Semiquantitative measurements
may be made by comparison to these reference spectra,
but such comparisons only give atomic concentrations
within a factor of 2 (or worse) since the measured AES
signal can be modified by a number of factors. More
precise quantitative measurements can be made by cali-
bration of the AES intensity for the atomic constituents
at a surface [89.5]. The AES sensitivity to atoms A on
a clean substrate (atoms B) can be determined if the ab-
solute quantity of A can be established by some other
means.
The chemical sensitivity of AES can also be ex-
ploited as a form of chemical microscopy (scanning
Auger microscopy) since the exciting electron beam that
is used may be focused to a very small size. The Auger
signal from the small target volume can be analyzed for
specific chemical components. By rastering the incident
electron beam, a chemical map of the surface can be
made.
89.3 Photon–Atom Interactions
89.3.1 Ultraviolet Photoelectron
Spectroscopy (UPS)
Photoelectron spectroscopy (PES) (Sect. 61.1) has been
historically divided into UV photoelectron spectroscopy
for low photon energies (generally the study of valence
electron states) and X-ray photoelectron spectroscopy
(study of core electron levels). The use of low en-
ergy photons (5 < hν<50 eV) for PES of solids has
the advantage that the photons have a negligible mo-
mentum (k ≈ 0). This allows straightforward band
mapping since the transitions are vertical in momentum
space:
hν = E
f
(k+ G) − E
i
(k) (89.4)
where k is the electron state wavevector and G is a re-
ciprocal lattice vector.
Most UPS studies are done using angle-resolved
photoelectron detection (also called angle resolved pho-
toelectron spectroscopy or ARPES). The kinetic energy
T
e
and emission angle θ of the photoelectrons are
measured, allowing the initial state binding energy and
momentum parallel to the surface
k
to be determined
from
k
=
2mT
e
2
sin θ. (89.5)
The mean free path of the photoelectrons from va-
lence levels allows both surface and bulk electron states
to be studied. Separation of the surface from bulk bands
can be accomplished in several ways [89.5]. Since a crys-
tal surface has two-dimensional symmetry, only the k
component of momentum is a good quantum number
for the surface states. Also, in transporting the surface or
Part G 89.3

Surface Physics 89.3 Photon–Atom Interactions 1347
bulk state photoelectron of momentum kthrough the sur-
face to the detector, only the k
component is conserved.
A bulk state disperses in three-dimensions, requiring
knowledge of k= k
+ k
⊥
. The magnitude of k
⊥
cannot
be determined unless the inner potential (change in po-
tential normal to the surface) is known by some other
means. This property allows bulk and surface states to
be distinguished since surface states remain fixed in en-
ergy for a constant k
, while bulk states disperse if k
⊥
is
changed while k
is kept constant. This is done by mea-
suring the photoelectron spectrum for a range of UV
photon energies at a fixed value of k
(often at θ = 0so
k
= 0). Bulk states disperse since k
⊥
varies as the pho-
ton energy is changed, but the surface states remain at
a fixed binding energy in the photoelectron spectrum.
The photoelectron transition matrix element be-
tween initial and final states |i and | f due to the
incident photon vector potential A is
I ∝
f
A· p+ p· A
i
2
≈
f
2A· p
i
2
. (89.6)
The spatial variation of A near the surface (the surface
photoeffect)can be neglected, although this is not strictly
valid at these low photon energies [89.26].
Due to the low photoelectron kinetic energies in
most UPS work, precise calculation of photoelectron
spectrum intensities is rather difficult due to multiple
scattering and phase shifts sensitive to valence electrons.
However, the initial electron state symmetries can be de-
termined using the symmetry properties of the matrix
element (89.6). Selection rules allow the state symme-
tries to be determined by measuring the photoemission
spectrum along high symmetry directions of the surface
using polarized UV radiation [89.5].
The valence electron states of adsorbed molecules
can also be studied using UPS. Peaks in the photo-
electron spectrum due to valence levels of adsorbed
molecules tend to have larger linewidths (≈ 1 eV typ-
ically) than in the gas phase due to solid state and
instrumental effects, so vibrational structure is seldom
resolved. The positions of the molecular valence states
are shifted in energy due to the surface work function
and solid state relaxation (dielectric screening) effects.
In addition to these rigid shifts, chemical shifts are also
observed due to bonding (chemisorption) interactions
between the molecule and surface. The molecular char-
acter of the valence orbitals is often retained from the
gas phase, so the shifted levels can be identified by using
the symmetry properties of the photoemission matrix el-
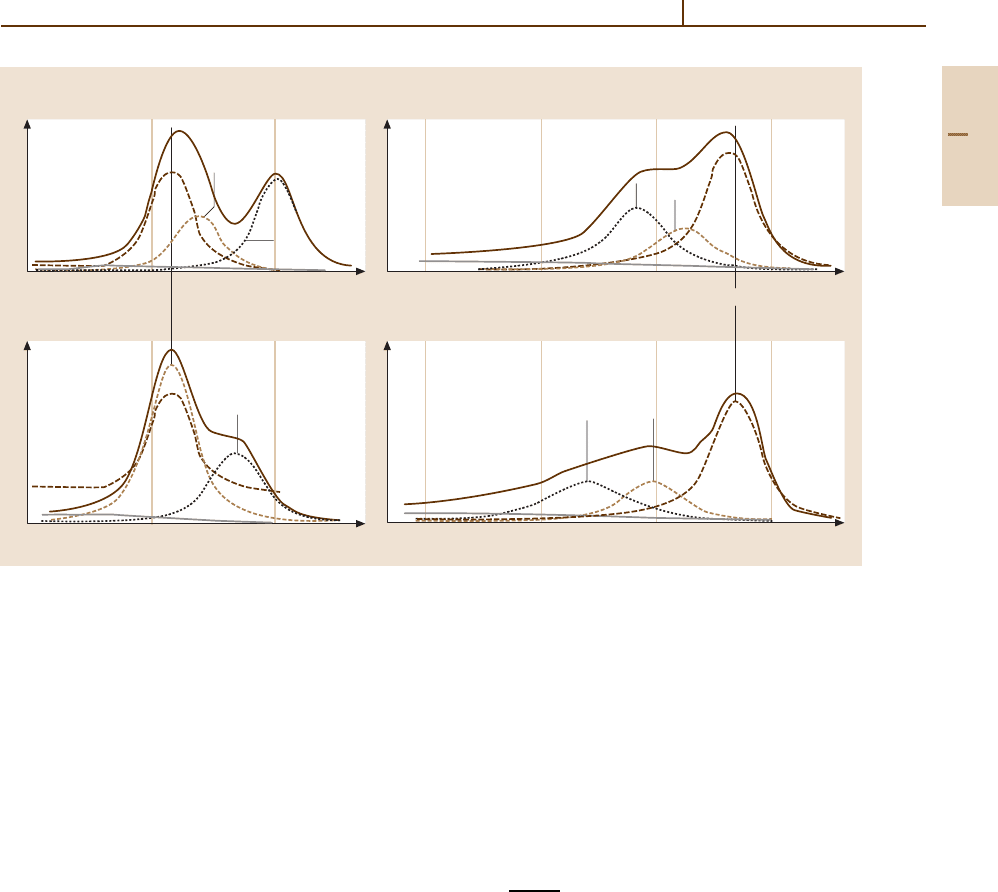
ement. For example, photoemission from gas phase CO
shows three valence states: 5σ ,1π and 4σ in order of
increasing binding energy. A series of photoemission
spectra [89.25] for gas phase CO, solid CO and CO
chemisorbed on several transition metal surfaces (in or-
der of increasing CO–surface binding energy) is shown
in Fig. 89.2. For solid CO and weakly chemisorbed
CO/Ag(111) all three valence orbitals of molecular CO
are well resolved, though the adsorbed CO spectrum is
shifted rigidly in energy by relaxation effects. Since CO
is chemisorbed more strongly on Cu(111) and Pd(111),
the 1π and 5σ valence states shift relative to one another.
The strongly overlapping 1π and 5σ states observed for
chemisorbed CO can be individually resolved by using
the photoemission selection rules and linearly polarized
UV radiation [89.25]. The origin of the chemical shift
between the 5σ and 1π levels has been ascribed to bond
formation involving the CO 5σ level and σ -symmetry
d-electron states on the transition metal surfaces [89.2].
89.3.2 Inverse Photoemission
Spectroscopy (IPES)
Inverse photoemission spectroscopy is properly classi-
fied as an incident electron technique but is included here
(eV)
20 15 10 5
0 =
E
vac
CO/Pd(111)
E
AD
= 142 kJ/mol
CO/Cu(111)
E
AD
= 47 kJ/mol
E
F
Pd
E
F
Cu
CO/Ag(111)
E
AD
= 19 kJ/mol
E
F
Ag
CO
SOLID
E
COND
= 7 kJ/mol
CO
GAS
4σ 5σ1π
s.u.
Fig. 89.2 AsetofUPS spectra for CO adsorbed on various
surfaces, as well as gas phase and solid CO. The peak
labeled “su” is a shake-up satellite peak. After [89.25]
Part G 89.3

1348 Part G Aplications
as a natural companion to UPS. IPES utilizes low energy
electrons (5–50 eV) incident on a surface. Transitions
from a high-lying initial electron state in the contin-
uum to a lower unoccupied state cause a UV photon
to be emitted. By detecting the emitted photon inten-
sity as a function of energy, the joint density of states is
measured [89.7]. IPES is used to study the unoccupied
portion of surface and bulk bandstructure, particularly
the region between the Fermi level and the vacuum level,
which is difficult to access by other means. This allows
the study of unoccupied states of adsorbed molecules
(e.g., antibonding molecular levels) as well as intrinsic
surface states such as the Rydberg-like states of electrons
trapped in the image potential at the surface [89.7].
89.3.3 X-Ray Photoelectron
Spectroscopy (XPS)
X-ray photoelectron spectroscopy allows the study of
the energy of atomic core levels via the Einstein photo-
electric equation T
e
= hν − E
b
,whereT
e
is the kinetic
energy of the photoemitted electron and E
b
is the
binding energy of the core level. Atoms bound in dif-
ferent chemical environments (e.g., at particular sites
on a surface) experience different chemical shifts, and
so are measured at slightly different binding energies.
This sensitivity to chemical environment allows XPS to
characterize the different types of binding sites for sim-
ilar atoms, and measure their abundance by measuring
the relative intensities of XPS emission from different
species.
Shifts in T
e
arise from two sources: intra-atomic
and inter-atomic relaxation shifts. The intra-atomic re-
laxation E
a
is due to screening of the core hole by other
electrons in the emitter atom. The inter-atomic relax-
ation E
r
is important in solids (particularly for metals)
and is due to screening of the core hole by the dielectric
response of the surrounding medium. These relaxation
shifts (on the order of a few eV) tend to increase the ki-
netic energy of the emitted photoelectron, so the kinetic
energy in the adiabatic limit is
T
e
= hν − E
b
+ E
a
+ E
r
. (89.7)
Since photoemission is a rapid process, nonadiabatic
processes lead to shakeup and shakeoff features in which
other electrons are excited to higher energy levels, caus-
ing the photoelectron to have lower kinetic energy [89.5]
(see Sect. 62.4.4). It is also possible to excite discrete
excitations of the solid, such as plasmons and a contin-
uum of low energy electron-hole excitations, causing the
XPS peak to have an asymmetric lineshape. The over-
all XPS distributions of T
e
thus contain contributions
from the adiabatic channel, and lower energy photoelec-
trons in a series of discrete peaks or a continuum from
nonadiabatic processes.
The chemical abundance of a species can be deter-
mined from the sum rule that the total XPS cross section
is proportional to the sum of the adiabatic peak and
all the shake-up and shake-off components. However it
is usually only convenient to measure the intensity of
the adiabatic peak. If comparisons are made between
chemical species in different chemical environments,
the shake-up and shake-off intensities may differ. Hence
the adiabatic channel intensities might not reflect the
true abundance. Very often this problem can be mini-
mized by careful calibration, and chemical analyses can
be made to an accuracy of a few percent.
The surface sensitivity of XPS is due to the short in-
elastic MFP for the photoelectrons, which can be made
to have energies in the range 10–1000 eV by appropriate
choice of the X-ray wavelength. The XPS signal is pro-
portional to exp[−z/(λ cos θ)],wherez is the depth of
the emitter, λ is the inelastic mean free path of the pho-
toelectron and θ is the angle measured from the surface
normal.
The popularity of XPS as a surface analysis tech-
nique is due to the availability of convenient and
sufficiently intense monochromatic X-rays from lab
sources (usually Al and Mg K
α
X-ray lines at 1486.6
and 1253.6 eV). The increasing availability of con-
tinuously tunable X-rays from synchrotron radiation
sources allows improved measurements due to the abil-
ity to tune the X-rays to slightly above threshold,
where the XPS cross-sections are maximized, the in-
elastic MFP is shorter (hence more surface specific)
and electron monochromators can operate with higher
resolution [89.5].
As an example of the chemical sensitivity of XPS,
photoelectron spectra of the 4f
7/2
core levels of W(111)
and Ta(111) are shown in Fig. 89.3. The spectra show
discrete peaks from both bulk and surface atoms. The
binding energies of the surface atoms are affected by the
adsorption of hydrogen.
XPS can also be used to give local structural in-
formation due to elastic scattering of the photoemitted
electron in the region near the emitter [89.5]. One form
of this is the use of forward scattering from a buried
emitter. A high energy XPS photoelectron is focused in
the direction of nearby atoms due to a lower effective
potential close to the atomic core. Angular scanning of
the XPS detector will then detect a more intense signal
along the bond axis. This method has proved useful in
Part G 89.3
Surface Physics 89.3 Photon–Atom Interactions 1349
32.0 31.5 31.0
32.0 31.5 31.0
Binding energy (eV)
Binding energy (eV)
23.0 22.5 22.0 21.5
Binding energy (eV)
23.0 22.5 22.0 21.5
Binding energy (eV)
a)
Intensity (arb. units)
b
S
2
S
1
W(111) 4f
7/2
hv = 70 eV
b)
Intensity (arb. units)
S'
1
W(111) + 11LH
2
c)
Intensity (arb. units)
b
S
2
S
1
Ta(111) 4f
7/2
hv = 66 eV
d)
Intensity (arb. units)
b
S'
2
S'
1
Ta(111) + 500LH
2
∇
Fig. 89.3 XPS spectra of the 4f
7/2
states of W(111) and Ta(111). Contributions from the bulk (b) and surface (S1,S2)
atoms can be distinguished, and the surface atom peaks are shifted by the adsorption of hydrogen. After [89.28]
studying the orientation of adsorbed molecules and the
structure of hetero-epitaxial thin films [89.27].
89.3.4 X-Ray Absorption Methods
X-ray absorption methods [89.8] measure the decay of
the core hole rather than the intensity of emitted pho-
toelectrons, since that XPS process is complicated by
a number of possible final state processes, as discussed
above. To obtain surface sensitivity, the X-ray absorp-
tion is measured using a low energy electron emitting
channel such as an Auger electron emission or the to-
tal electron yield, which are proportional to the overall
X-ray absorption. These methods require an intense and
tunable source of monochromatic x-radiation near the
excitation edge for the core level of a particular atom,
and so are usually performed using synchrotron radia-
tion. Synchrotron sources have the additional benefit of
linearly polarized light, which is crucial for NEXAFS
and useful for SEXAFS discussed next.
SEXAFS: Measurement of Bond Lengths
The surface extended X-ray absorption fine structure
(SEXAFS) technique is most commonly used to mea-
sure the bond lengths for atoms adsorbed on a surface.
SEXAFS utilizes the elastic backscattering of the emit-
ted XPS photoelectron from nearby atoms that surround
the emitter. Elastically scattered waves arrive back at the
emitter and add coherently (constructively and destruc-
tively) to the outgoing wave, thus modifying the matrix
element for the transition to the final state [89.29]. Exper-
imentally, the cross section σ(hν) for X-ray absorption
above the threshold photon energy is modified by an
oscillatory structure. The ‘atomic’ contribution to the
absorption cross section can be removed using
χ =
σ − σ
0
σ
0
, (89.8)
where σ and σ
0
are the measured surface and free atom
X-ray absorption cross sections. If only single scattering
events are involved in backscattering to the emitter then
the fine structure function χ is
χ(k) =
N
i=1
A
i
(k) sin
2kR
i
+ φ
i
(k)
, (89.9)
where k is the magnitude of the photoelectron wavevec-
tor and the sum is done for N ‘shells’ of atoms
surrounding the emitter. The distance R
i
is the radius
of the ith shell and 2kR
i
is the associated phase factor
Part G 89.3
1350 Part G Aplications
for the backscattered photoelectron, with an amplitude
A
i
(k). The phase shift φ
i
(k) is required due to the
backscattering path through the potential surrounding
the emitter and scattering from the atom at R
i
.Ifthe
φ
i
could be ignored, then a simple Fourier transform of
χ(k) would reveal the radial distribution function and
the bond lengths R
i
[89.29]. In practice, the phase shifts
cannot be neglected, but very often these can be found by
studying chemically similar systems in which the bond
lengths are known. The phase shifts for photoelectrons
well above the absorption edge (having kinetic ener-
gies greater than ≈50 eV) are dominated by the atomic
ion cores, and so are not sensitive to the valence elec-
tronic structure. The phase shifts can also be calculated
in a straightforward way since this problem is essen-
tially the same as done in LEED multiple scattering
calculations.
The amplitudes A
i
(k) due to scattering from shell i
at distance R
i
from a point source emitter are [89.29]
A
i
(k) =
N
∗
i
kR
2
i
| f
i
(π, k)| exp
− 2
u
2
i
k
2
×exp
− 2R
i
/λ
, (89.10)
4500 4600 4700 4800
02 684
2468
2468
Photon energy (eV)
a)
Intensity (arb. units)
S = Cu(111)(√3 × √3) R30°–I
B = Bulk CuI
R(Å)
c)
Intensity (arb. units)
I–Cu
S
B
k
2
χ(k)
b)
S
B
k
2
χ(k)
d)
k(Å
–1
)
k(Å
–1
)
S
B
ε
hω
Fig. 89.4 (a) X-ray absorption data taken near the iodine edge for bulk CuI and Cu(111)–I. (b) The extracted fine structure
function χ(k), shown multiplied by k
2
to enhance the high k structure. (c) The Fourier transform of χ(k) showing the
location of the first shell of Cu atoms from the I emitters. (d) Back-transformed k
2
χ(k) after applying a filter to extract
the nearest neighbor data. After [89.30]
where N
∗
i
is an effective number of atoms and
u
2
i
is the
mean-square displacement of the atoms in shell i.The
backscattering amplitude | f
i
(π, k)| has been separated
from its phase factor φ
i
(k) in (89.9). The inelastic mean
free path for the photoelectrons reduces the contribution
from successive shells by a factor exp(−2R
i
/λ),andit
is this term that allows the kinematic single-scattering
approach to be used. Multiple scattering paths involve
longer trajectories and so are more strongly attenuated
by this exponential factor. If the near-edge energy region
of the absorption cross section is not included in the
analysis, then the scheme outlined in (89.9)and(89.10)
is reasonable. The near-edge region (within ≈ 50 eV of
the absorption edge) is troublesome, not only because
of multiple scattering, but also because the phase shifts
φ
i
(k) for low energy photoelectrons are more sensitive to
valence electron distributions, and so are more sensitive
to the details of the local chemical environment.
The SEXAFS method is illustrated with the data
of Fig. 89.4 for iodine adsorbed on Cu(111). Panel (a)
shows the measured X-ray absorption σ(hν) for both
the surface system Cu(111)–I and bulk CuI. The ex-
tracted χ(k) and their Fourier transforms are shown in (b)
Part G 89.3
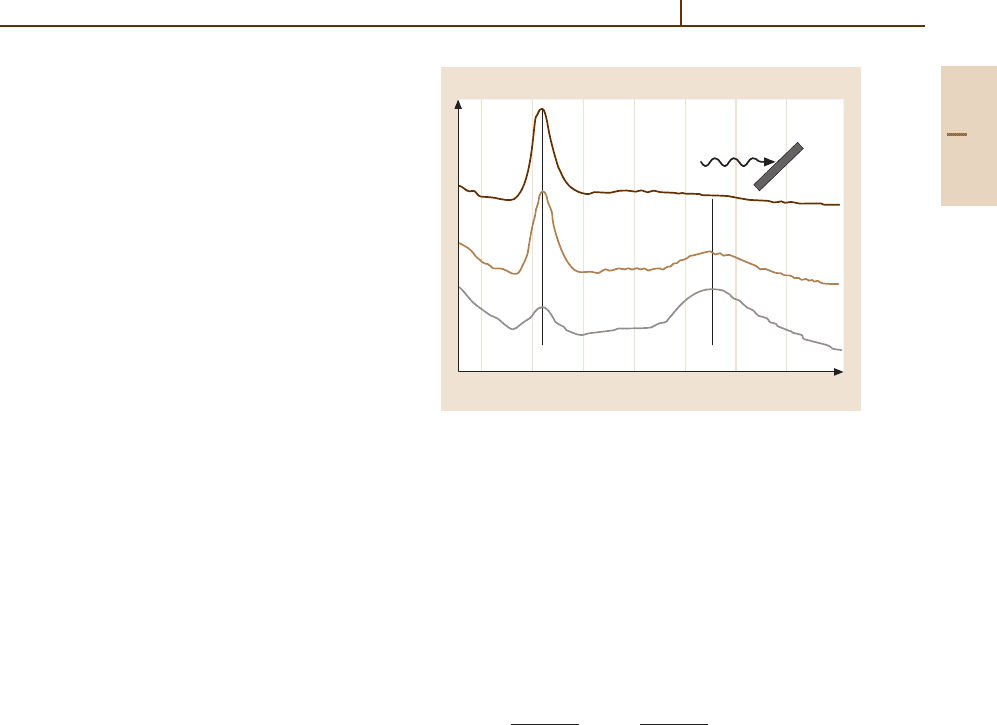
Surface Physics 89.4 Atom–Surface Interactions 1351
and (c). The Cu–I nearest neighbor bondlength is clearly
shownby the peak in (c), and is found to be 0.07 Å longer
than in bulk CuI. SEXAFS is most commonly applied to
atomic adsorption systems since molecular systems are
difficult to analyze, as they can contain several similar
bond lengths, and the shells containing different atoms
are difficult to model.
NEXAFS: Molecular Orientation at Surfaces
In SEXAFS, the X-ray absorption close to the excita-
tion edge is avoided due to the problems of multiple
scattering, and phase shifts that are chemically sensi-
tive to valence electrons. The study of near-edge X-ray
absorption fine structure (NEXAFS) can avoid these
difficulties by using only the symmetry properties of
the transition matrix element without concern for the
absolute amplitudes [89.32]. For isolated molecules,
unoccupied molecular states having σ or π symme-
try are very commonly found close to or just below
the threshold for photoemission. These ‘molecular res-
onances’ often remain for adsorbed molecules, and
the symmetry properties of the absorption intensity
I ∝|f | A· p|i|
2
can be used to determine the molecular
orientation.
For the overall matrix element to be nonzero, it
must be totally symmetric. Using linearly polarized syn-
chrotron radiation, the direction of polarization A can
be varied by rotating the crystal. For example, the K-
edge X-ray absorption of CO on the Ni(100) surface in
Fig. 89.5 shows final state resonances A (π-symmetry)
and B (σ-symmetry). The intensity of these features de-
pends of the direction of polarization of the incident
X-rays. X-rays with a polarization vector parallel to
the surface (θ = 90
◦
) strongly excite the π-symmetry
absorption while the σ-symmetry absorption is absent.
For grazing incidence X-rays polarized normal to the
surface, the σ-resonance is prominent. From dipole se-
lection rules, this polarization dependence of the X-ray
absorption is evidence that the CO molecule is adsorbed
280 290 300 310
Photon energy (eV)
Carbon Auger yield (arb. units)
AB
45°
10°
θ
= 90°
CO on Ni(100)
C K-edge
X-rays
θ
Fig. 89.5 Near-edge X-ray absorption data from the C K-
edge from CO adsorbed on Ni(100) as function of incident
photon angle θ. The molecular π (peak A) and σ (peak B)
resonances are observed. The polarization dependence of
the absorption allows the CO orientation to be determined.
After [89.31]
with its bond perpendicular to the plane of the Ni(100)
surface.
The σ-resonance final state in NEXAFS corresponds
to multiple scattering of the photoelectron along the
bond axis. The overall phase shift for this final state
is approximately
E − V(r) dr ≈
E − V(r) R = const. (89.11)
where R is the bond length. This sensitivity of the final
state phase shift to bond lengths allows the σ-resonance
energy to be used as a measure the molecular bond
length [89.33]. A plot of the σ -resonance energy vs.
1/R
2
shows a linear relationship, as is found in the case
of simple hydrocarbons in the gas phase and adsorbed
on a Cu(100) surface [89.32].
89.4 Atom–Surface Interactions
89.4.1 Physisorption
The binding interactions between atoms and surfaces
can be classified as physisorption (long range attrac-
tive dispersion forces) and chemisorption, in which
chemical bonds are formed. The long-range dispersion
force between a polarizable atom and a conducting
surface give rise to the leading term of the van der
Waals potential V(z) ∝−1/z
3
. At smaller atom-surface
separations, the location of the reference image plane
needs to be included, resulting in an attractive potential
V(z) =−C
v
/|z − z
i
|
3
where z
i
is the distance from the
last atomic plane to the image plane, typically 2–3 Å.
In principle, the constant C
v
is calculable from the
dielectric properties of the substrate and the atomic po-
larizability, but experiments have found values of C
v
Part G 89.4

1352 Part G Aplications
40% less than expected [89.34]. The cause of this dis-
crepancy has not been clarified, although contributions
from surface roughness have been suggested.
At larger atom-surface separations, retardation ef-
fects must be taken into account in the dispersion
interaction (the Casimir–Polder force, Sect. 79.2.4), and
here theory predicts a 1/z
4
interaction potential. This
form for the potential has been confirmed experimen-
tally [89.35].
As the atom approaches the surface, wave function
overlap eventually causes repulsion. In the absence of
chemical bond formation, the repulsive potential is sim-
ply proportional to the surface electron density n(z),
resulting in the overall physisorption potential
V(z) = Kn(z) −
C
v
|z − z
i
|
3
. (89.12)
The surface charge density n(z) decreases exponentially
above the surface [89.2], and the constant K can be de-
termined to reasonable accuracy by an effective medium
theoretical approach [89.36]. This shallowphysisorption
potential well has been studied experimentally in atomic
beam (often He) scattering experiments. Under certain
scattering conditions, it is possible for an incident atom
to be ‘selectively adsorbed’ on the surface by making
transitions into and out of bound states of the potential
well of (89.12). The incident atom with wavevector k
is diffracted by a surface reciprocal lattice vector G
hk
into a bound state E
n
of the potential well [89.2]. The
diffracted atomic beam intensities due to scattering via
selective adsorption show strong variations which can be
related to the quantum numbers (n, h, k), and give infor-
mation on the shape of the physisorption potential well.
89.4.2 Chemisorption
Many atoms and molecules will chemisorb when
brought sufficiently close to a surface, forming chem-
ical bonds (covalent or ionic) that are much stronger than
the physisorption bond [89.3]. In chemisorption, there
is charge transfer between the adsorbate and the sur-
face, modifying the electronic structure of both. Valence
electronic levels of the adsorbate are shifted in energy
and also broadened by resonant interactions with the
delocalized valence electrons at the surface (e.g., free
electron-like s–p states). For many transition metal sur-
faces, interactions with the more localized d–states are
also important.
The adsorbate-surface bond energies are most com-
monly studied by thermal desorption spectroscopy
(TDS) [89.37, 38]. In this method, the adsorbate cov-
ered surface is heated using a linear temperature ramp,
and the desorption rate of a particular species is meas-
ured using a mass spectrometer. By using a range of
heating rates β, not only can the desorption energy be
measured, but the kinetics governing the desorption pro-
cess can be uncovered. For example, in the simplest
case of a coverage-independent adsorption energy and
first order kinetics, if the peak desorption rate occurs at
a temperature T
0
then [89.39]
ν
β
=
E
d
k
B
T
2
0
exp
E
d
k
B
T
0
(89.13)
where E
d
is the desorption energy and ν is the ‘attempt
frequency’ for desorption. Since both ν and E
d
are un-
known, values for both can be found by measuring TDS
spectra using two different heating rates β or by estimat-
ing ν (often ν ≈ 10
13
s
−1
). More complex desorption
kinetics are studied by utilizing the full desorption pro-
file, and a range of heating rates and initial adsorbate
coverages. These kinetic data are also applicable to ad-
sorption if the adsorption and desorption are reversible
processes. For irreversible adsorption systems, it is pos-
sible to measure the adsorption energies directly [89.40]
by monitoring the ir radiation emitted from a surface
as a submonolayer quantity of atoms or molecules is
adsorbed.
89.5 Recent Developments
Surface Physics has both rapidly matured and ex-
panded its connections with other disciplines in the
last eight years. A number of notable review works
have been published recently [89.41–45]. The appli-
cation of surface science techniques has expanded
rapidly in a number of technological areas, such as
semiconductor devices, catalysis, and magnetic ma-
terials. The emerging field of nanotechnology has
drawn heavily from the techniques of surface sci-
ence. Important advances have been made in a large
number of areas, including biosurfaces [89.46,47], clus-
ter science [89.48], carbon fullerene materials [89.49,
50], electrochemistry [89.51], and surface photochem-
istry [89.52].
Continuing advances have been made in the appli-
cation of light from synchrotron radiation sources — so
Part G 89.5
