Drake G.W.F. (editor) Handbook of Atomic, Molecular, and Optical Physics
Подождите немного. Документ загружается.


Applications of Atomic and Molecular Physics to Astrophysics 82.7 Shocked Gas 1243
infrared region. CO, SiO and possibly H
+
3
have been
identified. In the absence of grains, molecules must
be formed through either three-body or radiative pro-
cesses. In the supernova ejecta, the densities are too low
for three-body processes to be effective and molecules
are formed through radiative association reactions. The
molecules are removed by reactions with He
+
and the
molecular abundances put a constraint on how much he-
lium can be mixed back into the region with carbon and
oxygen [82.37].
82.7 Shocked Gas
Shock waves occur in compressible fluids when the pres-
sure gradients are large enough to generate supersonic
motion, or when a disturbance is propagating through
the fluid at supersonic velocities. Because information
about the disturbance cannot propagate upstream in the
fluid faster than the speed of sound, the fluid cannot re-
spond dynamically until the shock arrives. The shock
then compresses, heats, and accelerates the fluid. The
boundary separating the hot compressed gas and the up-
stream gas is the shock front in which the energy of
directed motion of the shock is converted to random
thermal energy.
Shocks are ubiquitous in the interstellar medium
where they are driven by the ionization fronts of ex-
panding Hii regions or nebulae, by outflowing gas
accompanying stellar birth and evolution, and by super-
nova explosions. If the shock velocity is above 50 km/s,
the shock gas is excited, dissociated, and ionized. The
subsequent recombination and cooling radiation pro-
duces photons that may ionize and dissociate the gas
components ahead of and behind the shock. This pre-
cursor radiation modifies the effects of the shock and
influences its dynamical and thermal evolution. Fast
shocks destroy all molecules by dissociating H
2
by col-
lisions with H, H
2
and He and with electrons. Exchange
reactions with H atoms destroy the other molecular
species. At low densities, radiative stabilization occurs
and dissociation is less efficient. Molecules reform in
the cooling postshock gas. Slower shocks do not cause
ionization or dissociation, but the chemical composition
and the ion composition are modified by reactions taking
place in the warm gas. The response of the interstellar
gas to slow shocks is significantly affected by the pres-
ence of a magnetic field. In some ionization conditions,
a magnetic precursor may occur in which a magne-
tosonic wave carries information about the shock, and
the ionized and neutral components of the gas react dif-
ferenly to the shock. Many different kinds of shock have
been identified [82.38].
A very fast shock with a velocity of hundreds of
km/s such as are driven by supernova explosions, cre-
ates a hot dilute cavity in the interstellar medium with
a temperature of millions of degrees. The density is low
and the gas cools and recombines slowly. Overlapping
supernova-induced cavities may be responsible for the
hot gas that occupies a considerable volume of the in-
terstellar medium in the Galaxy and in some external
galaxies. The conditions are far from coronal equilib-
rium as the gas cools more rapidly than it recombines.
The cooling radiation appears as soft X-rays and UV
emission lines with a characteristic spectrum.
As the gas cools below 10 000 K, molecular for-
mation occurs. Molecular hydrogen is formed on the
surfaces of grains as in molecular clouds and by the neg-
ative ion sequence that is effective in the early Universe
Sect. 82.8. With the formation of H
2
in a still warm gas,
the chemistry is driven by exothermic and endothermic
reactions with H
2
. Thus OH is produced by the reac-
tion of O atoms, and H
2
O by the further reaction of
OH with H
2
. Enhanced abundances of other neutral and
ionic molecules are the products of subsequent reactions
with OH. The reactions of S
+
and S with OH lead to
SO
+
and SO, and their simultaneous presence may be
an indicator of a dissociative shock. There are in addi-
tion physical indicators of shocks, such as asymmetric
line profiles indicating high velocities.
In a nondissociative shock in a molecular gas, reac-
tions with warm H
2
dominate the chemistry as it does in
the cooling zone of a dissociative shock. The composi-
tion is controlled by the post shock temperature and the
H/H
2
ratio. The warm H
2
changes the ionic composition
by converting C
+
into CH
+
. Evidence for a nondissocia-
tive shock is the infrared emission from H
2
.Thethermal
emission from collisionally excited vibrational levels in
shock-heated gas is readily distinguished from that dis-
cussed in Sect. 82.3 arising from UV pumping in a PDR.
Emission from H
2
has been detected in numerous ob-
jects in the Galaxy and in many distant external galaxies.
In external galaxies, X-rays may contribute to the H
2
in-
frared sprectrum through heating the gas and through
excitation by photoelectron pumping to excited states
followed by a downward cascade [82.39,40].
Part G 82.7
1244 Part G Applications
82.8 The Early Universe
Molecules appeared first in the Universe after the adi-
abatic expansion had reduced the matter and radiation
temperature to a few thousand degrees and recombi-
nation occurred, creating a nearly neutral Universe. The
small fractional ionization that remained was essential to
the formation of molecules. Molecular hydrogen formed
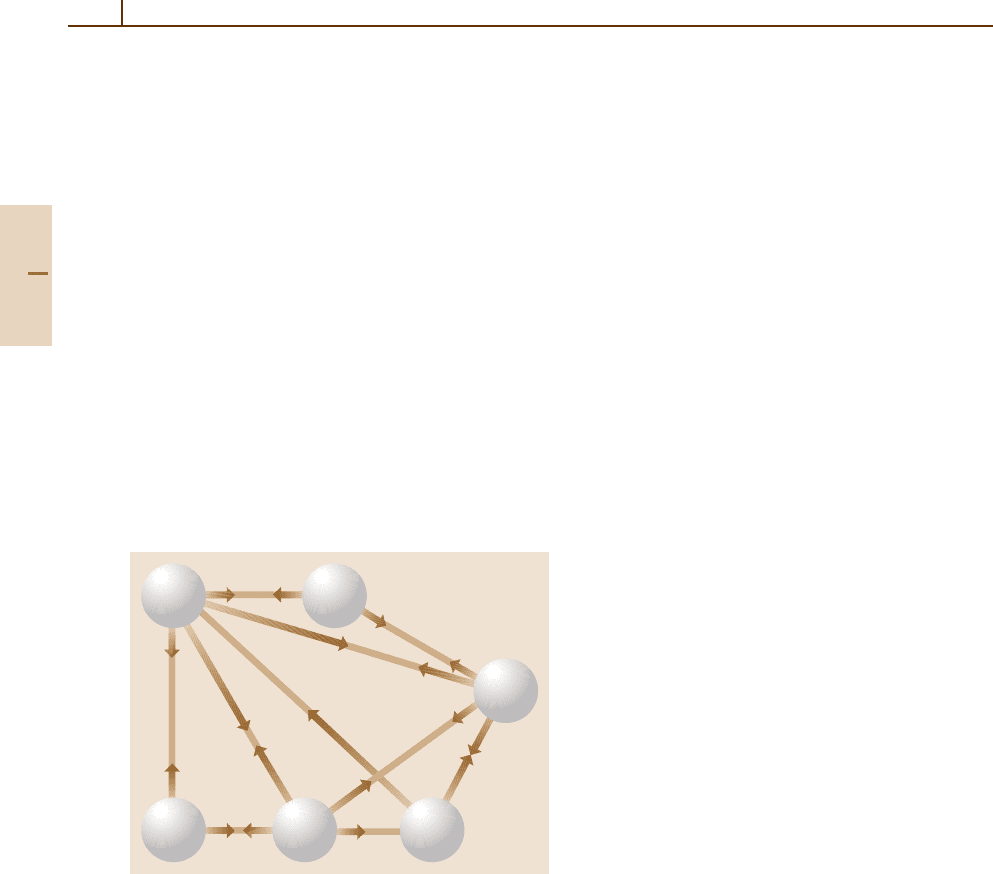
through the sequences
H
+
+ H → H
+
2
+ hν (82.25)
H
+
2
+ H → H
2
+ H
+
(82.26)
and
H+ e
−
→ H
−
+ hν (82.27)
H
−
+ H → H
2
+ e
−
, (82.28)
the protons and electrons acting as catalysts. Many other
atomic and molecularprocesses occurred Fig. 82.3,some
involving excited hydrogen atoms. Thus
H
∗
+ H
2
→ H
+
3
+ e
−
(82.29)
was a source of H
+
3
.
H
H
–
H
+
H
+
2
H
+
3
H
2
e
H
H
H
H
2
e
e
e
H
+
,
H
–
e
H(n = 2)
H(n = 2)
H(n = 2)
ϑ
ϑ
ϑ
ϑ
e,
ϑ
ϑ
Fig. 82.3 Diagram showing the important reactions in the
production of hydrogen molecules in the early Universe
The Universe contained trace amounts of deuterium
and
7
Li nuclei with which heteronuclear molecules
could be made. Molecules with dipole moments may
leave an imprint on the cosmic blackbody background
radiation that occupies the Universe. The deuterated
molecules HD form from
D
+
+ H
2
→ HD+ H
+
, (82.30)
and H
2
D
+
from
D+ H
+
3
→ H
2
D
+
+ H , (82.31)
HD
+
+ H
2
→ H
2
D
+
+ H , (82.32)
and
HD+ H
+
2
→ H
2
D
+
+ H . (82.33)
Lithium hydride is formed through
Li + H → LiH + hν
(82.34)
Li+ H
−
→ LiH+ e
−
(82.35)
Li
−
+ H → LiH+ e
−
. (82.36)
There are many destruction processes, of which
LiH+ H → Li + H
2
(82.37)
may be the most severe, though its rate coefficient
is uncertain. The chemistry of the early Universe is
summarized in [82.41].
The formation of molecules was a crucial step in
the fragmentation of the first gravitationally collapsing
objects which separated out of the cosmic flow. Three-
body recombination
H+ H + H → H
2
+ H (82.38)
Li+ H + H → LiH + H (82.39)
may be a major source of molecules as the density
increases.
82.9 Recent Developments
While the core atomic and molecular process outlined
are still unchanged, our understanding of the astro-
physical environment has been greatly enhanced by
a number of recent satellites. The Wilkinson Microwave
Anisotropy Probe (WMAP) has given us the best map
of the universe at the time of recombination and given
us general confirmation of the Big Bang model. Per-
haps the most surprising result is that stars seem to have
formed much sooner than would have been expected,
about 180 million years after the big bang [82.42]. This
makes it even more difficult to understand how the uni-
verse goes from the relative uniformity at the time of
recombination to the collapse and formation of the first
objects so quickly, a problem which is certainly con-
trolled by atomic and molecular processes. The relevant
atomic and molecular processes have been recently re-
Part G 82.9

Applications of Atomic and Molecular Physics to Astrophysics References 1245
viewed by Lepp, Stancil and Dalgarno [82.43]. Two
recent X-ray satellites, Chandra and XMM-Newton,
both launched in 1999, have greatly increased our ability
to detect hot ionized gas in stars, supernova remnants,
active galaxies and other regions [82.44]. In particular,
Chandra has allowed us for the first time to directly ob-
serve the hot gas between galaxies [82.45]. The Infrared
Space Observatory (ISO) has provided a tremendous
amount of data on cold regions in our own galaxy and
allowed us to directly observe the icy mantles of dust
grains [82.46].
Since the detection of molecules in SN 1987A, there
have been many more observations of CO molecules in
Type II supernova and theymay even occur in every Type
II [82.47]. CO has also been observed in a Type Ic super-
nova [82.48]. It remains a puzzle as to why the molecules
are not rapidly removed by helium ions. A recent calcu-
lation of the O+ He
+
system [82.49] finds that radiative
charge transfer is much faster then direct charge transfer
for temperatures below 10
6
K, but still too slow to sig-
nificantly reduce the helium ion abundance in supernova
ejecta. The most likely explanation remains that mixing
is not complete in supernova ejecta, and the molecules
survive in regions of relatively low helium abundance.
The state of modeling photoionized clouds has been
recently reviewed by Ferland [82.50]. He also highlights
the great need for atomic and molecular data for analyz-
ing these clouds. New satellite data along with continued
ground observations continually raise new astrophysi-
cal puzzles, puzzles which are controlled and probed by
atomic and molecular processes. The astrophysical com-
munity owes a great debt to both atomic and molecular
laboratory measurements and theoretical models of en-
ergy levels, reaction rates, and transition probabilities.
In order to continue to progress in our understanding of
the universe we will need to continue to fund the under-
standing of the atomic and molecular processes which
control it.
82.10 Other Reading
Astronomy is one of the oldest sciences and one
of the fastest evolving. Advances in technology are
rapidly increasing the sensitivity and resolution of
our instruments and so new observations and more
sophisticated models lead to an ever greater under-
standing of the Universe. This means that books
will often be somewhat dated when they appear.
However, the series Annual Review of Astronomy
and Astrophysics is a good source of recent review
articles.
In addition, good introductions or overviews of
a particular field are given in [82.1, 51–56]. Many
sources of atomic and molecular data are listed and dis-
cussed in [82.7]. For details on atomic spectroscopy,
see [82.57, 58]. For details on molecular spectroscopy,
see [82.59,60].
References
82.1 D. E. Osterbrock: Astrophysics of Gaseous Nebulae
and Active Galactic Nuclei (Univ. Science Books, Mill
Valley 1989)
82.2 P. F. Winkler, G. W. Clark, T. H. Markert, K. Kalata,
H. W. Schnopper, C. R. Canizares: Astrophys. J. Lett.
246,27L(1981)
82.3 R. F. Reilman, S. T. Manson: Astrophys. J. Suppl. Ser.
40, 815 (1979)
82.4 B. L. Henke, P. Lee, T. J. Tanaka, R. L. Shimabukoro,
B. K. Fujikawa: Atom. Nucl. Data Tables 27, 1 (1982)
82.5 M. Balucinska-Church, D. McCammon: 400,699
(1992)
82.6 D. A. Verner, D. G. Yakovlav, J. M. Band, A. B. Trzhas-
kavskaya: Atom. Nucl. Data Tables 55, 233 (1993)
82.7 Special issue of Revista Mexicana de Astronomia
y Astrofisica, March 23 (1992)
82.8 H. Nussbaumer, P. J. Storey: Astron. Astrophys. 178,
324 (1978)
82.9 H. R. Ramadan, Y. Hahn: Phys. Rev. A 39, 3350
(1989)
82.10 N. R. Badnell: Phys. Scr. T 28, 33 (1989)
82.11 M. C. Bacchus-Montabonel, K. Amezian: Z. Phys. D
25, 323 (1993)
82.12 P. Honvault, M. C. Bacchus-Montabonel, R. McCar-
roll: J. Phys. B 27, 3115 (1994)
82.13 B. Herrero, I. L. Cooper, A. S. Dickinson, D. R. Flower:
J. Phys. B 28, 711 (1995)
82.14 C. Mendoza: Planetary Nebulae IAU Symp. 103,ed.
by D. R. Flower (Reidel, Dordrecht 1983) p. 143
82.15 M. Brocklehurst, M. Salem: Comp. Phys. Commun.
13, 39 (1977)
82.16 M. Salem, M. Brocklehurst: Astrophys. J. Suppl. Ser.
39, 633 (1979)
82.17 P. G. Martin: Astrophys. J. Suppl. Ser. 66, 125 (1988)
82.18 P. J. Storey, D. G. Hummer: Mon. Not. R. Astron. Soc.
272, 41 (1995)
Part G 82

1246 Part G Applications
82.19 M. Arnaud, R. Rothenflug: Astron. Astrophys.
Suppl. 60,425(1985)
82.20 T. J. Gaetz, E. E. Salpeter: Astrophys. J. Suppl. Ser.
52,155(1983)
82.21 E. van Dishoeck, J. Black: Astrophys. J. Suppl. Ser.
62, 109 (1987)
82.22 W. G. Roberge, D. Jones, S. Lepp, A. Dalgarno: As-
trophys. J. Suppl. Ser. 77, 287 (1991)
82.23 A. Sternberg, A. Dalgarno: Astrophys. J. Suppl. Ser.
99, 565 (1995)
82.24 R. Gredel, S. Lepp, A. Dalgarno, E. Herbst: Astro-
phys. J. 347, 289 (1989)
82.25 T. J. Millar, A. Bennett, J. M. C. Rawlings, P. D. Brown,
S. B. Charnley: Astron. Astrophys. Suppl. 87,585
(1991)
82.26 P. R. A. Farquhar, T. J. Millar: CCP7 Newsletter 18,6
(1993)
82.27 R. L. Kurucz: Astrophys. J. Suppl. Ser. 40, 1 (1979)
82.28 M. J. Seaton: J. Phys. B 20, 6363 (1987)
82.29 A. E. Lynas-Gray, M. J. Seaton, P. J. Storey: J. Phys.
B 28, 2817 (1995)
82.30 M. J. Seaton, Y. Yan, B. Mihalas, A. K. Pradhan:
Mon. Not. R. Astron. Soc. 266, 805 (1994)
82.31 C. A. Iglesias, F. J. Rogers: Astrophys. J. 443,460
(1995)
82.32 A. Omont: Circumstellar Chemistry. In: Chemistry in
Space, ed. by J. M. Greenberg, V. Pirronello (Kluwer
Academic, Dordrecht 1991) p. 171
82.33 G. A. Mamon, A. E. Glassgold, A. Omont: Astrophys.
J. 323, 306 (1987)
82.34 L. A. M. Nejad, T. J. Millar: Astron. Astrophys. 183,
279 (1987)
82.35 T. J. Millar, E. Herbst: Astron. Astrophys. 288, 561
(1994)
82.36 R. McCray: Ann. Rev. Astron. Astrophys. 31,175
(1993)
82.37 W. Liu, S. Lepp, A. Dalgarno: Astrophys. J. 396,679
(1992)
82.38 B. T. Draine, C. F. McKee: Ann. Rev. Astron. Astro-
phys. 31, 373 (1993)
82.39 S. Lepp, R. McCray: Astrophys. J. 269, 560 (1983)
82.40 R. Gredel, A. Dalgarno: Astrophys. J. 446,852
(1995)
82.41 A. Dalgarno, J. Fox: Ion Chemistry in Atmospheric
and Astrophysical Plasmas. In: Unimolecular and
Bimolecular Reaction Dynamics, ed. by C.-Y. Ng,
T. Baer, I. Powis (Wiley, New York 1994)
82.42 C. L. Bennett et al.: Astrophys. J. Suppl. 148, 1 (2003)
82.43 S. Lepp, P. Stancil, A. Dalgarno: J. Phys. B 35,R57
(2002)
82.44 F. Paerels, S. Kahn: Ann. Rev. Ast. Appl. 41,291
(2003)
82.45 F. Nicasto et al.: Nature 433, 495 (2005)
82.46 C. Cesarsky, A. Salama: ISO Science Legacy: A Com-
pact Review of ISO Major Achievements (Springer,
2005)
82.47 J. Spyrimilo, B. Leibundgut, R. Gilmozzi: Ast. Ap.
376, 188 (2001)
82.48 C. Gerardy et al.: PASJ 54, 905 (2002)
82.49 L. B. Zhao et al.: Astrophys. J. 615, 1063 (2004)
82.50 G. Ferland: Ann. Rev. Ast. Appl. 41, 517 (2003)
82.51 C. W. Allen: Astrophysical Quantities (Athlone, Lon-
don 1973)
82.52 K. R. Lang: Astrophysical Formula (Springer, Berlin,
Heidelberg 1980)
82.53 W. W. Duley, D. A. Williams: Interstellar Chemistry
(Academic, London 1984)
82.54 L. Spitzer: Physical Processes in the Interstellar
Medium (Wiley, New York 1978)
82.55 A. Dalgarno, D. R. Layzer: Spectroscopy of Astro-
physical Plasmas (Cambridge Univ. Press, Cam-
bridge 1987)
82.56 T. Hartquist: Molecular Astrophysics (Cambridge
Univ. Press, Cambridge 1990)
82.57 R. D. Cowan: The Theory of Atomic Structure and
Spectra (Univ. California Press, Berkeley 1981)
82.58 I. I. Sobelman: Atomic Spectra and Radiative Tran-
sitions (Springer, Berlin, Heidelberg 1979)
82.59 G. Herzberg: Molecular Spectra and Molecular
Structure (Prentice-Hall, New York 1939)
82.60 P. F. Bernath: Spectra of Atoms and Molecules (Ox-
ford Univ. Press, Oxford 1995)
Part G 82
1247
Comets
83. Comets
With the exception of the in situ measurements
made by the Giotto and Vega spacecraft at
comet 1P/Halley (the P/ signifies a periodic comet)
during March 1986, all determinations of the
volatile composition of the coma are derived
from spectroscopic analyses. Detailed modeling
is then used to infer the volatile composition
of the cometary nucleus. This chapter focuses
on the principal atomic and molecular processes
that lead to the observed spectrum as well as
the needs for basic atomic and molecular data in
the interpretation of these spectra. The largely
collisionless and low density coma, with no
gravity or magnetic field, is a unique spectroscopic
laboratory, as evidenced by the discovery
of C
3
before its identification in terrestrial
laboratories [83.1]. Many key discrepancies remain
to be resolved concerning the basic molecular
composition and the elemental abundances of
both the volatile and refractory components of the
cometary nucleus, as well as the comet-to-comet
variation (particularly between “new” and evolved
periodic comets) of these quantities. These issues
(and many others) are discussed in the recent
83.1 Observations .......................................1247
83.2 Excitation Mechanisms.........................1250
83.2.1 Basic Phenomenology................1250
83.2.2 Fluorescence Equilibrium ...........1250
83.2.3 Swings and Greenstein Effects ....1251
83.2.4 Bowen Fluorescence ..................1252
83.2.5 Electron Impact Excitation ..........1253
83.2.6 Prompt Emission .......................1253
83.2.7 OH Level Inversion.....................1254
83.3 Cometary Models .................................1254
83.3.1 Photolytic Processes ..................1254
83.3.2 Density Models .........................1255
83.3.3 Radiative Transfer Effects ...........1256
83.4 Summary ............................................1256
References ..................................................1257
analytical review of Festou et al. [83.2, 3]orin
the compendia of Halley results [83.4]. The former
also contains a comprehensive bibliography. Other
sources concentrating largely on the physics and
chemistry of comets include the volumes edited
by Wilkening [83.5]andHuebner [83.6]andthe
pre-Halley review of Mendis et al. [83.7].
Comets are small bodies of the solar system believed
to be remnants of the primordial solar disk. Formed
near the orbits of Uranus and Neptune and subsequently
ejected into an “Oort cloud” of some 40 000 AU in ex-
tent, these objects likely preserve a record of the volatile
composition of the early outer solar system, and so are
of great interest for the physical and chemical mod-
eling of solar system formation. The comets arrive in
the inner solar system as a result of galactic pertur-
bations. The cometary volatiles are vaporized as their
orbits bring them closer to the sun and it is solar ra-
diation that initiates all of the processes that lead to
the extended coma. Gas vaporization also leads to the
release of dust into the coma, and the scattering of sun-
light by dust is the major source of the visible coma
and dust tail of comets. Somewhat fainter, and much
more extended, is the plasma tail, resulting from pho-
toionization by solar extreme UV radiation of the neutral
volatiles and their subsequent interaction with the solar
wind.
83.1 Observations
In a review in 1965, Arpigny [83.8] summa-
rized the known molecular and atomic emis-
sions detected in the visible region of the spec-
trum (here defined as 3000 to 11 000 Å) as
follows:
radicals: OH, NH, CN, CH, C
3
,C
2
,andNH
2
ions: OH
+
,CH
+
,CO
+
2
,CO
+
,andN
+
2
;
metals: Na, Fe .
Part G 83

1248 Part G Applications
The only known atomic feature was the O i forbid-
den red doublet at 6300 and 6364 Å. From the radicals
and ions one could infer the presence of their progen-
itor “parent” molecules such as H
2
O, NH
3
,HCN,CO
and CO
2
, directly vaporizing from the comet’s nucleus.
The metals, seen only in comets passing close to the
sun, were assumed to come from the vaporization of
refractory grains. The inventory of metals was soon
expanded to include K, Ca
+
, Ca, V, Cr, Mn, Ni and
Cu, from observations of the sun-grazing comet Ikeya-
Seki (C/1965 S1) [83.9,10]andH
2
O
+
was identified in
comet Kohoutek (C/1973 E1). This latter comet was also
the first to be extensively studied at wavelengths both
shortward and longward of the visible spectral range.
The first parent molecule to be directly identified
was CO, which fluoresces in the Fourth Positive system
A
1
Π
u
− X
1
Σ
+
in the VUV [83.11], although the in
situ neutral mass spectrometer measurements made of
Halley disclosed the presence of an extended, domi-
nant source of CO [83.12] whose origin is still being
debated [83.13]. Ideally, the molecular species should
be detectable through their radio and sub-mm rotational
transitions or through the detection of vibrational bands
or individual ro-vibrational lines in the near IR. Water
was first directly detected through ro-vibrational lines
near 2.7 µmincometHalleyandagainincometWilson
(C/1986 P1) [83.14, 15]. However, due to the low col-
umn densities of the other expected species, typically ≈1
or less than that of H
2
O, the direct detection of species
such as H
2
CO, H
2
SandCH
3
OH has only recently been
made possible by the development of more sensitive
instrumental techniques together with the fortuitous ap-
paritions of two bright comets, C/1996 B2 (Hyakutake)
and C/1995 O1 (Hale-Bopp) in 1996 and 1997. To date,
more than two dozen parent molecules have been iden-
tified [83.16]. Isotope ratios, particularly the D/H ratio,
in molecules such as HDO have been determined from
sub-millimeter observations [83.17].
The ultimate result of solar photolysis (and to a lesser
degree, the interaction with the solar wind) is the re-
duction of all of the cometary volatiles to their atomic
constituents. The atomic inventory is somewhat easier
to derive as the resonance transitions of the cosmically
abundant elements H, O, C, N and S all lie in the VUV
and, in principle, the total content of these species in the
coma can be determined by an instrument with a suitably
large field of view. Of course, a fraction of the atomic
species of each element will be produced directly in ionic
form, and will not be counted using this approach. In ad-
dition, another fraction exists in the coma in the solid
grains, and this component will also not be included,
except for a small amount volatilized by evaporation
or sputtering by energetic particles. The composition of
the grains, though not the absolute abundance, has been
determined from in situ measurements made by the Hal-
ley encounter spacecraft [83.18], and can be inferred,
though not unambiguously, from reflection spectroscopy
of cometary dust in the 3–5 µm range.
The advent of space-borne platforms for obser-
vations in the VUV has produced a wealth of new
information about the volatile constituents of the coma.
The A
2
Σ
+
− X
2
Π(0, 0) band of the OH radical at
≈ 3085 Å was well known from ground-based spectro-
scopic observations, but as this wavelength lies very
close to the edge of the atmospheric transparency win-
dow, the strength of this feature (relative to that of
other species) was not appreciated until 1970 when
comet Bennett (C/1969 Y1) was observed from space by
the Orbiting Astronomical Observatory (OAO-2). The
OAO-2 spectrum also showed a very strong, broadened
H i Ly-α emission from H, the other principal dissocia-
tion product of H
2
O. The broad shape of Ly-α seen in
the OAO-2 spectrum is due to the large spatial extent
of the atomic H envelope, the result of a high velocity
acquired in the photodissociation process and a long life-
time against ionization. Later, at the apparition of comet
Kohoutek (C/1973 E1), atomic O and C were identified
in the spectra and direct UV images of the H coma, as
well as of the O i and C i emissions, were obtained from
sounding rocket experiments. These experiments were
repeated for comet West (C/1975 V1) and led to the first
detection of CO [83.11].
Between 1978 and 1996, over 50 comets were
observed spectroscopically over the wavelength range
1200–3400 Å by the International Ultraviolet Explorer
(IUE) satellite observatory [83.19, 20]. Most of the
spectra were obtained at moderate resolution (∆λ =
6–10 Å), although high dispersion echelle spectra (∆λ
=0.2–0.3 Å) are useful for some studies, particu-
larly those of fluorescence equilibrium (Sect. 83.2.2).
For Halley alone, over 200 UV spectra were obtained
from September 1985 to July 1986. The launch of the
Hubble Space Telescope (HST) in 1990, together with
subsequent enhancements to the spectroscopic instru-
mentation that were made on-orbit, marked another
advance in sensitivity as well as the ability to observe
in a small field-of-view very close to the nucleus. This
yielded the first detection of CO Cameron band emis-
sion, a direct measure of CO
2
being vaporized from
the nucleus [83.21]. For an overview of a cometary
spectrum, a composite spectrum of 103P/Hartley 2 span-
ning the region from H i Ly-α to 7000 Å taken with
Part G 83.1
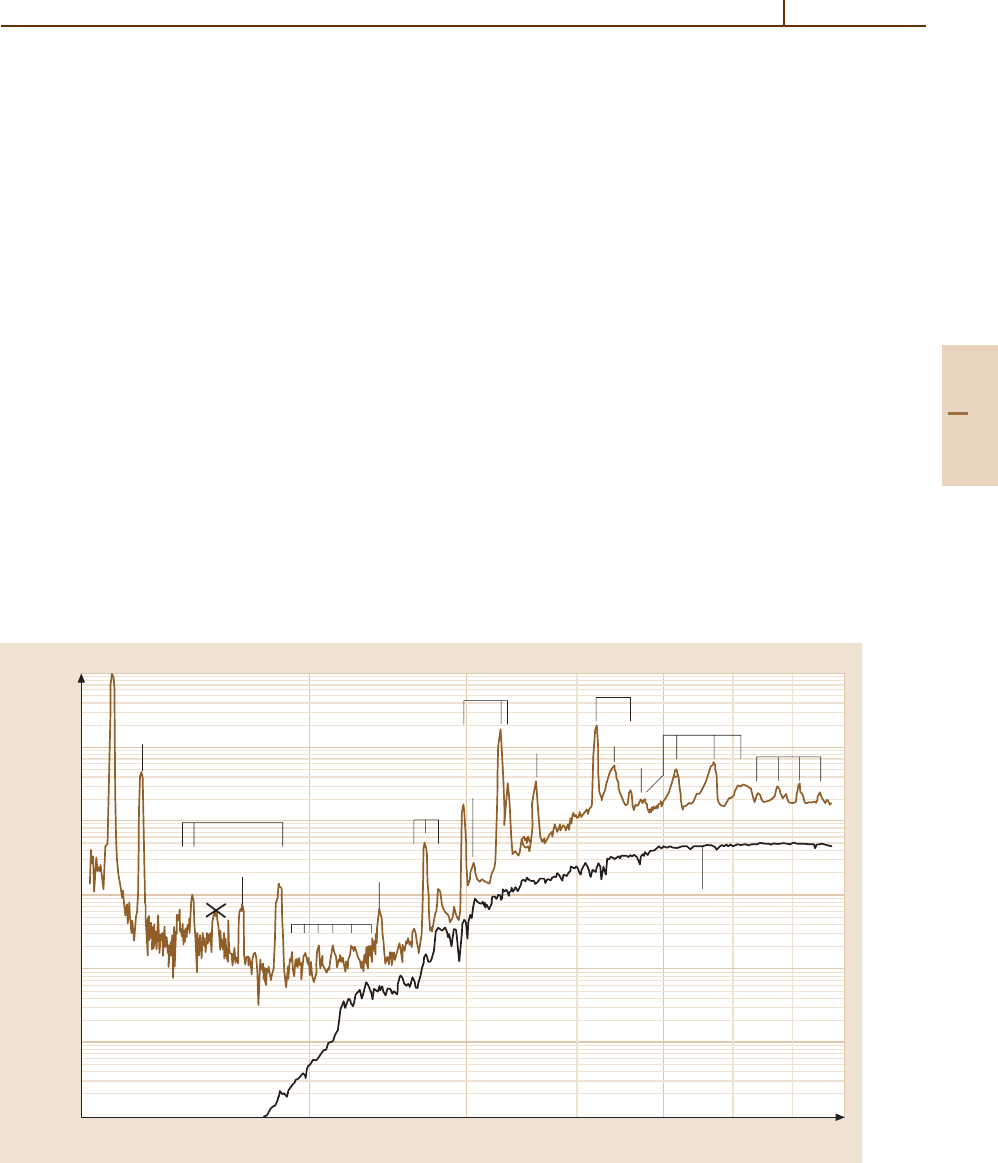
Comets 83.1 Observations 1249
the Faint Object Spectrograph of HST, using five sep-
arate gratings, is shown in Fig. 83.1. The launch of the
Far Ultraviolet Spectroscopic Explorer (FUSE) in 1999
provides access to the spectral region between 900 and
1200 Å at very high spectral resolution, and has led to
the detection of H
2
(Sect. 83.2.4), upper limits on Ar
and N
2
, and some three dozen unidentified emission
lines [83.22,23].
Several other satellite observatories have contributed
unique cometary observations in the UV and sub-mm
spectral windows. The Solar and Heliospheric Ob-
servatory (SOHO) has two valuable instruments: The
SWAN (Solar Wind Anisotropies) instrument provides
skymapsinHi Ly-α at 1
◦
resolution and has ob-
served over 20 comets since 1996 [83.25]. The UVCS
(Ultraviolet Coronograph Spectrometer) provides far-
uv spectra and images of comets close to the Sun,
where HST and FUSE are prohibited from observing,
and has recently detected C
++
in the tail of comet
C/2002 X5 (Kudo-Fujikawa) [83.26]. The direct de-
tection of H
2
O in the fundamental rotational line at
557 GHz in several comets was made by the Submil-
limeter Wave Astronomy Satellite (SWAS) [83.27]and
the Odin satellite [83.28]. This line cannot be ob-
served from ground-based telescopes because of the
10 000.00
1000.00
100.00
10.00
1.00
0.10
0.01
1100 2000 3000 5000 8000
Wavelength (Å)
Brightness (Rayleighs/Å)
H
O
C
S
CO
Cameron
C
2
CS
Co
2
+
OH
NH
C
3
CN
CH
C
2
NH
2
Sun
Fig. 83.1 Composite FOS spectrum of comet 103P/Hartley 2. After [83.24]
strong absorption by water vapor in the terrestrial
atmosphere.
Prior to 1996, X-rayshad not been detected in comets
and the conventional wisdom wasthat they were unlikely
to be produced in the cold, rather thin cometary atmo-
sphere. The discovery of soft X-rayemission (E <2keV)
from comet Hyakutake (C/1996 B2) by the Röntgen
Satellite (ROSAT) thus came as a surprise [83.29].
Since then, X-ray emission has been detected from over
a dozen comets using ROSAT and four other space
observatories, the Extreme Ultraviolet Explorer, Bep-
poSAX,theChandra X-ray Observatory (CXO), and
Newton-XMM [83.30]. The earliest observations were
at very low spectral resolution, making it difficult to se-
lect amongst the possible excitation mechanisms: charge
exchange, scattering of solar photons by attogram dust
particles, energetic electron impact and bremsstrahlung,
collisions between cometary and interplanetary dust, and
solar X-ray scattering and fluorescence. The more re-
cent CXO observations, at higher spectral resolution,
favor the charge exchange of energetic minor solar wind
ions such as O
6+
,O
7+
,C
5+
,C
6+
, and others, with
cometary gas, principally H
2
O, CO, and CO
2
, as the pri-
mary mechanism. This mechanism would explain why
the X-ray intensity appears to be independent of the gas
Part G 83.1
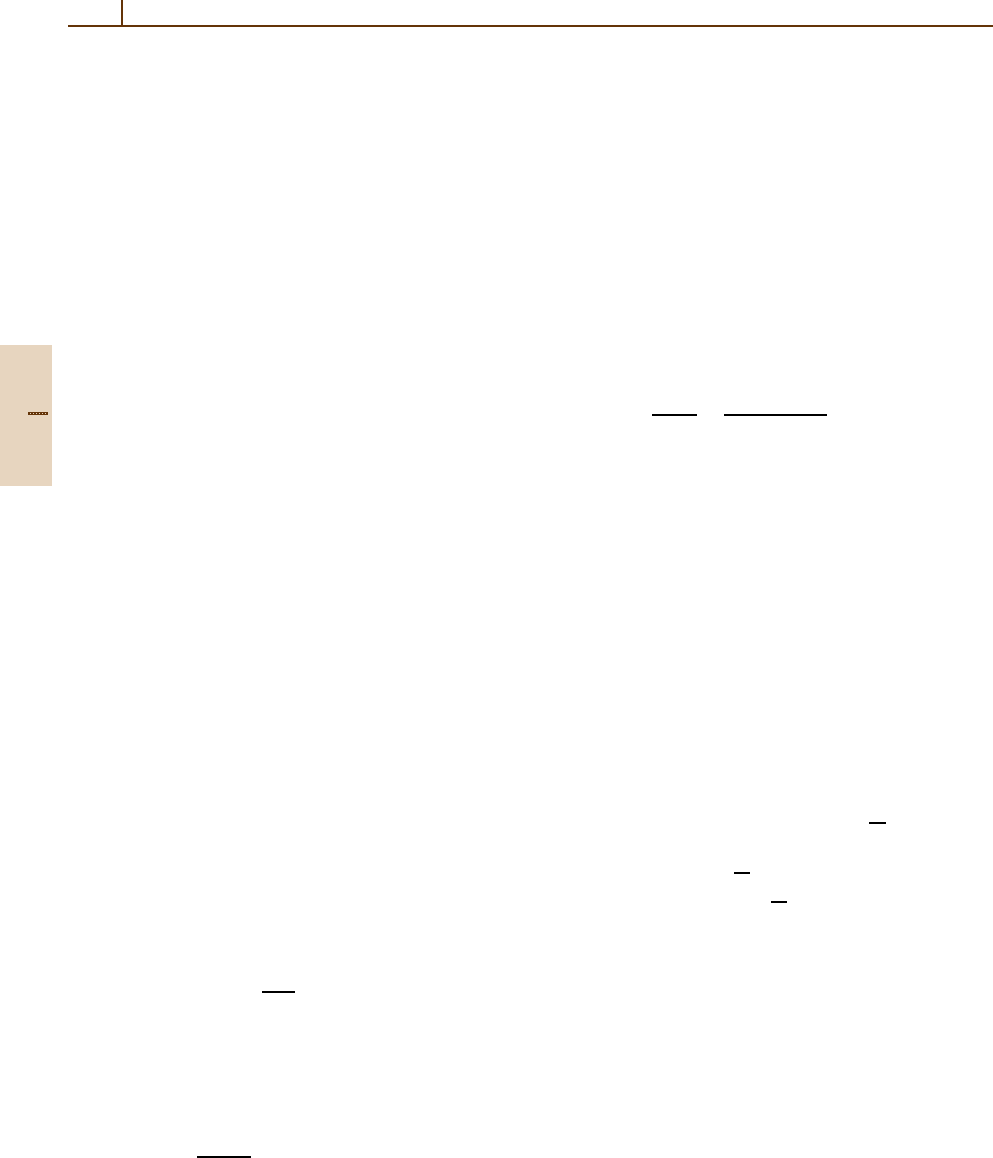
1250 Part G Applications
production rate of the comet, and that the peak emis-
sion is offset from the location of the comet’s nucleus.
This conclusion is also supported by recent laboratory
work on the charge transfer of highly ionized species
with cometary molecules [83.31] and by theoretical cal-
culations of state specific cascades [83.32]. The X-ray
emission thus tells us more about the solar wind than
about the gaseous composition of comets.
83.2 Excitation Mechanisms
83.2.1 Basic Phenomenology
Coma abundances may be derived from spectropho-
tometric measurements of either the total flux or the
surface brightness in a given spectral feature. The un-
certainty in the derived abundances includes not only
the measurement uncertainty, but also uncertainties in
the atomic and molecular parameters and, in the case
of surface brightness measurements, uncertainties in the
model parameters used. Thus relative abundances, de-
rived from observations of different comets with the
same instrument and under similar geometrical condi-
tions, are often more reliable.
Atoms and ions in the cometary coma emit radiation
primarily by means of resonance re-radiation of solar
photons. For the cosmically abundant elements H, C, N,
O, and S, their strongest resonance transitions are in the
VUV. The few exceptions are noted below. Assume that
the coma is optically thin in these transitions. The total
number of species i in the coma is
M
i
= Q
i
τ
i
(r), (83.1)
where Q
i
is the production rate (atoms or molecules s
−1
)
of species i and τ
i
(r) is its lifetime at heliocentric dis-
tance r, τ
i
(r) = τ
i
(1 AU)r
2
.Ther dependence arises
from photolytic destruction processes induced by solar
UV radiation, and to a lesser degree by the solar wind,
as described in Sect. 83.3.1.
The luminosity, in photons s
−1
, in a given transition
at wavelength λ,isthen
L
iλ
= M
i
g
iλ
(r), (83.2)
where the fluorescence efficiency, or “g-factor”, g
iλ
(r) =
g
iλ
(1 AU)r
−2
,is
g
iλ
(1 AU) =
πe
2
mc
2
λ
2
f
λ
πF
˜
ω
photons s
−1
atom
−1
, (83.3)
where f
λ
is the absorption oscillator strength, πF
is
the solar flux per unit wavelength interval at 1 AU and
˜
ω
is the albedo for single scattering, defined for a line in
an atomic multiplet as
˜
ω =
A
j
j
A
j
, (83.4)
where A
j
is the decay rate. If a given multiplet is not
resolved, then
˜
ω = 1. For diatomic molecules, fluores-
cence to other vibrational levels becomes important and
the evaluation of
˜
ω depends on the physical conditions in
the coma, as discussed in Sect. 83.2.2. Thus, for a comet
at a geocentric distance ∆, the total flux from the coma
for the transition is
F
iλ
=
L
iλ
4π∆
2
=
Q
i
g
iλ
(r)τ
i
(r)
4π∆
2
, (83.5)
and the product g
iλ
(r)τ
i
(r) is independent of r.
Unfortunately, the scale lengths (the product of life-
time and outflow velocity) of almost all of the species of
interest in the UV are ≈10
5
–10
6
km at 1 AU. Thus, total
flux measurements require fields of view ranging from
several arc-minutes to a few degrees. This has been done
only in the case of a few isolated sounding rocket exper-
iments. Most information about the UV spectra comes
from observations made by orbiting satellite observa-
tories whose spectrographs have small apertures (e.g.,
10
×20
for IUE) and thus sample only a very small
part of the total coma. In this case, again assuming an
optically thin coma, the measured flux F
iλ
in the aper-
ture can be converted to an average surface brightness
B
iλ
(in units of Rayleighs):
B
iλ
= 4π10
−6
F
iλ
Ω
−1
, (83.6)
where Ω is the solid angle subtended by the aperture.
The brightness, in turn, is related to
N
i
, the average
column density of species i within the field of view by
B
iλ
= 10
−6
g
iλ
(r)N
i
. (83.7)
The evaluation of Q
i
from N
i
requires the use of a model
of the density distribution of the species i (Sect. 83.3.2).
A similar treatment can be applied to the excitation
of the near infrared vibrational transitions of cometary
parent molecules since the direct pumping by solar IRra-
diation far exceeds the indirect pumping of ground state
vibrational levels through electronic transitions excited
by the solar UV flux [83.16]. However, this does not ap-
ply to the rotational transitions which are controlled by
collisional excitation, primarily collisions with H
2
O. In
this case, the observed rotational temperature may be re-
garded as a reliable measure of the kinetic temperature
of the coma gas.
Part G 83.2
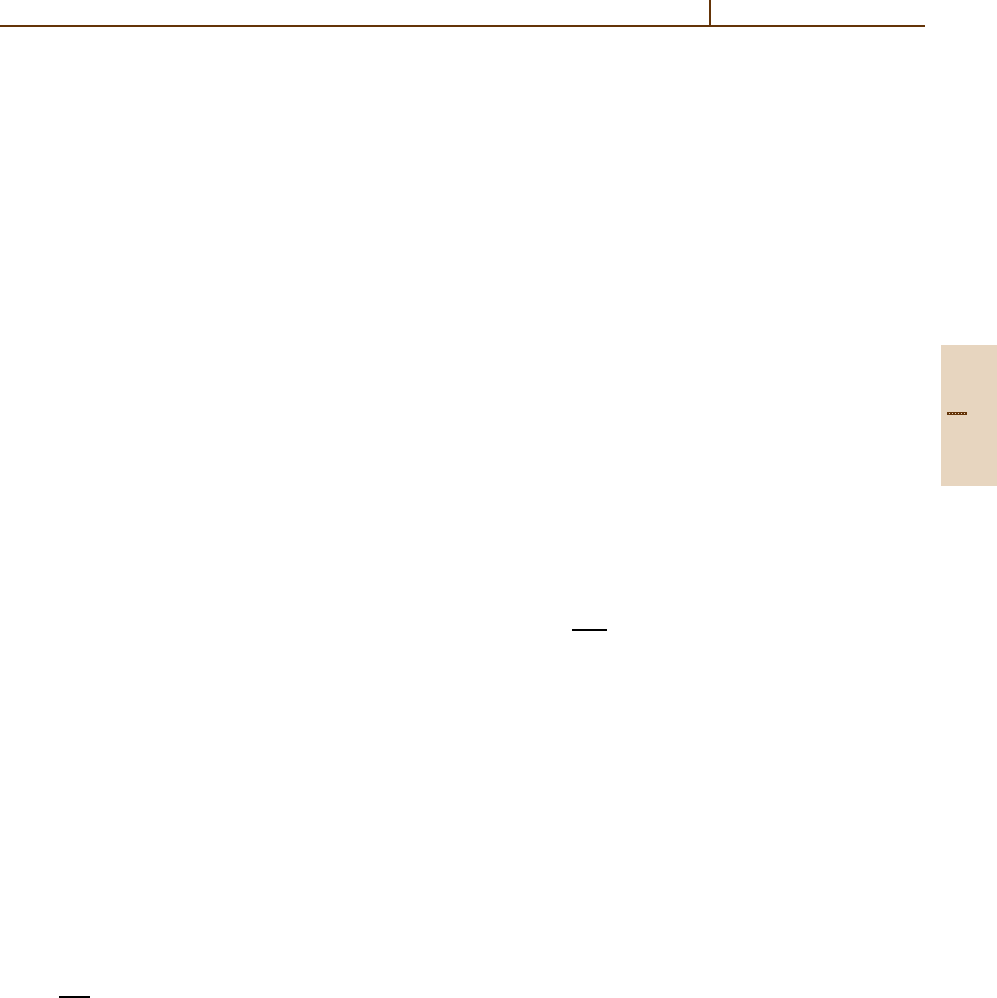
Comets 83.2 Excitation Mechanisms 1251
83.2.2 Fluorescence Equilibrium
In the case of low resolution spectroscopy, where an
atomic multiplet or molecular band is unresolved, the
evaluation of the g-factor (83.3) does not require knowl-
edge of the population of either atomic fine structure
levels or molecular rotational levels in the ground state
of the transition. Furthermore, the assumption that the
solar flux does not vary over the multiplet or band allows
us to use the total transition oscillator strength. This as-
sumption is more often than not invalid because of the
Fraunhofer structure of the solar spectrum in the near
uv and visible region and the emission line nature of
the spectrum below 2000 Å. For high resolution spectra,
the g-factors for each individual line must be calculated
separately and the relative populations of the ground
state levels must be included. There are three cases to be
considered:
1. The g-factor, or probability of absorption of a solar
photon, is less than the probability that the species
will be dissociated or ionized, i. e., g
iλ
<(τ
i
)
−1
.
In this case, the ground state population is not af-
fected by fluorescence and a Boltzmann distribution
at a suitable temperature (typically ≈200 K at 1 AU)
corresponding to the production of the species may
be used. This is often the case in the far UV,where
the solar flux is low, such as for the Fourth Posi-
tive band system of CO. For atomic transitions from
triplet ground states, such as is found with O, C
and S, downward fine structure transitions are fast
enough to effectively depopulate all but the lowest
fine structure level.
2. The species undergoes many photon absorption and
emission cycles in its lifetime, and the ground state
population is determined (usually after 5 or 6 cycles)
by the fluorescence branching ratios. This is the con-
dition of fluorescence equilibrium, which applies for
almost all radicals observed in the visible and near
UV regions. The general procedure is to solve a set
of coupled equations of the form
dn
a
dt
=−n
a
N
b=1
p
ab
+
N
b=1
n
b
p
ba
, (83.8)
where n
a
is the relative population of level a and p
ab
and p
ba
are transition rates out of and into this level,
respectively. The n
a
are normalized to unity. The
steady state, obtained after many cycles, is given
by dn
a
/dt = 0. Since the downward transition rates
are determined only by quantum mechanics, while
the absorption rates depend on the magnitude of the
solar flux, the steady state population varies with
distance from the sun with higher rotational levels
(as for the case of CN [83.8]) populated closer to the
sun. In some cases, where only a few cycles occur,
the equations are integrated numerically. As the g-
factor varies with time, it also effectively varies with
the position of a species in the coma. Care must also
be taken when spectra taken with small apertures are
analyzed as the transit time for an atom or molecule
to cross the aperture may be
g
iλ
(r)
−1
. In practice,
these considerations are often not important.
3. The same as 2. except that the density is sufficiently
high that collisional transitions must be included in
addition to the radiative transitions between lev-
els. However, as the collisional rates are poorly
known, in practice a “collision sphere” is defined
such that a molecule traveling radially outward from
this sphere suffers only one collision with other
molecules or atoms. Outside this sphere, fluores-
cence equilibrium is assumed to hold, while inside
a thermal distribution of ground state levels is used.
A rough estimate of the radius R
c
of the colli-
sion sphere, based on the radial outflow model of
Sect. 83.3.2 is given by [83.2,3]
R
c
= 10
3
Q
10
29
km , (83.9)
where Q isthe total production rate in molecules s
−1
.
83.2.3 Swings and Greenstein Effects
Swings [83.33] first pointed out that because of the
Fraunhofer absorption lines in the visible region of the
solar spectrum, the absorption of solar photons in a mo-
lecular band would vary with the comet’s heliocentric
velocity
˙
r, leading to differences in the structure of
a band at different values of
˙
r when observed at high
resolution. In (83.8), this corresponds to evaluating the
p
ba
= p
ba
(
˙
r). For typical comets observed near 1 AU,
˙
r can range from –30 to +30 km/s, while in certain
cases of comets with small perihelia the range can be
twice as large. This effect of the Doppler shift between
the sun and the comet is commonly referred to as the
Swings effect. Even for observations at low resolution,
the Swings effect must be taken into account in the cal-
culation of the total band g-factor, and this has been
done for a number of important species such as OH, CN
and NH. A particularly important case, that of the OH
A
2
Σ
+
− X
2
Π (0,0) band at ≈ 3085 Å, which is often
used to derive the water production rate of a comet,
Part G 83.2
1252 Part G Applications
is illustrated in Fig. 83.2, which also shows the de-
pendence of fluorescence equilibrium on heliocentric
distance [83.34].
While this effect was first recognized in the spectra
of radicals in the visible range, a similar phenomenon
occurs in the excitation of atomic multiplets below
2000 Å, where the solar spectrum makes a transition to
1.00 AU; unquenched
1.00 AU; quenched
0.25 AU; unquenched
0.25 AU; quenched
8
6
4
2
0
–60 –40 –20 0 20 40 60
Heliocentric velocity (km/s)
L/N × r
H
2
(10
–15
erg/s × molecule)
Fig. 83.2 OH (0,0) band g-factor as a function of heliocen-
tric velocity. After [83.34]
6
5
4
3
2
1
0
8
6
4
2
–0.3 –0.2 –0.1 0 0.1 0.2 0.3
r
°
(km/s)
80 40 0 –40 –80
g(× 10
–6
/s × atom)
Solar
flux
(×10
9
photons/
cm
2
× s
× Å)
∆λ(Å)
OI
λ 1302.2 Å
g(Lyβ)
× 10
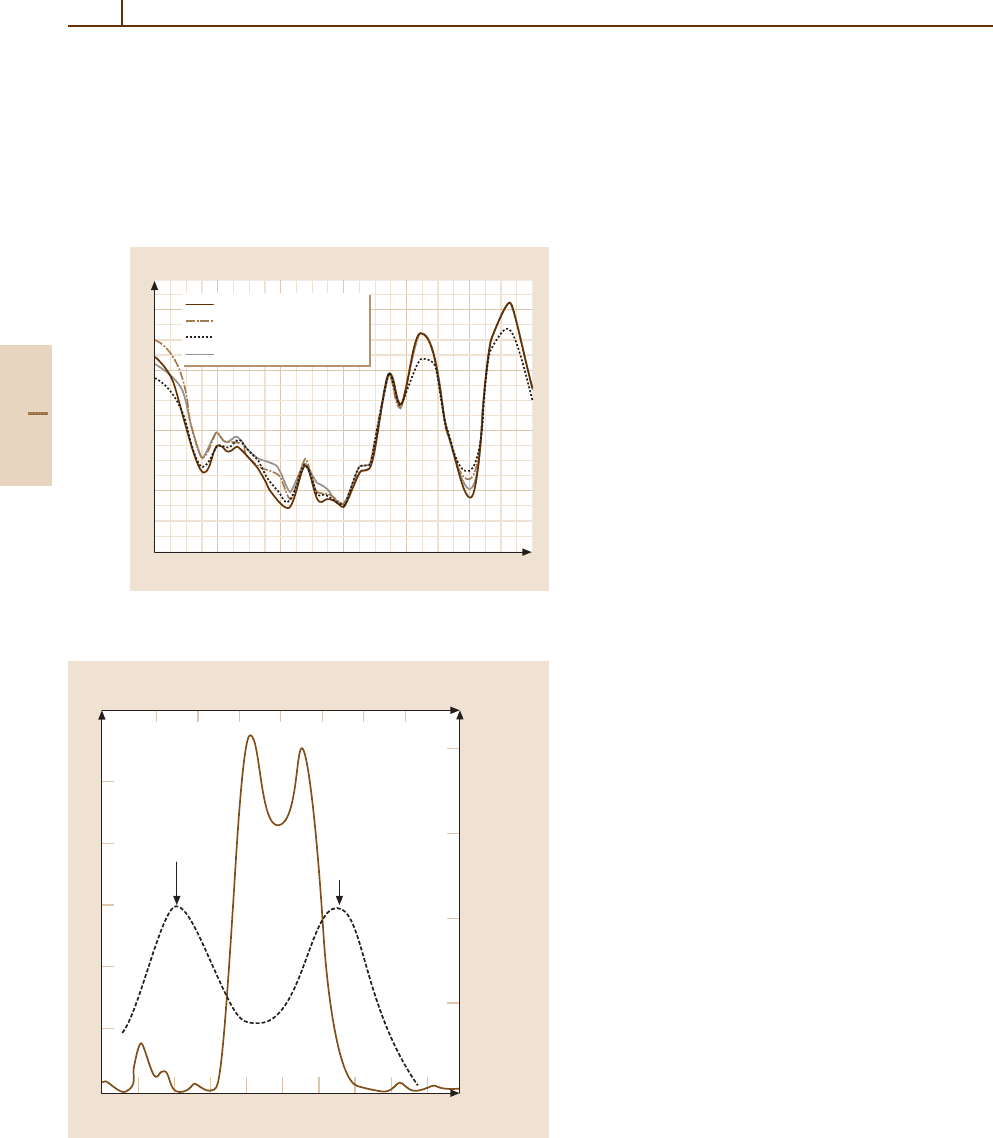
Fig. 83.3 Solar flux and fluorescence efficiency for O i λ1302 as
a function of heliocentric velocity. After [83.35]
an emission line spectrum. For example, the three lines
of O i λ1302 have widths of ≈ 0.1 Å, corresponding to
a velocity of ≈25 km/s, so that knowledge of exact so-
lar line shapes is essential for a reliable evaluation of
the g-factor for this transition [83.35], as illustrated in
Fig. 83.3.
A differential Swings effect occurs in the coma since
atoms and molecules on the sunward side of the coma,
flowing outward towards the sun, have a net velocity that
is different from those on the tailward side, and so, if
the absorption of solar photons takes place on the edge
of a line, the g-factors will be different in the two direc-
tions. Differences of this type appear in long-slit spectra
in which the slit is placed along the sun-comet line (the
Greenstein effect [83.36]). Again, an analogue in the far
UV has been observed in the case of O i λ1302 [83.37],
as can be seen in Fig. 83.3. Although it is also possible
to explain the observation by nonuniform outgassing,
this was considered unlikely as all of the other observed
emissions had symmetric spatial distributions. The mea-
surement of the Greenstein effect leads immediately to
a determination of the mean outflow velocity of the given
species.
83.2.4 Bowen Fluorescence
Figure 83.3 also demonstrates that for heliocentric ve-
locities > 30 km/s, the Doppler shift reduces the solar
flux at the center of the absorption line to a very small
value, so that the O i λ1302 line is expected to appear
weakly, if at all, in the observed spectrum. Thus, it was
a surprise that this line appeared fairly strongly in two
comets, Kohoutek (C/1973 E1) and West (C/1975 V1),
whose values of
˙
r were both > 45 km/s at the times of
observation. The explanation invoked the accidental co-
incidence of the solar H i Ly-β line at 1025.72 Å with the
O i
3
D −
3
P transition at 1025.76 Å, cascading through
the intermediate
3
P state as shown in the simplified en-
ergy level diagram of Fig. 83.4 [83.35]. This mechanism,
well known in the study of planetary nebulae, is referred
to as Bowen fluorescence [83.38]. The g-factor due to
Ly-β pumping is an order of magnitude smaller than
that for resonance scattering, as shown in Fig. 83.3,but
sufficient to explain the observations and to confirm that
H
2
O is the dominant source of the observed oxygen in
the coma.
Ly-β is also coincident with the P1 line of the (6,0)
band of the H
2
Lyman system
B
1
Σ
+
u
− X
1
Σ
+
g
lead-
ing to fluorescence in the same line of several (6,v
)
bands, the strongest being that of the (6,13) band at
1608 Å [83.39]. This line is, however, difficult to ob-
Part G 83.2
