Drake G.W.F. (editor) Handbook of Atomic, Molecular, and Optical Physics
Подождите немного. Документ загружается.


982 Part E Scattering Experiment
66.63 J. M. Farrar, T. P. Schafer, Y. T. Lee: AIP Conference
Proceedings No. 11, Transport Phenomena,ed.by
J. Kestin (American Institute of Physics, New York
1973)
66.64 R. Schinke, J. Bowman: Molecular Collision Dynam-
ics, ed. by J. Bowman (Springer, Berlin, Heidelberg
1983)
66.65 S. Bosanac: Phys. Rev. A 22, 2617 (1980)
66.66 G. Hall, K. Liu, M. J. McAuliffe, C. F. Giese,
W. R. Gentry: J. Chem. Phys. 81, 5577 (1984)
66.67 X. Yang, A. Wodtke: Int. Rev. Phys. Chem. 12,123
(1993)
66.68 I. V. Hertel: Adv. Chem. Phys. 50, 475 (1982)
66.69 W. H. Breckenridge, H. Umemoto: Adv. Chem. Phys.
50, 325 (1982)
66.70 A. G. Suits, P. de Pujo, O. Sublemontier, J. P. Vis-
ticot, J. Berlande, J. Cuvellier, T. Gustavsson,
J. M. Mestdagh, P. Meynadier, Y. T. Lee: J. Chem.
Phys. 97, 4094 (1992)
66.71 J. Los, A. W. Kleyn: Alkali Halide Vapors,ed.by
P. Davidovits, D. L. McFadden (Academic, New York
1979) Chap. 8
66.72 E. A. Gislason: Alkali Halide Vapors, ed. by P. Davi-
dovits, D. L. McFadden (Academic, New York 1979)
Chap. 13
66.73 R. E. Olson, F. T. Smith, E. Bauer: Appl. Optics 10,
1848 (1971)
66.74 W. B. Miller, S. A. Safron, D. R. Herschbach: Discuss.
Faraday Soc. 44, 108 (1967)
66.75 W. B. Miller: . Ph.D. Thesis (Harvard Univ., Harvard
1969)
66.76 J. M. Farrar, Y. T. Lee: J. Chem. Phys. 65, 1414 (1976)
66.77 R. J. Buss, P. Casavecchia, T. Hirooka, Y. T. Lee:
Chem. Phys. Lett. 82,386(1981)
66.78 L. Schnieder, K. Seekamp-Rahn, E. Wrede,
K. H. Welge: J. Chem. Phys. 107, 6175 (1997)
66.79 B. Strazisar, C. Lin, H. F. Davis: Science 290,958
(2000)
66.80 H. L. Kim, M. A. Wickramaaratchi, X. Zheng,
G. E. Hall: J. Chem. Phys. 101, 2033 (1994)
66.81 M. Brouard, S. P. Duxon, P. A. Enriquez, J. P. Si-
mons: J. Chem. Phys. 97, 7414 (1992)
66.82 T. P. Rakitzis, S. A. Kandel, R. N. Zare: J. Chem. Phys.
107, 9382 (1997)
66.83 D. A. Blank, N. Hemmi, A. G. Suits, Y. T. Lee: Chem.
Phys. 231, 261 (1998)
66.84 P. A. Willis, H. U. Stauffer, P. Z. Hinrichs, H. F. Davis:
J. Chem. Phys. 108, 2665 (1998)
66.85 M. Ahmed, D. S. Peterka, A. G. Suits: Phys. Chem.
Chem. Phys. 2, 861 (2000)
66.86 G. Capozza, E. Segoloni, F. Lenori, G. G. Volpi,
P. Casavecchia: J. Chem. Phys. 120, 4557 (2004)
66.87 A. T. J. B. Eppink, D. H. Parker: Rev. Sci. Instrum. 68,
3457 (1997)
66.88 T. N. Kitsopoulos, C. R. Gebhardt, T. P. Rakitzis: Rev.
Sci. Instrum. 72, 3848 (2001)
66.89 D. Townsend, M. P. Minitti, A. G. Suits: Rev. Sci.
Instrum. 74, 2530 (2003)
66.90 J. J. Lin, J. Zhou, W. Shiu, K. Liu: Rev. Sci. Instrum.
74, 2495 (2003)
66.91 J. Zhou, J. J. Lin, K. Liu: J. Chem. Phys. 119, 8289
(2003)
Part E 66
983
Ion–Molecule
67. Ion–Molecule Reactions
The observation of ion–molecule reactions has
a history that goes back to the beginning of the
twentieth century, when J. J. Thomson discovered
that operating his positive ray parabola appara-
tus in a hydrogen atmosphere produced signals
at a mass to charge ratio of 3, which he correctly
attributed to the species H
3
[67.1]. Later studies
showed that this species was produced by a re-
action between the primary ionization product
H
+
2
and molecular hydrogen. Most ion-molecule
reactions proceed without an activation bar-
rier and their cross sections are governed by the
long range attractive potential of the approach-
ing reactants (Sect. 64.2.4). Reaction rates based
on long range potential capture models [67.2]
predict rates in excess of 10
−9
cm
3
molecule
−1
s
−1
,
corresponding to thermal energy cross sections
(Sect. 47.1.7)of10
−16
–10
−15
cm
2
.Theimportance
of ion-molecule reactions in such widely di-
verse areas as planetary atmospheres, (Sect. 84.1),
electrical discharges and plasmas (Sect. 87.1.4),
particularly in semiconductor processing, in
the formation of molecules in interstellar
space (Chapt. 82), and in flames and combustion
67.1 Instrumentation.................................. 985
67.2 Kinematic Analysis............................... 985
67.3 Scattering Cross Sections ...................... 987
67.3.1 State-to-State Differential Cross
Sections ................................... 987
67.3.2 Velocity–Angle Differential Cross
Sections ................................... 988
67.3.3 Total Cross Sections with
State-Selected Reactants............ 989
67.3.4 Product–State Resolved Total
Cross Sections ........................... 989
67.3.5 State-to-State Total Cross
Sections ................................... 990
67.3.6 Energy Dependent Total Cross
Sections .................................. 990
67.4 New Directions:
Complexity and Imaging ...................... 991
References .................................................. 992
systems (Sect. 88.1), has borne out that pre-
diction. This chapter discusses applications of
single-collision scattering methods to the study
of reactive collision dynamics of ionic species with
neutral partners.
A number of different physical processes can be cate-
gorized as ion-molecule reactions, with examples such
as
A
±
+ BC → A+ BC
±
, charge transfer
A
±
+ BC → A+ (B+ C)
±
, dissociative charge
transfer
A
±
+ BC → AB
±
+ C . particle transfer
The ± superscript indicates charge appropriate to anions
and cations. The parentheses indicate that the charge can
reside on either the B or C fragment. Particle transfer
reactions often involve the transfer of a hydrogen atom
or a proton, but heavy particle <transfer processes are
often important ones as well. An interesting example in
which new carbon-carbon bonds are formed, termed a
condensation reaction, is the following:
C
+
+ CH
4
→ C
2
H
+
3
+ H .
An additional process unique to anionic systems is de-
tachment, occurring when the intermediate collision
complex is internally excited above its autodetachment
threshold:
A
−
+ BC →[ABC
−
]
∗
→A+ BC + e
−
,
detachment
A
−
+ BC →[ABC
−
]
∗
→ABC + e
−
.
associative detachment
For exothermic reactions at low collision energies where
the long range attraction dominates the interaction po-
tential, cross sections are consistent with Langevin
orbiting, generally having E
−1/2
energy dependence.
Part E 67
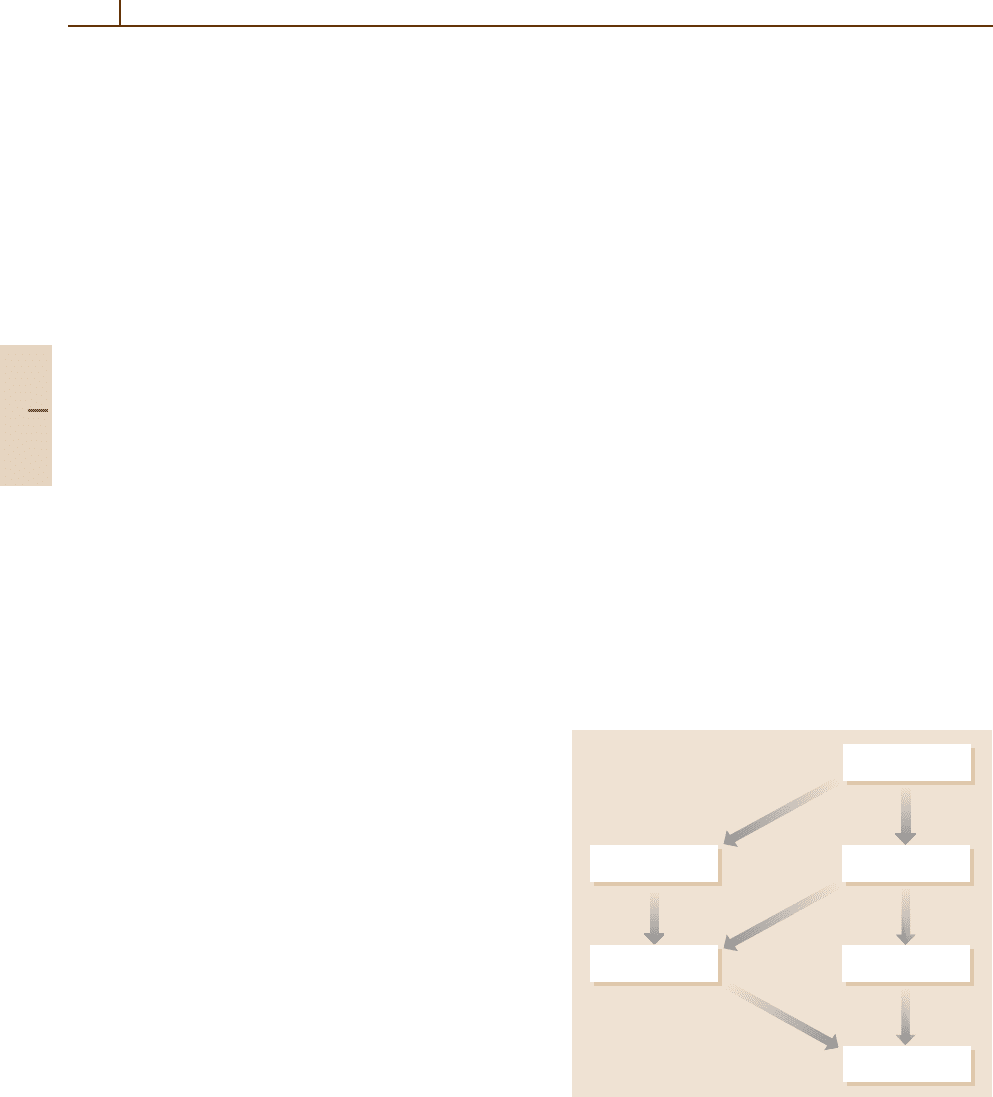
984 Part E Scattering Experiment
At higher energies, cross sections drop below this limit,
as surface crossings and short-range repulsive features
become important. Endothermic reactions exhibit cross
section thresholds, as illustrated in Sect. 67.3.4 on the
N
+
+ D
2
system.
Scattering measurements probe the potential energy
surface, or surfaces, governing the collision dynamics
with techniques that measure the fluxes from specific
reactant quantum states into product quantum states,
scattering angles, and product translational energies.
Scattering experiments define more precisely than in
a bulb the initial and final conditions in a collision
[67.3]. A scattering experiment measures a cross section
(Sect. 91.1.1) rather than a rate constant. The measured
cross section represents an average over initial condi-
tions and a sum over final states inherent in the technique
used.
Cross sections of various forms can be defined with
respect to the rate of formation of product states un-
der single-collision conditions. The total rate of product
formation dN
±
/dt for a beam of ions with number den-
sity n
1
intersecting a gas of number density n
2
within a
scattering volume ∆V, defined by the overlap of the ion
beam with the target gas, is given by
dN
±
/dt = σv
rel
n
1
n
2
∆ V , (67.1)
where σ is the total reaction cross section (Sect. 47.1.7)
and v
rel
is the relative speed of the collision partners. Us-
ing the experimental ion beam current I
±
, cross section
σ, neutral number density n
2
, and attenuation length L,
this expression converts to the particularly useful form
dN
±
/dt = 6.25 × E − 7σI
±
n
2
L (67.2)
for computing signal levels, where σ isexpressedinÅ
2
,
I
±
in nA, n
2
in cm
−3
,andL in cm. An ion beam of
current 1 nA, intersecting a target of length 1 cm at a
pressure of 10
−3
Torr, corresponding to a number den-
sity of 3.5×10
13
cm
−3
at STP, and reacting with a cross
section of 1 Å
2
yields a total rate of product formation
of 2 × 10
7
s
−1
.
The detector observes only a fraction of this total
rate. If reaction products are scattered isotropically over
4π steradians in the laboratory, and the detector entrance
slit of area dS is located a distance r from the collision
center, subtending a solid angle dS/r
2
, the fraction of the
total signal scattered into the slit is dS/(4πr
2
) [67.4].
The fraction of the collision volume, or of the product
state distribution accessible to a particular experimen-
tal method, determines the tradeoffs between resolution
and signal level, and therefore the feasibility of a given
experiment.
In an “ideal” experiment, one collides reactants, with
well specified quantum numbers collectively denoted
n, at a precisely defined relative velocity v
rel
, resolv-
ing products in quantum states n
scattered through
center-of-mass scattering angle θ. The resulting detailed
differential cross section (DCS) (Sect. 47.1.1) is denoted
by σ(n
,θ| n, v
rel
). However, most experiments involve
at least partial averages over initial states and/or sum-
mations over final states. Figure 67.1 shows the result
of averaging σ(n
,θ| n, v
rel
) over θ to yield the state-to-
state cross section at fixed v
rel
, denoted by σ(n
|n, v
rel
).
Averaging this cross section over a Maxwell–Boltzmann
distribution of molecular speeds at a specified temper-
ature T yields the detailed state-to-state rate constant
k(n
|n, T), while summation over the final states n
and
averaging over the initial states n yields the thermal
rate constant k(T). These latter two quantities are ther-
mally averaged, multiple collision properties and not
the subject of this chapter, although they play an im-
portant role in practical applications. Another pathway
for averaging the detailed DCS σ(n
,θ|n, v
rel
) arises
from averaging over n and summing over n
to yield
cross sections differential in product velocity v
rel
and
θ at fixed v
rel
, denoted by σ(v
rel
,θ|v
rel
).Anaverage
over θ and v
rel
yields the velocity dependent total cross
section σ(v
rel
), and its Maxwell-Boltzmann average pro-
duces the thermal rate constant k(T) once again. The
subject of this chapter is a discussion of the various
cross sections σ shown in Fig. 67.1. As one moves from
σ(n⬘,θⱍn,V)
σ(n⬘ⱍn,V)
k(n⬘ⱍn;T)
k(T)
σ(V)
σ(V⬘,θⱍV)
n⬘, n
n⬘, n
v
v
θ
n⬘, n
θ, v⬘
Fig. 67.1 Relationships among differential and total cross
sections, and rate constants. Brackets denote averages over
indicated variables, or averages over initial states and sum-
mations over final states
Part E 67
Ion–Molecule Reactions 67.2 Kinematic Analysis 985
more highly averaged quantities to the detailed cross
sections, more sophisticated reactant preparation and
product detection schemes are required to extract the
desired information, at the expense of decreased sig-
nal levels. Technological advances, particularly in laser
preparation of quantum state-selected reactants and in
state-specific product detection, have made the “ideal”
experiment a near reality in favorable circumstances,
particularly in neutral-neutral interactions, e.g., in ex-
periments on H + H
2
and its isotopic variants. This
chapter discusses the cross sections σ(n
,θ| n, v
rel
),
σ(v
rel
,θ|v
rel
), σ(n
| n, v
rel
),andσ(v
rel
), emphasizing
the dynamical information that can be extracted from
each kind of measurement.
67.1 Instrumentation
Instrumentation for studying ion-molecule reactions
is quite diverse, and numerous literature sources
are available for further discussion [67.5]. A typ-
ical instrument has an ionization source, a primary
mass selector, a collision region, and detector,
consisting of a mass spectrometer or employ-
ing a spectroscopic technique allowing molecular
identification.
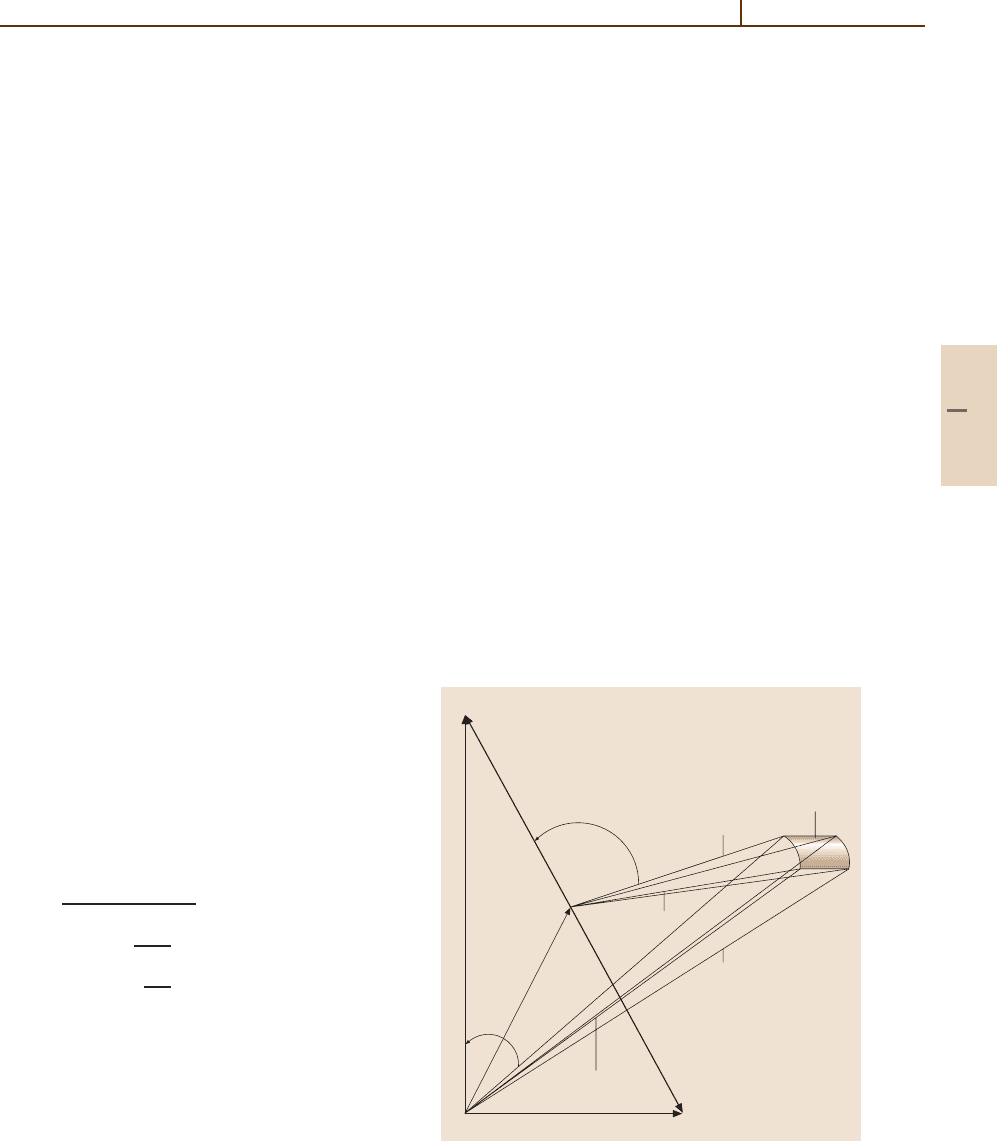
67.2 Kinematic Analysis
The transformation of laboratory measured speeds,
angles and intensities to their center of mass (cm) coun-
terparts can be accomplished with appropriate geometric
constructions [67.6,7]. The geometric relationships can
be understood by considering a kinematic Newton di-
agram for the collision process A+ BC,asshown
in Fig. 67.2. The diagram is constructed for the spe-
cial case in which the reactant beams intersect at 90
◦
.
The laboratory scattering angle and velocity are Θ
and v respectively, while the corresponding center of
mass quantities are θ and u. The beam velocity vec-
tors v
A
and v
BC
define the initial conditions, and the
relative velocity vector v
rel
is defined by their vec-
tor difference. The velocity c of the center of mass,
or centroid, of the collision system is determined by
conservation of linear momentum; the vector c divides
the v
rel
in inverse proportion to the masses of A and
BC:
c =
m
A
v
A
+ m
BC
v
BC
M
,
(67.3)
u
A
=−
m
BC
M
v
rel
, (67.4)
u
BC
=
m
A
M
v
rel
, (67.5)
where M is the total mass of the reactants with masses
m
A
and m
BC
. An observer moving away from the lab-
oratory origin in the direction of the center of mass
vector c would see the reactants A and BC approach
along the direction of v
rel
, with the products retreat-
ing along the direction of v
rel
. The angle θ between
these vectors defines the center of mass scattering an-
gle. For a single Newton diagram, detection of products
at recoil speed u and scattering angle θ requires that
measurements be made at laboratory coordinates (v,
Θ). For monoenergetic incident beams, the measure-
ment of both laboratory scattering angle and speed
results in a unique lab to cm coordinate transforma-
tion. Linear momentum conservation also relates the
center of mass speeds of products AB and C to the
relative velocity of the separating products according
v
A
dS
u
A
c
v
BC
u
BC
v
AB
u
AB
dω
Θ
d
⍀
θ
Fig. 67.2 Kinematic Newton diagram showing laboratory
and center of mass velocities and scattering angles
Part E 67.2

986 Part E Scattering Experiment
to
u
AB
=
m
C
M
v
rel
, (67.6)
u
C
=−
m
AB
M
v
rel
. (67.7)
The final relative kinetic energy of the separating prod-
ucts is
T
rel
=
1
2
µ
v
rel
· v
rel
, (67.8)
where µ
is the reduced mass of the prod-
ucts.
The total energy of a collision system is computed
from the energies of the incident reactants and the energy
accessible to the products:
E
tot
= T
rel
+ E
int
− ∆D
o
0
= T
rel
+ E
int
; (67.9)
T
rel
and E
int
refer to the incident kinetic and inter-
nal excitation energies of the reactants and the primed
quantities correspond to the products. ∆ D
o
0
is the zero
point energy difference of reactants and products. Spec-
ification of the vibrational and rotational energy of a
product determines the relative velocity v
rel
with which
the products separate, according to
v
rel
=
2
µ
(T
rel
+ E
int
− ∆D
o
0
− E
int
)
1/2
.
(67.10)
The quantization of internal energy of the product AB
leads to a series of concentric circles about the cen-
troid that describe the loci of final translational speeds
for AB produced in specific internal states. An exam-
ple of the kinematic resolution of product vibrational
states in the reaction O
−
+ HF → F
−
+ OH is shown
in Fig. 67.3 [67.8, 9].
At a collision energy of 0.47 eV (45.0kJ/mol), the
laboratory flux distribution shows structure that is at-
tributable to the formation of OH in v
= 0, 1, and 2
states. The kinematic Newton diagram at the bottom of
the figure shows the concentric circles corresponding to
the formation of F
−
in concert with OH in specific vibra-
tional states having quantum numbers v
= 0, 1, and 2.
For laboratory scattering angles in the range 0
◦
≤ Θ ≤
11
◦
and 80
◦
≤ Θ ≤ 90
◦
energy scans only intersect the
kinematic circles corresponding to OH in v
= 0and1
states, while data in the intermediate range of angles 18
◦
≤ Θ ≤ 70
◦
show contributions from all three states. The
laboratory speeds at which these vibrational states ap-
pear, for a given Θ, are marked in the figure, showing
clear correspondence with the structure in the experi-
mental data. Rotational excitation in the products will
broaden the contributions from individual vibrational
states in experiments lacking rotational resolution. The
best resolution achievedin electrostatic energy analyzers
is approximately 5 meV (40 cm
−1
); and consequently,
scattering measurements of product fluxes based on ki-
netic energy analysis generally do not have rotational
state resolution. However, photoelectron spectroscopy
measurements on H
2
[67.10] have yielded spectra of
H
+
2
energy levels with rotational resolution.
The transformation of intensities and cross sections
between laboratory and cm coordinate systems relies on
conservation of flux in a transformation between two
coordinate systems moving with respect to one another.
Figure 67.2 shows the nature of this conservation: in
laboratory coordinates, the flux into solid angle dΩ is
given by I
lab
(Ω) dΩ, where the laboratory intensity I
lab
is the flux per unit solid angle, while the corresponding
flux in the cm frame is I
cm
(ω) dω,whereI
cm
is the cm
flux per unit solid angle. The solid angles subtended by
the detector in the laboratory and cm frames expressed
in velocity space may be computed from the surface
element dS subtended by the detector:
dω =
dS
u
2
, dΩ =
dS
v
2
. (67.11)
The flux equality I
lab
(Ω) dΩ = I
cm
(ω) dω leads to the
intensity transformation
I
lab
(v, Θ) =
v
2
u
2
I
cm
(u,θ). (67.12)
The widths of the beam velocity distributions must
be accounted for in extracting accurate cm cross sections
from laboratory data. In the case where the laboratory
flux at a given Θ is comprised of contributions from
individual quantum states of the products, the laboratory
flux is recovered from the relation
I
lab
(v, Θ) =v
2
∞
0
dv
2
f
2
(v
2
)
∞
0
dv
1
f
1
(v
1
)
v
rel
u
2
×
n
σ(n
,θ|n, v
rel
)δ(u − u
n
)
.
(67.13)
In this expression, the velocity distributions for the pri-
mary ion beam and the secondary neutral beam are
given by f
1
(v
1
)and f
2
(v
2
) respectively. The final prod-
uct internal states are labeled by the index n
;thecm
cross section for producing collective quantum state
n
is given by σ(n
,θ|n, v
rel
) and the cm speed cor-
responding to the formation of this state is given by
Part E 67.2
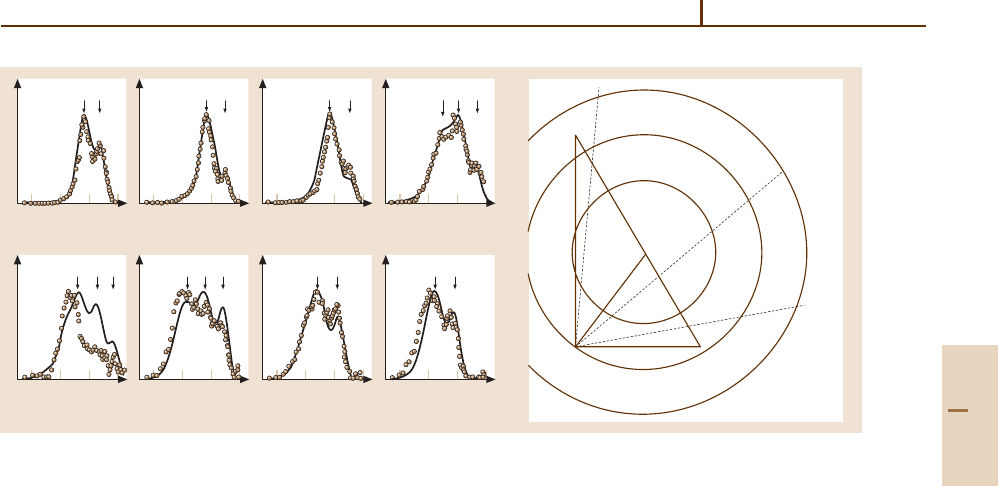
Ion–Molecule Reactions 67.3 Scattering Cross Sections 987
18°
5 15 25 35 5 15 25 35 5 15 25 35 5 15 25 35
5 15 25 35 5 15 25 35 5 15 25 35 5 15 25 35
9° 11°0° 10 1 0 1 0 21 0
1021021010
Lab velocity (100 m/s)
Lab velocity (100 m/s)
Newton
Diagram
CM
v
HF
v
0
–
v
rel
v⬘= 2
v⬘= 1
v⬘= 0
80° 90°70°26°
Fig. 67.3 F
−
reactive fluxes in the O
−
+ HF system at a collision energy of 45 kJ/mol. After [67.8, 9] by permission
u
n
. The solution of this equation for σ(n
,θ|n, v
rel
) can
be accomplished both by forward convolution integra-
tion fitting procedures [67.11] and by iterative unfolding
[67.12]. Forward convolution methods assume paramet-
ric forms for σ(n
,θ|n, v
rel
) that are substituted into
(67.13). The parameters are then varied until the cal-
culated fluxes agree with the data within experimental
error. Iterative deconvolution methods generally extract
the cm cross section summed over final states,
n
σ(n
,θ|n, v
rel
), (67.14)
but the finite energy of the detection scheme also deter-
mines whether quantum states are completely resolved
or if the data represent a summation over product energy
levels.
67.3 Scattering Cross Sections
Measurements of the single-collision cross sections
shown schematically in Fig. 67.1 require a variety of
sophisticated techniques. Each of these are discussed in
the context of measurement techniques and information
content, with illustrative examples.
67.3.1 State-to-State Differential Cross
Sections
Information most diagnostic of the potential energy sur-
face for chemical reaction comes from σ(n
,θ|n, v
rel
).
The experimental data of Fig. 67.3 on the O
−
+
HF system provide an example of a case in which
n
refers to product OH vibrations in the ground
electronic state, but without resolution of product
rotations. Iterative deconvolution of the laboratory
fluxes results in a flux distribution that is a sum
of cross sections, as described in (67.14). Fig-
ure 67.4 shows this distribution in cm velocity space
as a function of u and θ. In this representation,
the relative velocity vector lies along the 0
◦
- 180
◦
line, and the symmetric peaks of the data near
0
◦
(forward scattering) and 180
◦
(backward scatter-
ing) indicate that the reaction proceeds through a
transient collision complex living for at least sev-
eral rotational periods. The symmetry of the flux
distribution with respect to 90
◦
is the most impor-
tant diagnostic for the participation of a long-lived
transient complex. Whether the distribution is forward-
backward peaked, as in the present example, or
more isotropic reflects the geometry of the com-
plex (i. e., oblate or prolate symmetric top) and the
manner in which angular momentum is partitioned
in the products [67.13]. In Fig. 67.4, the forward-
backward scattering is indicative of the fact that
the orbital angular momentum L of the approach-
ing reactants is partitioned preferentially into orbital
angular momentum of the products, L
, through
a near-linear [O···H···F]
−
intermediate. The vari-
ous peaks in the flux distribution of Fig. 67.4 can
Part E 67.3
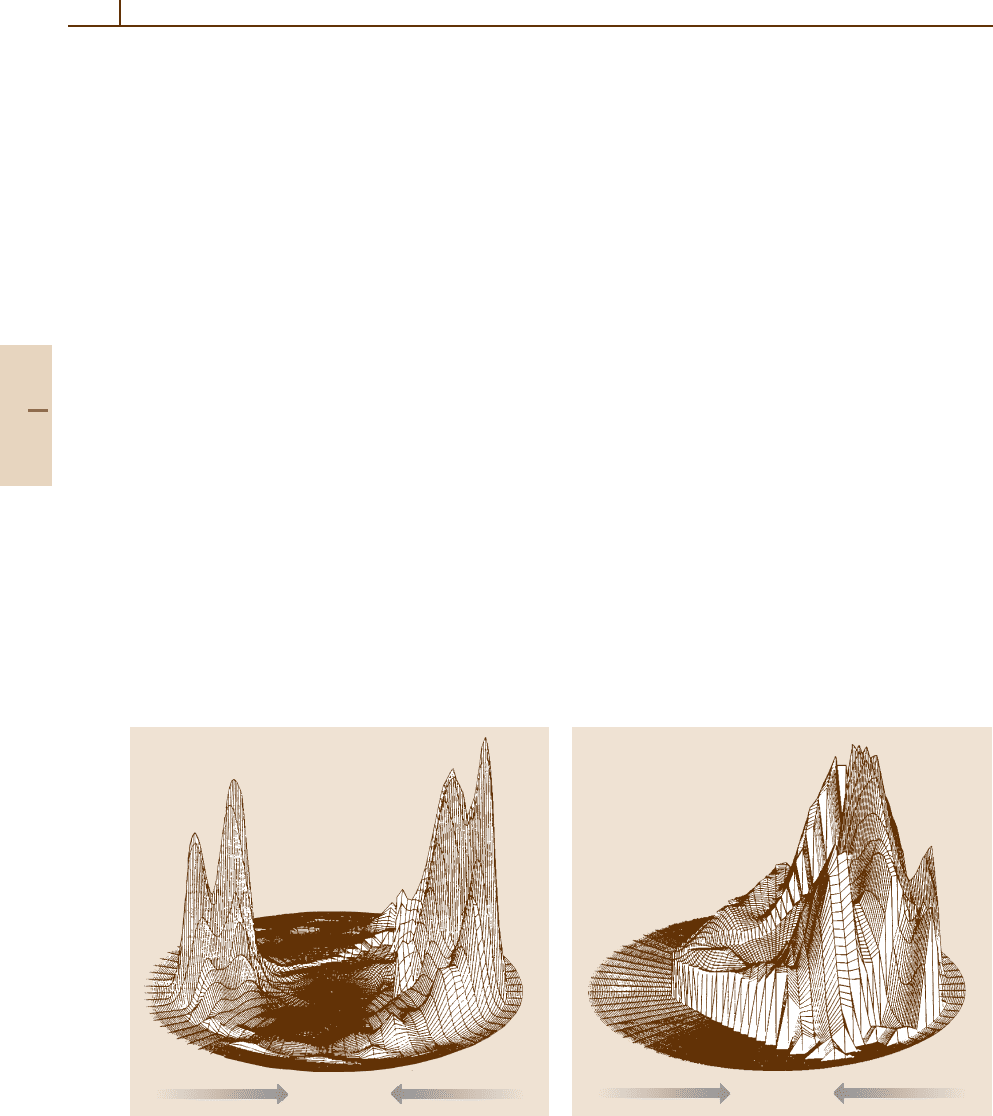
988 Part E Scattering Experiment
be assigned to OH vibrations v
= 0, 1, and 2.
Integration of the cross sections over the appro-
priate angular and velocity ranges yields the result
P(v
= 0) : P(v
= 1) : P(v
= 2) = 0.38 : 0.43 : 0.18.
Crossed–beam experiments (Sect. 60.2.2) with suffi-
cient angular and kinetic energy resolution yield angular
distributions for individual vibrational states. Similarly,
laser-induced fluorescence experiments [67.15] yield ro-
tational distributions. Recent work on the Ar
+
+ N
2
→ Ar + N
+
2
(v
= 0, J
) system illustrates this capabil-
ity [67.16] (Sects. 64.2.1 and 38.4.1). However, these
experiments probe N
+
2
in the collision volume, thereby
averaging over all possible scattering angles, and are
more properly examples of product-state resolved cross
sections, discussed in Sect. 67.3.4. Experiments that
provide complete product vibrational-rotational state
specification and product scattering angles have yet to
be carried out.
In the above examples the neutral beam is produced
by supersonic expansion, producing reactants in their
ground vibrational states, with rotational temperatures of
only a few kelvins. Experiments with reactants prepared
in excited states, either by laser absorption [67.17]or
photoionization, and with full final state selection and
angular resolution, remain a major goal of ion-molecule
chemistry.
The production of reagent ions in selected vibra-
tional and vibrational-rotational states by resonance en-
hanced multiphoton ionization (REMPI) (Sect. 74.1.2)
90°
0°
180°
O
–
HF
Fig. 67.4 Axonometric plot of F
−
fluxes in velocity space
in the reaction O
−
+ HF → OH + F
−
at 45 kJ/mol. Data
from [67.8,9]
has been accomplished in a number of systems [67.17,
18]. The use of photoelectron spectroscopy to assess the
purity of the state-selection process is essential [67.19].
67.3.2 Velocity–Angle Differential Cross
Sections
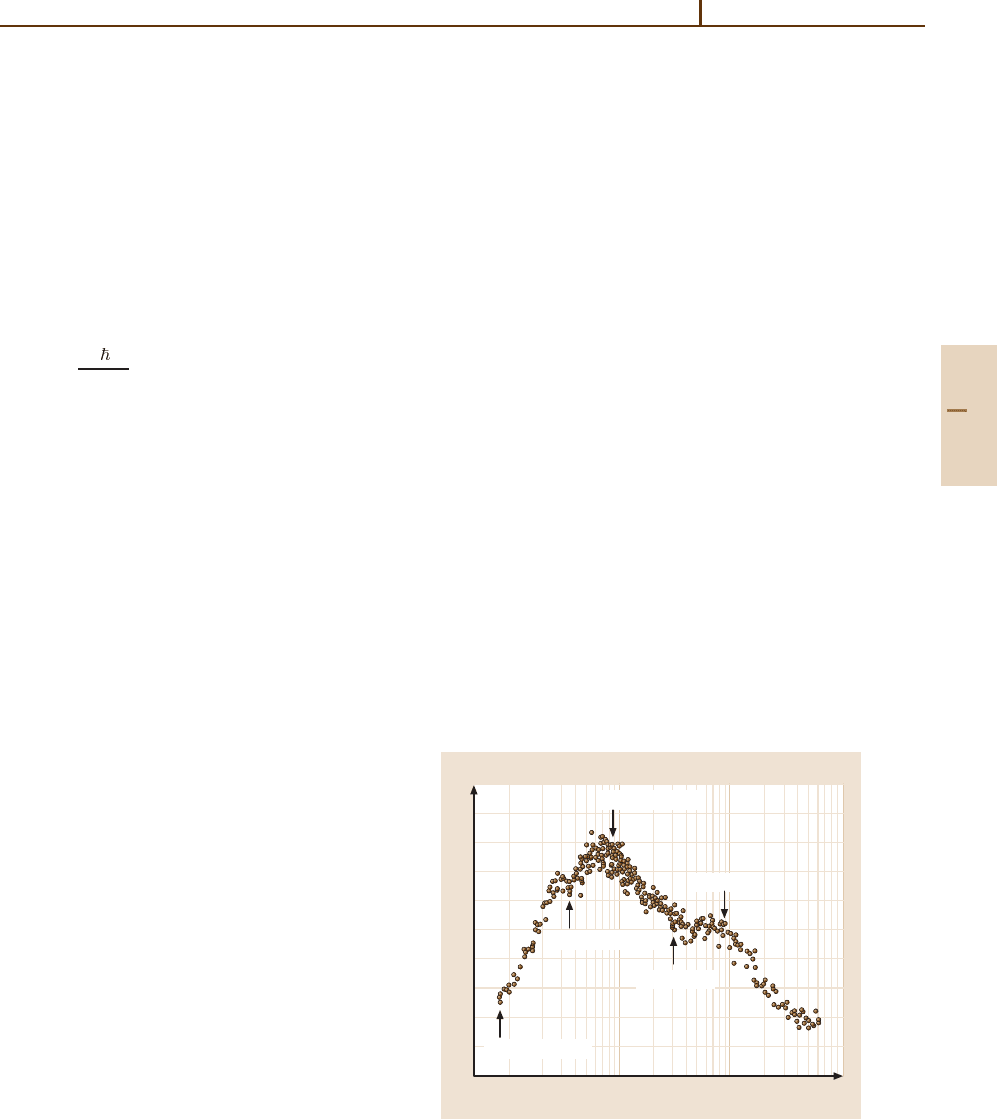
The velocity-angle DCS σ(v
rel
,θ|v
rel
) obtained by
measuring kinetic energy distributions as a function
of Θ can be plotted as a distribution in u and
θ. Figure 67.5 shows σ(v
rel
,θ|v
rel
) for the reaction
C
+
+ H
2
O →[COH]
+
+ H at a collision energy of
2.14 eV (206.5kJ/mol) [67.14]. The scattering is pre-
dominately backward, and the data indicate a reaction
taking place in part through a transient complex, but
principally through low impact parameter direct colli-
sions leading to backward scattered products. Collisions
leading to such backward scattered products are called
rebound collisions and are dominated by the repulsive
part of the potential surface. Although the reaction of C
+
with H
2
O is exothermic, high kinetic energy release in
the rebound component suggests that a potential energy
barrier is in the exit channel, i. e., it acts as the products
separate.
At lower collision energies, the forward peak in
the flux distribution becomes more pronounced for the
C
+
+ H
2
O reaction, suggesting that the reaction is me-
diated by a collision complex living for a fraction of a
rotational period. Under these conditions, it is possible
H
2
O C
+
90°
0°
180°
Fig. 67.5 Axonometric plot of [COH]
+
fluxes in velocity
space in the reaction C
+
+ H
2
O → [COH]
+
+ Hat2.14 eV
(206.5kJ/mol). Data from [67.14]
Part E 67.3
Ion–Molecule Reactions 67.3 Scattering Cross Sections 989
to extract collision complex lifetimes from the angular
distributionasymmetry through the osculating model for
chemical reactions [67.20]. In this model, the lifetime is
a parametric function of the complex’s rotational period,
evidenced by the forward-backward asymmetry
I(180
◦
)/I(0
◦
) = 1/ cosh(τ
R
/2τ) . (67.15)
In this expression, τ is the lifetime of the complex and τ
R
is the rotational period, estimated from the moment of
inertia I and the maximum orbital angular momentum
L
max
of the complex. The total reaction cross section σ
can be used to estimate this latter quantity from
σ =
π
2
2µT
rel
(L
max
+ 1)
2
. (67.16)
In the present case, the lifetime estimate for the
[CHOH]
+
complex is less than 1× 10
−13
s(Chapt.35).
At high energies, rebound collisions dominate
the dynamics of the C
+
+ H
2
O reaction. Another
limit often encountered in high energy ion-molecule
reactions is the stripping process in which the in-
coming ionic projectile removes a particle from its
molecular collision partner, and the new ionic prod-
uct travels in the same direction as the incident
ionic reactant. The spectator stripping limit [67.22]
for a reaction of the type A+ BC → AB + C cor-
responds to the case in which the cm speed of
product C is unchanged from the cm speed of
reactant BC,i.e.,C “spectates” as the reaction
occurs.
67.3.3 Total Cross Sections with
State-Selected Reactants
State-selected cross sections σ(n
|n, v
rel
) were first
measured by Chupka and collaborators [67.23, 24]in
the endothermic reaction of H
+
2
+ He → HeH
+
+
H, with H
+
2
prepared in states v ≥ 2 by photoioniza-
tion, and HeH
+
detected without product state analysis.
Thus, n
represents a summation overallaccessible prod-
uct states in state-selected reactant experiments. At a
given vuv ionization wavelength, H
+
2
is prepared, sub-
ject to Franck-Condon factor limitations (Sect. 33.6.1),
in all possible vibrational states up to the maximum al-
lowed by the photon energy. Therefore, extraction of
cross sections for individual vibrational states requires
VUV wavelength dependent studies in which the num-
ber of reactant vibrational states is increased smoothly
from the ground state up to the limit allowed by in-
strumental resolution or photon sources. A number of
reactive and charge transfer systems have been studied
with photoionization techniques at the reactant state-
selected level with unresolved product states. Data are
typically in the form of P(v
) vs. v
, as a function of colli-
sion energy. A comprehensive review by Ng is available
[67.25].
67.3.4 Product–State Resolved Total Cross
Sections
The resolution of product states in cross section mea-
surements can be accomplished in a variety of ways.
We will first discuss the case in which the reactants have
not been state-selected. In principle, spectroscopic meth-
ods such as laser-induced fluorescence can be used for
complete product quantum state specification. Earlier
reference to N
+
2
vibration-rotation state-resolved charge
transfer experiments [67.16] provides an excellent ex-
ample of the state of the art of such methods, and their
extension to chemical reactions represents an impor-
tant future development. A beautiful example is mass
spectrometric detection of N
+
2
in the Ar
+
+ N
2
charge
transfer system [67.21]. Guided ion-beam production
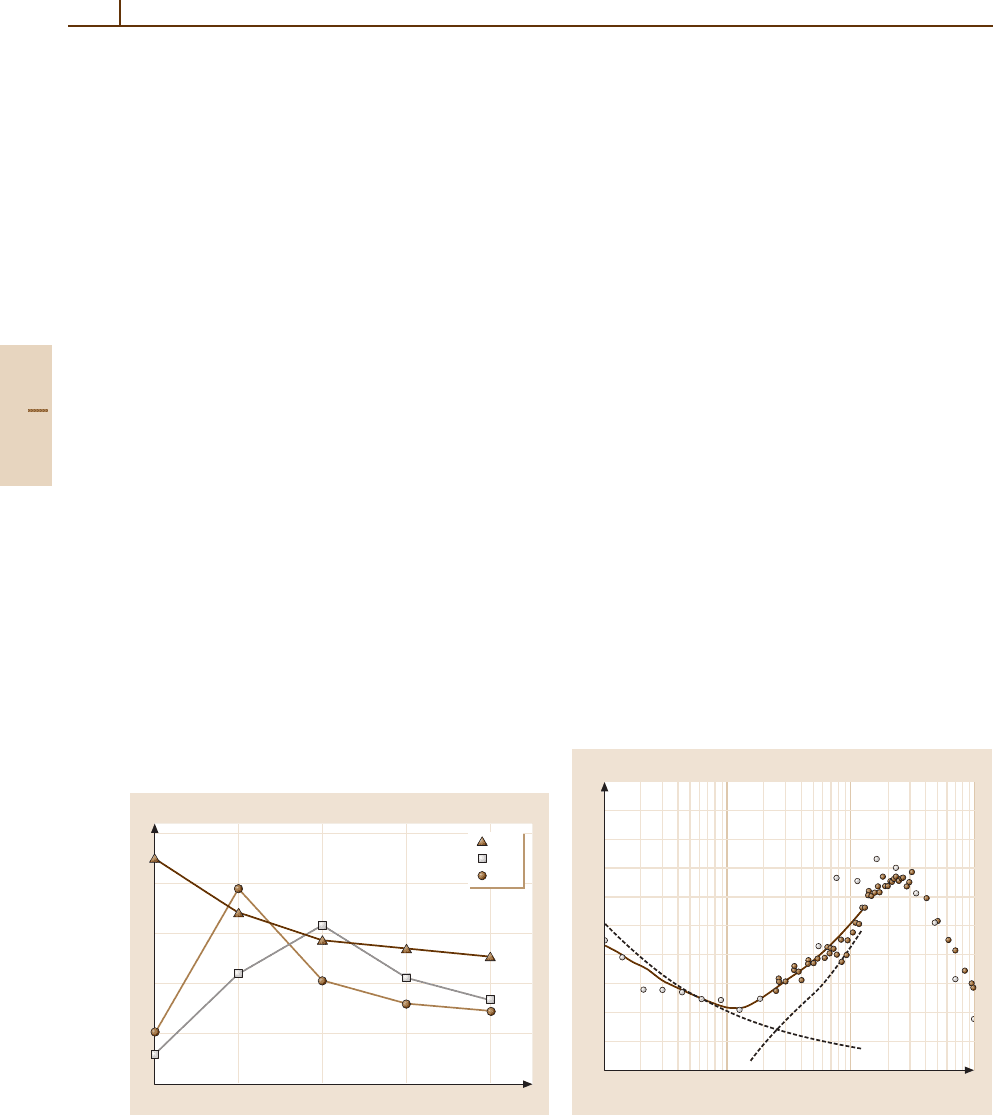
and detection methods [67.26] in conjunction with a su-
personic beam of N
2
allow 4π detection of the charge
transfer products as a function of collision energy, yield-
ing accurate total cross sections. The results, plotted in
Fig. 67.6, show thresholds for the production of excited
vibrational states of N
+
2
X
2
Sigma
g
, v
= 1, 2, and 4) as
well as the formation of the excited B
2
Σ
g
state. The
detection of chemiluminescence from electronically ex-
10
8
6
4
2
0
0.1 1 10 100
Cross section (arb. units)
Collision energy (eV)
N
2
+
(X
2
Σ
g
1
v = 4)
N
2
+
(X
2
Σ
g
1
v = 2)
N
2
+
(B
2
Σ
u
+
)
N
2
+
(X
2
Σ
g
1
v = 1)
D(N
+
–N)
Fig. 67.6 Energy dependent cross section for production
of N
+
2
in states as indicated in the Ar
+
+ N
2
charge
transfer system. After [67.21] by permission
Part E 67.3
990 Part E Scattering Experiment
cited states of ion-molecule reactions is also a powerful
method for determining such cross sections; methods
and results are reviewed in [67.27].
67.3.5 State-to-State Total Cross Sections
The most detailed probe of an ion-molecule reaction
is the state-to-state cross section. The measurement
of angularly-resolved state-to-state cross sections has
only been achieved for reactants in their ground states,
but true state-to-state total cross sections have now
been measured in a few favorable cases. A particu-
larly novel method for product-state determination with
reactant-state selection has been developed [67.25]. In
the differential reactivity method, product vibrational
states are distinguished by their differing cross sections
for charge transfer with selected molecules. Although
the method is limited at present because state-selected
charge transfer cross sections have been measured in
only a few systems, the technique has great potential.
The charge transfer reaction H
+
2
(v)+ H
2
→ H
2
+ H
+
2
(v
),inwhichH
+
2
is prepared in v = 0and1byVUV
photoionization, provides a particularly good example
of its power. The H
+
2
charge transfer products, formed
in states v
= 0− 3, are first mass-analyzed, accelerated
to 10 eV, and then passed through a collision cell con-
taining N
2
, Ar, or CO, where charge transfer occurs
once again. The N
+
2
,Ar
+
,orCO
+
products are mass
analyzed and the cross sections σ
m
for forming these
ions by charge transfer from the H
+
2
reaction products
are measured. The charge transfer cross sections for H
+
2
with these gases have different dependences on v
,as
2
1
0
01234
v⬘
Relative cross section (arb. units)
Co
Ar
N
2
Fig. 67.7 Cross sections for charge transfer of H
+
2
(v
) vs.
v
with N
2
, Ar, and CO at a collision energy of 10 eV. After
[67.25] by permission of John Wiley & Sons, Inc
shown in Fig. 67.7, and therefore, the gases can be used
to probe the product states of H
+
2
formed in the sym-
metric charge exchange reaction. Letting X
v
denote the
fraction of H
+
2
formed in the vibrational state v
, n de-
notethe number density of the neutral collision gas, and l
denote the attenuation length, the following set of simul-
taneous equations can be solved for X
v
, since thecross
sections σ
v
are known and the σ
m
are measured:
X
0
+ X
1
+ X
2
+ X
3
= 1 (67.17)
and, for a particular ion (e.g., N
+
2
,Ar
+
,CO
+
),
X
0
nlσ
0
+ X
1
nlσ
1
+ X
2
nlσ
2
+ X
3
nlσ
3
= nlσ
m
.
(67.18)
[An equation like (67.18) will exist for each collision
gas.] An extensive set of data at collision energies from
2 eV up to 16 eV has been obtained [67.29]. When the
reactant ions are in the vibrational ground state, at low
kinetic energies the charge transfer product is also in
the ground state, indicating that resonant charge transfer
is the dominant process. At increasing collision ener-
gies, X
1
increases from 0.0 to 0.17. For vibrationally
excited H
+
2
reactants, inelastic relaxation to form H
+
2
in
v
= 0 is important at all collision energies, increasing in
magnitude with increasing collision energy. Of particu-
lar interest is the fact that inelastic relaxation forming
v
= 0 is substantially more important than inelastic
excitation producing v
= 1 or 2. This trend is pre-
dicted by theory, but underestimated at lower collision
energies.
10
8
6
4
2
0
1 10 100 1000
Collision energy (meV)
Cross section (Å
2
)
Fig. 67.8 Total cross section for ND
+
formation in the N
+
+ D
2
system, showing a low energy threshold at 15 meV.
After [67.28] by permission
Part E 67.3
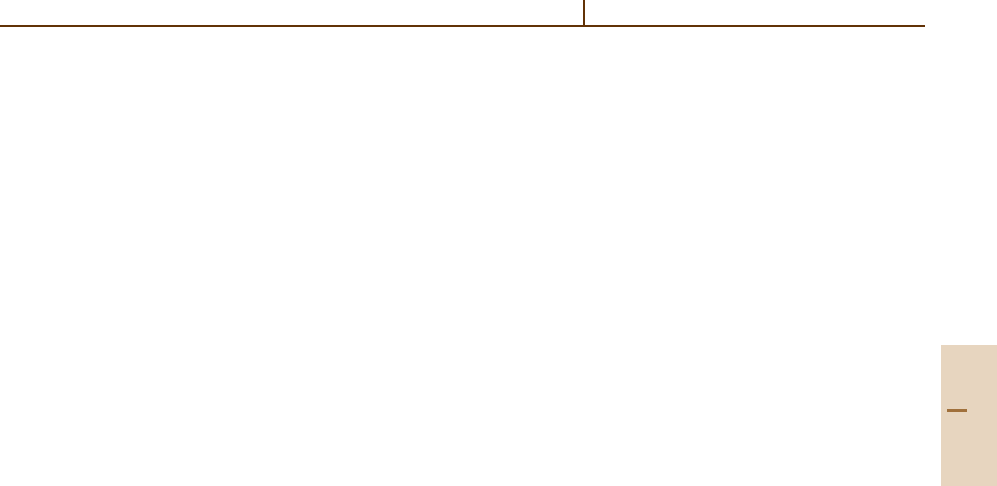
Ion–Molecule Reactions 67.4 New Directions: Complexity and Imaging 991
67.3.6 Energy Dependent Total Cross
Sections
Although non-state-selected cross sections, denoted
σ(v), lack information about product energy disposal,
important features of the potential energy surface can
be obtained from their measurement. Guided ion beam
methods have been especially important in the deter-
mination of accurate cross sections, particularly those
that employ a supersonic beam rather than a gas as a
neutral target. Energy-dependent cross sections provide
crucial information on ion and neutral thermochemistry
through accurate measurements of reaction thresholds,
and help to elucidate important potential surface fea-
tures such as thresholds, barriers, and crossings [67.30].
A particularly illustrative example of threshold forma-
tion concerns the reaction N
+
(
3
P) + H
2
→ NH
+
+ H
and its isotopic variants. This reaction is important at
low (< 0.1 eV) collision energies as the first step in
the chain reaction that leads to formation of ammo-
nia in dense interstellar clouds, but the bond energy of
NH
+
is uncertain enough to prevent knowing whether
the above reaction is endothermic or exothermic. Re-
cent total cross section measurements on the isotopic
variant N
+
(
3
P) + D
2
→ ND
+
+ D at very low col-
lision energies now appear to answer this question
[67.28]. Figure 67.8 shows experimental results per-
formed on two different instruments, one a guided
ion beam-crossed neutral beam machine and the other
a guided merged-beam apparatus. The data show a
very clear threshold at 15 meV, demonstrating that
the reaction is endothermic and allowing for a more
accurate estimate of the ND
+
bond energy. This
experiment shows that the high energy resolution af-
forded in total cross section measurements employing
crossed and merged beams, rather than thermal col-
lision cells, can answer thermochemical questions of
importance in a wide variety of applications, includ-
ing astrophysics, combustion, electrical discharges, and
atmospheric processes.
67.4 New Directions: Complexity and Imaging
The crossed beam technique provides the precise kine-
matic definition required to extract the most intimate
details of reactive collisions. Although the highest res-
olution examples of the technique involve systems
with three or four atoms, several recent examples of
systems of greater complexity have appeared in the
literature. The multiply-charged CF
2+
2
+ D
2
system
[67.31] is appreciably more complex experimentally
than its singly-charged analog, owing to the possibility
of forming two charged fragments with correlated prod-
uct distributions. The nine-atom C
2
H
+
2
+ CH
4
system
has been studied previously with guided ion beam-
gas cell methods under conditions where the reactant
ions are produced by multiphoton ionization [67.32].
Those early experiments, which yielded total reaction
cross sections for vibrational state-selected ions, gave
significant indications of mode-selectivity in complex
reactions. A more recent study [67.33], conducted with-
out reactant state-selection or product state analysis,
maps out product angular distributions and disposal of
energy in relative translation with ion trapping tech-
niques that measure longitudinal velocity components
directly and provide upper bounds on the transverse
velocity components.
The majority of extant crossed beam experimen-
tal studies construct three-dimensional velocity space
distributions with a series of one-dimensional sections
through the full distribution such that, in any interval of
time, only a single detection element in velocity space
can be measured. Recent advances in imaging methods
[67.34–36], in which many velocity space elements can
be observed in a single time interval, promise to increase
the sensitivity of the crossed beams method by orders
of magnitude. The signal gains that can be achieved
with the implementation of such multiplex advantage
methods will allow the lower reactant and product sig-
nal levels associated with increasing state-specification
and/or system complexity to be tolerated without undue
increases in data acquisition times.
Figure 67.9 shows a schematic of an instrument that
illustrates the imaging principle.
As indicated in the diagram, the locus of points of
reaction products with a constant center of mass speed
is a sphere whose radius increases with time. Individ-
ual quantum states of a given reaction product form a
set of nested spheres in velocity space. Projecting this
set of product spheres onto a detection plane allows all
product velocity elements to be observed in a single
time window. Application of the inverse Abel trans-
form [67.37] allows the full three-dimensional velocity
distribution to be extracted from the two-dimensional
projection on the detection plane. Velocity focusing
methods [67.38] allow reaction products originating
from spatially distinct regions of the collision volume
Part E 67.4
