Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

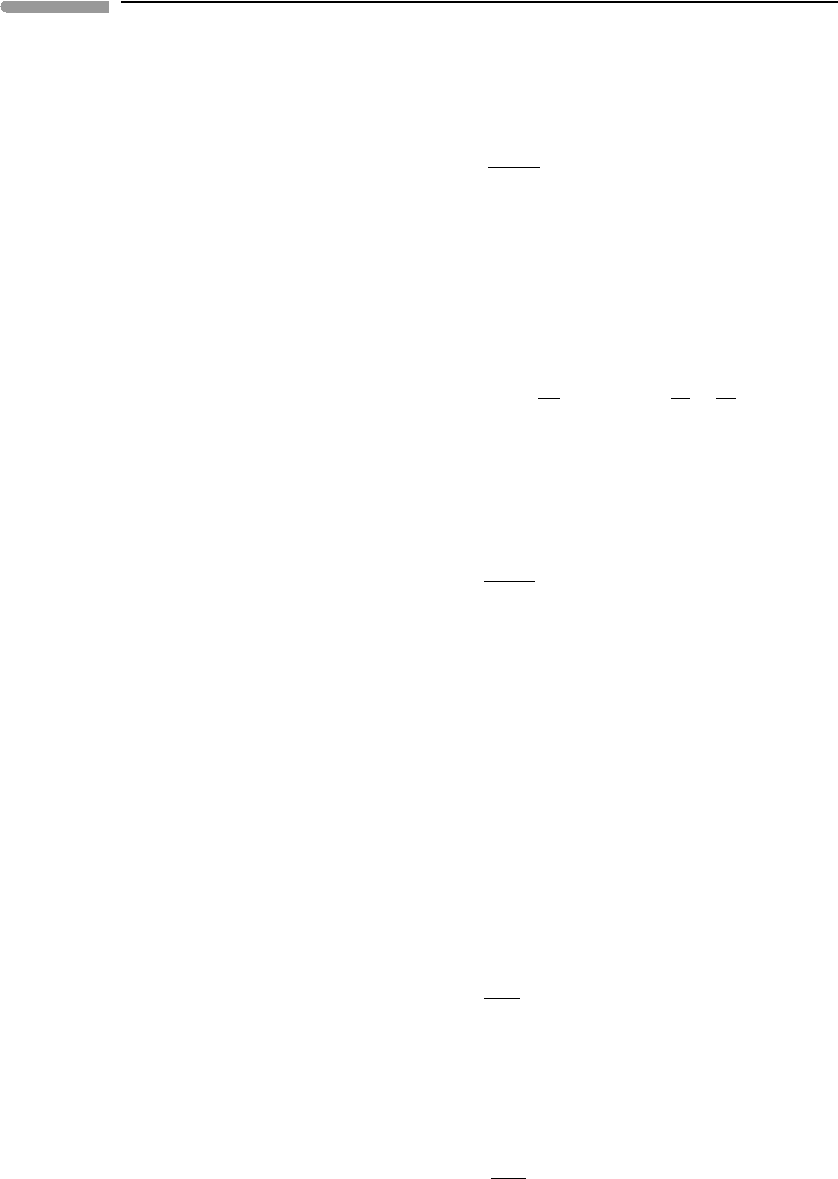
8 Energy in planetary processes
then
¯
f
g
always has a negative orientation – this is the mathematical expression of the fact that
gravity is always an attractive force. We can then write the inner product in equation (1.3)
as:
¯
f
g
·d
¯
x =−
GMm
x
2
dx, (1.4)
where the negative sign arises from the fact that we are calculating the inner product
between a vector and a one-form that subtend an angle of 180
◦
(Box 1.1). This is the work
performed by the gravitational force (the general three-dimensional case requires more
complex notation, but the physics are well summarized in (1.4)). The change in gravitational
potential energy that corresponds to a finite displacement between two positions, x
a
and x
b
can be obtained by substituting (1.4) into (1.3) and integrating:
U
g,b
−U
g,a
=−
b
a
¯
f
g
·d
¯
x = GMm
b
a
dx
x
2
=−GMm
1
x
b
−
1
x
a
. (1.5)
By convention, we define gravitational potential energy as being 0 when the objects are
separated by an infinite distance, i.e. we make U
g,a
=0asx
a
→∞. With this convention we
then define the gravitational potential energy of an object with mass m in the gravitational
field of a planet with mass M as:
U
g
=−
GMm
r
, (1.6)
where r is the distance between the centers of mass of the two bodies. For any finite value
of r, U
g
is negative, and U
g
approaches a maximum value of 0 as the separation between
the two bodies approaches infinity. When writing equation (1.4) I justified the negative
sign on purely mathematical grounds, as arising from the inner product of two oppositely
pointing vectors, but we can now see the physical meaning of this negative sign. Suppose
mass m is moved away from M (r > 0). In order for this to occur an external agent must
perform work. By conservation of energy this work becomes stored as potential energy in
the gravitational field. Therefore it must be U
g
> 0, which is what results from equation
(1.6) with r > 0. Conversely, if r < 0 the gravitational field gives up energy (U
g
< 0)
which is transferred to mass m and appears, for example, as kinetic energy.
I will introduce here two other equations that we use in the analysis of gravitationally
driven planetary heating in Chapter 2. First, we can see from equation (1.2) that the gravi-
tational acceleration, g, due to a body of mass M at a distance r from its center of mass is
given by:
g =−
GM
r
2
, (1.7)
where the negative sign expresses the fact that gravitational acceleration is always attractive
(directed towards the mass that causes it, where r is positive away from the mass). Recall
that the numerical value of g is the intensity of the gravitational field. We also define the
gravitational potential, Φ
g
, as the gravitational potential energy per unit mass:
Φ
g
=−
GM
r
. (1.8)

9 1.3 Mechanical energy
Gravitational potential is a scalar field (Box 1.1). Gravitational acceleration is a one-form
(or covariant vector) that is the gradient of the potential field. The magnitude of the one-form
is given by:
g =−
dΦ
g
dr
. (1.9)
Force is another one-form, that is the product of a scalar (mass) times acceleration (Newton’s
second law of motion). Mass, or inertia, is the scaling factor between force and acceleration;
this is the origin of the term scalar.
We are generally concerned with differences in gravitational potential energy between
different states of a system. For example, when tectonic processes elevate a mountain range,
or when lava flows build a volcano, gravitational potential energy is stored in the rocks.
How much potential energy is stored in a mountain range? This depends on the distance
that the rocks can move towards the center of the planet before they get to a level below
which they can move no further. How do we define such a level? Sea level may be a
good first approximation, but we can give a more general answer, that will also hold for
planets without oceans. We begin by looking at two additional questions. First, where did
this gravitational potential energy associated with topography come from? Conservation of
energy requires that we identify an energy source, which in this case entails conversion of
some of the planet’s internal heat to gravitational potential energy (more on this in Chapter
3). Second, what happens to this potential energy as the mountain loses elevation? The short
answer is that this gravitational potential energy ultimately becomes heat and is dissipated to
space, but the pathway may entail some intermediate steps, depending on how the mountain
loses elevation. In general, elevation is lost by a combination of three processes: erosion,
isostatic adjustment and tectonic collapse. During erosion, potential energy becomes heat
as a result of friction during sediment transport and also when particles come to their
final resting place in a sedimentary basin. Isostatic adjustment may return gravitational
potential energy to the mantle, either as heat or as mechanical energy. Tectonic collapse
results in gravitational potential energy either being dissipated directly as heat during ductile
deformation, or being stored as elastic energy (another type of mechanical energy that we
will discuss) to be eventually dissipated, ultimately as heat too, during earthquakes. All of
these processes drive towards converting the surface of the planet to one over which there
are no differences in gravitational potential energy. Such a surface is called an equipotential
surface. Awell-defined reference level for potential energy on Earth is thus the geoid, which
is defined as the equipotential surface that is as close as possible (e.g. in a least square sense)
to mean sea level (see Worked Example 1.1). In planets without oceans, we may choose
as our reference the equipotential surface that is as close as possible to the mean planetary
radius (and, if we were to follow the same convention used for Earth, we should call such
surfaces: areoid, aphrodoid, hermoid, selenoid, etc.). We will generally be concerned with
differences between the value of U
g
at the geoid and its value at any other level that we
may be interested in.
Worked Example 1.1 Gravitational potential energy and topography
(a) Consider a mass m of rock initially located on Earth’s geoid. Let R be the geoid’s mean
radius. The rock is then moved to an elevation h above the geoid, such that h R, i.e. we
stay close to the planet’s surface. The initial distance between the two centers of mass (the

10 Energy in planetary processes
rock’s and the Earth’s) is R, and the final distance, after the rock is raised, is R+h.We
first use equation (1.6) to show that the gravitational potential energy of the rock in its final
state, relative to the geoid, is given by mgh, the equation that you probably remember from
introductory physics.
Calling the gravitational potential energies at the geoid and at an elevation h above the
geoid U
g,geoid
and U
g,h
, we have:
U
g,h
−U
g,geoid
=−
GMm
R +h
−
−
GMm
R
=
GMmh
R
(
R +h
)
. (1.10)
If we stay close to the planet’s surface, then R(R+h) ≈R
2
. We can also consider the planet’s
gravitational acceleration to be constant over the interval R to R+h. Using equation (1.7)
to calculate gravitational acceleration at the geoid and substituting in (1.10):
U
g,h
−U
g,geoid
≈
GMmh
R
2
=−mgh =m|g|h. (1.11)
With our sign convention g is always a negative quantity. The “g”inmgh is thus the
magnitude of g, as shown in the last term of equation (1.11).
(b) The Sierra Nevada of California is the largest uninterrupted mountain range in the
United States. It is roughly 600 km long, 100 km wide and has a mean elevation of 1.5 km
(averaged over this horizontal extent). Assuming that this average elevation represents the
center of mass of the mountain range, and that the rocks making up the Sierra Nevada have
an average density of 2800 kg m
−3
, what is the potential energy stored in the Sierra Nevada
relative to the geoid? At the geoid, g ≈ 9.8ms
−2
. Plugging in these values into equation
(1.11) we find that the Sierra Nevada stores approximately 3.7 × 10
21
J of gravitational
potential energy, approximately equivalent to an explosive yield of one million megatons
(see Section 1.12.2).
(c) Uplift of the Sierra Nevada has occurred over the past 5 million years. Assuming a
constant rate of uplift over that time interval, the energy flux (energy per unit area per unit
time) that went into elevating the Sierra Nevada is approximately 3.9 ×10
−4
Wm
−2
=
0.39 mW m
−2
, where 1 W(Watt) = 1Js
−1
. Typical terrestrial heat fluxes are of the order
of 50–100 mW m
−2
, i.e. two orders of magnitude greater. There is plenty of energy in the
Earth to elevate mountain ranges.
1.3.2 Kinetic energy
Bodies in motion have kinetic energy, U
k
, that arises from their speed and is given by:
U
k
=
1
2
mv
2
, (1.12)
where v is the magnitude of the body’s velocity, i.e. its speed. Kinetic energy is a function
of the relative speed between a body and an observer. For example, if we observe, from
a location at rest on the Earth, an asteroid of mass m moving towards Earth with speed
v, the kinetic energy of the asteroid in our reference frame is given by equation (1.12)
and the Earth has no kinetic energy in our reference frame. Measured from a reference

11 1.3 Mechanical energy
frame attached to the asteroid, on the other hand, the asteroid has no kinetic energy, and
the Earth has kinetic energy given by equation (1.12), but with m in this case being the
mass of the Earth. Although the two values of kinetic energy are very different, if the
two bodies collide the result is unique: conversion to heat of an amount of energy equiva-
lent to most of the kinetic energy of the asteroid, as measured from the Earth’s reference
frame (Worked Example 1.2). In order to see why this is the case we need to introduce
the law of conservation of momentum, which is a law of nature that, in classical physics,
is distinct from energy conservation. In reality, energy and momentum conservation are
different manifestations of a single conservation law that arises from the geometrical
properties of spacetime, but this becomes an issue only for objects moving at relativistic
speeds.
Momentum,
¯
p, is a vector quantity, and is given by:
¯
p = m ¯v, (1.13)
where ¯v is the velocity vector. The law of conservation of momentum states that the total
momentum of a system is conserved. In contrast to energy, that has many different mani-
festations, momentum is unique and is always conserved; it cannot be converted to other
“types” of momentum. In a perfectly elastic collision both momentum and kinetic energy
are conserved, i.e., there is no conversion of kinetic energy into other types of energy.
The concept of elastic collisions will enter into our discussion of a thermodynamic vari-
able known as internal energy, later in this chapter. In an inelastic collision, in contrast,
momentum is conserved (as it must always be) but kinetic energy is not. Of course, the total
energy of the system must be conserved, so that during an inelastic collision some kinetic
energy is converted to other types of energy (ultimately heat). Conversion of kinetic energy
to heat during inelastic collisions takes place, for example, when celestial bodies collide.
This process was responsible for accretionary heating during the Solar System’s formative
period (Chapter 2). On a very different scale, inelastic collisions of subatomic particles with
atoms in a crystal are the cause of radioactive heating, one of the key sources of energy in
terrestrial planets (Chapter 2).
Worked Example 1.2 Dissipation of kinetic energy during collisions of celestial bodies
(a) An asteroid of mass m
a
moves directly towards Earth (mass M ) with velocity ¯v
a
,as
measured from Earth. Consider a reference frame relative to which the Earth is initially at
rest (i.e. the Earth’s initial velocity is zero), but that will remain fixed even if the state of
motion of the Earth were to change. Figure 1.2a shows the initial situation as seen from this
reference frame, choosing a leftward-directed velocity as positive. We consider the collision
of the two bodies as being perfectly inelastic, meaning that momentum, ¯p, is conserved,
but kinetic energy is not. After the collision, the Earth and the asteroid merge into a single
body of mass (m
a
+M), moving with velocity ¯v
f
relative to the same reference frame
as before, which has not been affected by the collision. We seek the magnitude and the
direction (leftwards or rightwards) of ¯v
f
, the change in the velocity of the Earth relative
to the external reference frame, and the change in kinetic energy of the Earth + asteroid
system resulting from the collision.
12 Energy in planetary processes
M
M+m
m
v
a
v
f
M
m
–v
a
v
f
*
M+m
(a)
(b)
fixed reference frame
fixed reference frame
time
time
Fig. 1.2 Asteroid (mass m) colliding with Earth (mass M), as seen from Earth (a) and from the asteroid (b).
The final velocity is calculated from momentum conservation:
¯
p
initial
= m ¯v
a
=
¯
p
final
=(m +M)¯v
f
¯v
f
=
m
m +M
¯v
a
.
(1.14)
The change in the Earth’s velocity is simply v
f
, as its initial velocity in the chosen frame of
reference is zero. The amount of kinetic energy that is converted to other forms of energy
(chiefly heat) is given by:
U
k, final
−U
k, initial
=
1
2
(m +M)|¯v
f
|
2
−m|¯v
a
|
2
=−
1
2
m|¯v
a
|
2
M
m +M
. (1.15)
If the asteroid is small compared to Earth, then the expression in parentheses ≈ 1, and the
heat dissipated is essentially equal to the kinetic energy of the asteroid before the collision.
13 1.4 Expansion work
(b) We repeat the calculation from the point of view of a reference frame that is initially
at rest relative to the asteroid, and that, as before, is not affected by the collision (Fig. 1.2b).
In this case the Earth’s initial velocity is −¯v
a
and the asteroid’s initial velocity is zero.
Conservation of momentum is in this instance:
¯
p
initial
= M
(
−¯v
a
)
=
¯
p
final
=(m +M)¯v
∗
f
¯v
∗
f
=
M
m +M
(
−¯v
a
)
,
(1.16)
where the asterisk is used to remind ourselves that the final velocity in this case is measured
relative to a different reference frame, initially fixed to the asteroid. The change in the
Earth’s velocity is then given by:
¯v
Earth
=−
M
m +M
(
¯v
a
)
−
(
−¯v
a
)
=
m
m +M
¯v
a
, (1.17)
which is the same as the change calculated in part (a), compare equation (1.14). Note
carefully the meaning of the signs: equation (1.16) shows that after the collision the Earth
is still moving towards the right, but at a lower speed than before the collision. Equation
(1.17) shows that the velocity change is positive, i.e. leftwards. As seen from the asteroid’s
reference frame, the Earth has slowed down by the same amount as the Earth has speeded
up (from rest) when seen from the Earth’s reference frame. You should verify that the loss
of kinetic energy as calculated from the asteroid reference frame also agrees with the loss
calculated from the Earth’s reference frame, equation (1.15).
1.3.3 Energy dissipation
Energy dissipation is the conversion of any kind of non-thermal energy to heat. A process
that accomplishes this conversion is called a dissipative transformation. In the example of
an asteroid colliding with Earth kinetic energy is dissipated, i.e. it is converted to heat. The
full import of this definition will become clear in Chapter 4. For now, it is important to
realize that, although energy is conserved during a dissipative process (this is the First Law
of Thermodynamics), a full reversal of a dissipative transformation is impossible. In other
words, it may be possible to reconvert some of the heat back to mechanical energy, but
there is always a fraction of the heat generated by dissipation that can never be converted
back to non-thermal energy (this is the Second Law of Thermodynamics). Transformations
between different types of non-thermal energy are called non-dissipative, meaning that the
inverse transformation is, in principle, possible.
1.4 Expansion work. Introduction to equations of state
1.4.1 The concept of expansion work
Change in volume of any substance entails an energy transfer, in other words, a system that
undergoes a volume change either absorbs or releases energy. Energy transfer mediated by a

14 Energy in planetary processes
change in volume is called expansion work. If a substance expands, it is performing mechan-
ical work on its environment and, by the law of conservation of energy, this energy must
come from somewhere. Expansion of volcanic gas during a pyroclastic eruption is a process
in which a large amount of energy is transferred from a magmatic system to the atmosphere
by performing expansion work on the latter. Other examples are perhaps less eye-catching
but not less important: discontinuous phase transitions, such as vaporization and freezing
of water, are accompanied by expansion work, as are many mineral reactions. For instance,
consider the difference in density between the Al
2
SiO
5
polymorphs kyanite and sillimanite.
When kyanite transforms to sillimanite the volume of a fixed amount of Al
2
SiO
5
increases,
because its density decreases. This mineral transformation performs expansion work on its
environment. The converse is also true, when a system is compressed it absorbs mechanical
energy from its environment and this energy is stored in the substance. Transformation of
sillimanite to kyanite absorbs mechanical energy from the environment, and this energy is
stored as chemical energy in the crystal of kyanite (the expression “chemical energy” is for
the time being quite vague, it will take on a more precise meaning in subsequent sections
and chapters).
Dissipative compressions are an important source of heat in a wide range of planetary
processes. For example, air masses that descend rapidly in the atmosphere and protostellar
clouds of gas (such as the one that gave rise to our Solar System over 4.55 Ga) that contract
under their own gravitational pull, heat up as a result of compression, i.e. compression trans-
forms mechanical energy into thermal energy. In contrast, elastic materials, such as rocks
at relatively low temperatures, are able to undergo approximately non-dissipative changes
in volume, storing compression work (= negative expansion work) as elastic energy.
Expansion work, then, plays an important role in many planetary processes, but how do
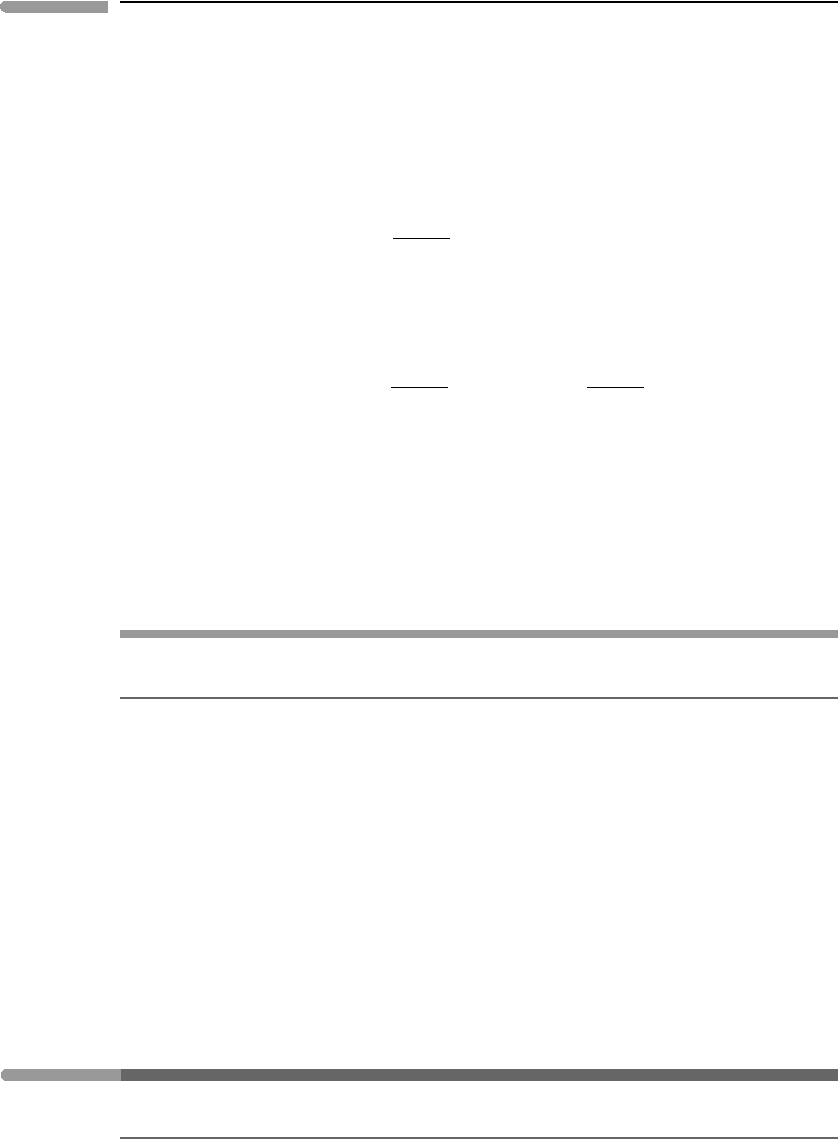
we measure it? Figure 1.3 shows a system of arbitrary shape expanding into an environment
that exerts a uniform pressure P. If the system’s surface area is A, then as the system expands
it must overcome a total force PA over its entire surface. Although PA is an oriented object
we are not using vector notation to represent it. This is careless notation, that we can justify
informally by noting that during uniform expansion pressure and force have the same
orientation as displacement, so that the inner product of the two objects equals the scalar
product of their magnitudes (Box 1.1).
Consider an infinitesimal expansion that causes the surface of the system to move out-
wards by an amount dx (Figure 1.3). Then the total amount of work performed by the system
during this infinitesimal transformation is:
dW = P Adx. (1.18)
The product Adx is the change in volume of the system, dV (because this is an infinitesimal
expansion, the total surface area can be considered to remain constant). The differential
expression for expansion work is then:
dW = PdV . (1.19)
The SI unit of pressure is the pascal (Pa), defined as a pressure of 1 N m
−2
, so that a volume
change of 1 m
3
against a pressure of 1 Pa performs 1 J of work. Another unit of pressure
that I find much preferable is the bar, where 1 bar = 10
5
Pa. One good reason to prefer the
bar over the pascal is that atmospheric pressure on Earth at sea level is approximately 1 bar,
so it is easy to develop an intuitive feeling for the magnitude of a pressure expressed in bar,
15 1.4 Expansion work
P
dx
A
P+dP
P
P+dP
P+dPP+dP
P
P
Fig. 1.3
Expansion work. The shell defined by the two spheres is the increase in volume dv =Adx.
kbar or mbar. A convenient unit of volume is, then, J bar
−1
, where1Jbar
−1
= 10
−5
m
3
= 10 cm
3
. Expressing volume in J bar
−1
and pressure in bar, W comes out in J.
1.4.2 Quasi-static, reversible and irreversible processes
In the derivation of the expansion work formula I have been rather sloppy with the sign
convention, and this sloppiness must be corrected before we can advance any further. In
order to do so, we need to address another issue which is often the source of much confusion
and consternation when encountering thermodynamics for the first time. I stated that the
system expands “into an environment that exerts a uniform pressure P ” – but what is the
pressure inside the system? Classical thermodynamics is concerned only with states of
equilibrium. Because of this constraint, the only situation that can be rigorously addressed
with classical thermodynamics is one in which the pressure inside the system equals the
external pressure. But if the two pressures are the same, then why is the system expanding
at all? Here lies the problem, if the two pressures are the same then the system is static,
and there is no work being performed. Classical thermodynamics deals with this riddle by
inventing an idealized type of transformation called a quasi-static transformation. This is
a process that takes a system from an initial equilibrium state to a final equilibrium state by
means of an infinite number of intermediate equilibrium states that are infinitesimally close
to one another. Such a transformation is, of course, physically impossible. In our example of
an expanding system, we could visualize a situation in which the pressure inside the system
is always greater than the external pressure by an infinitesimal amount, dP. By this we mean
that the internal pressure is barely higher than the external pressure, so that the system does

16 Energy in planetary processes
expand, but not so much higher as to make any difference in the amount of expansion work
given by equation (1.19), i.e. we would get the same numerical result whether we used the
internal or the external pressure in our calculations.
A concept that is distinct to that of a quasi-static transformation, but is sometimes con-
fused with it, is that of a reversible transformation. By definition this is a process during
which there is no energy dissipation. A reversible transformation must be quasi-static, but
the converse is not true: a quasi-static transformation may dissipate energy, and thus be
irreversible. An example of this would be infinitesimally slow expansion of a gas (i.e. a
quasi-static process) accomplished by displacing a piston that slides inside a cylinder with
friction. The irreversibility arises from frictional heating. We will come across other exam-
ples that will gradually make the concepts of dissipation and irreversibility clear, and will
define these concepts rigorously using the Second Law of Thermodynamics (Chapters 4
and 12).
In contrast, the concept of a quasi-static transformation can be clarified by means of an
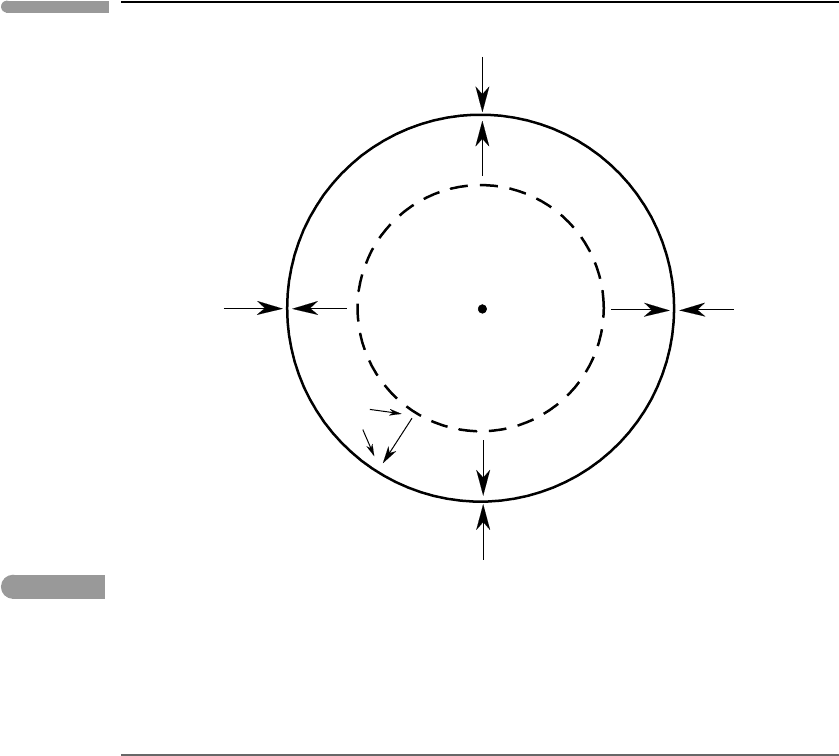
example. A gas is a collection of moving molecules. Consider what happens at the micro-
scopic level when a gas is subjected to an increase in pressure. The gas is at equilibrium if,
as a result of interactions among its molecules (i.e. collisions), the distribution of molecular
kinetic energies (or, equivalently, molecular speeds) is smooth, with a unique peak value
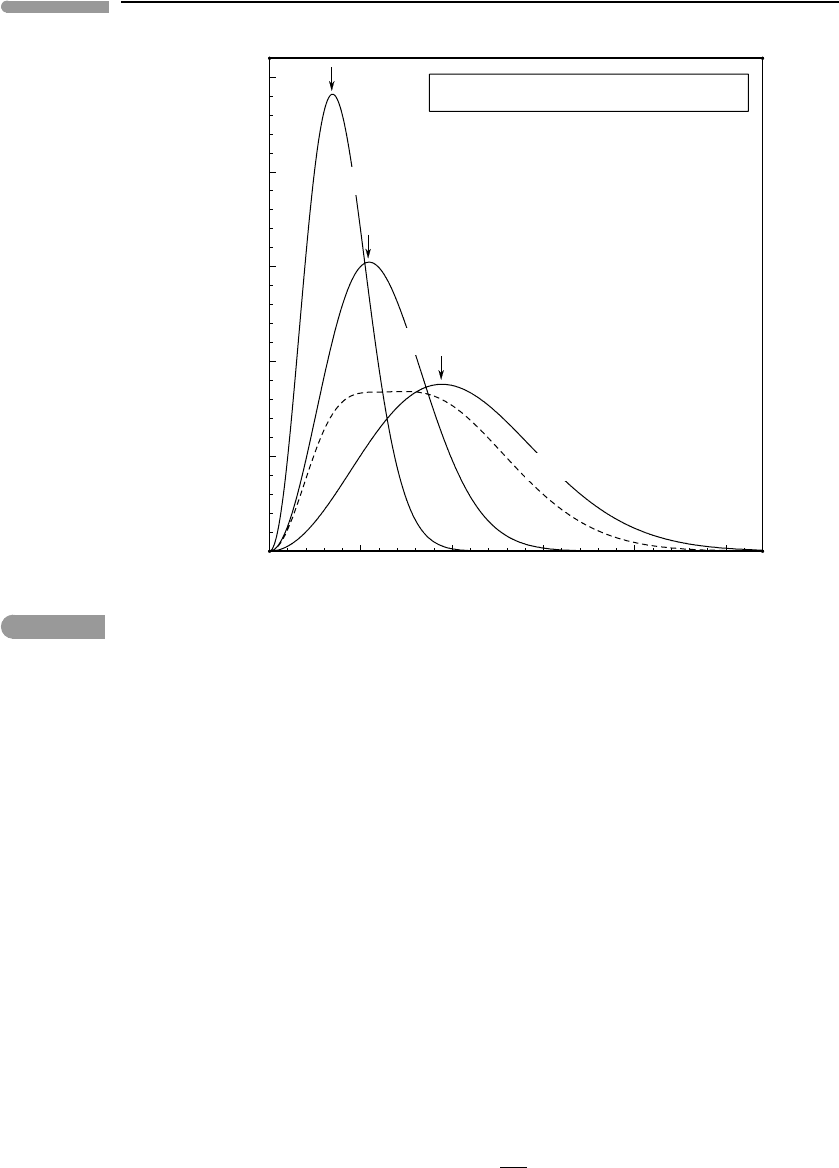
(Fig. 1.4) that specifies the most probable value of kinetic energy. The temperature of the
gas is a measure of this most probable kinetic energy value. Equilibrium in the gas is pos-
sible only if there has been sufficient time to allow its molecules to exchange information
throughout the entire volume occupied by the gas, so that all molecules “know” what the
most probable kinetic energy is. Information is transported by molecular collisions, so that
it travels at a rate comparable to molecular speeds. If the gas is now compressed at a rate
that is very slow relative to most molecular speeds then the gas will remain at equilib-
rium throughout the compression, because information about perturbations in one part of
the system reach the entire system before the magnitude of the perturbations can change
significantly. This is an example of a quasi-static transformation. In contrast, if the gas is
compressed at a rate that is much faster than the speed with which most molecules move,
then the gas cannot be at equilibrium. In this case a perturbation in one part of the system
can grow significantly before the rest of the system “knows” about it. Molecules in different
parts of the system will have different speed distributions, so that the speed distribution for
the entire volume of gas will not display a unique maximum value (dashed curve in Fig.
1.4). The fast compression is not quasi-static and, therefore, it is an irreversible transforma-
tion. During such an irreversible transformation it is not possible to define the temperature
nor the pressure of the gas, as there is no unique most probable molecular kinetic energy
(Fig. 1.4). Sonic booms, which occur when air is compressed at a rate that is faster than
characteristic molecular speeds, are examples of irreversible transformations of this type.
1.4.3 Measuring expansion work. Material properties and equations of state
We are now able to come to a rigorous definition of the sign of the expansion work. If the
pressure exerted by the system, P (we assume a quasi-static expansion), and the change in
volume, dV = Adx, have the same orientation, then PdV is a positive quantity. The work
performed by the system on the environment during expansion, dW , is a positive quantity.
This work represents energy transferred from the system to its environment, so that the
energy content of the environment changes by an amount dW , and the energy content of
the system that is expanding changes by an amount −dW . It is important to understand
17 1.4 Expansion work
0 500 1000 1500 2000 2500
0
0.001
0.002
Molecular speed (m s
–1
)
Probability density
Distribution of molecular speeds for N
2
200 K
500 K
1500 K
Fig. 1.4
Maxwell–Boltzmann probability density distribution for molecular speeds in N
2
(assumed to be an ideal gas) in the
absence of a gravitational field. The areas under all the curves are unity. As temperature increases the probability
distribution becomes wider but there is always a unique most probable speed, that depends on temperature only
(arrows). The dashed line shows a hypothetical non-equilibrium distribution of molecular speeds – the most probable
speed, and hence the temperature, cannot be defined in this case.
exactly what equation (1.19) is measuring and how to interpret the sign of the expansion
work that one calculates upon integrating this equation. Because P is always a positive
quantity, a positive value of W means that the system has expanded and performed work
on its environment; the system’s energy content has therefore decreased. Compression, i.e.
a decrease in the system’s volume, results in a negative value of W which implies that
the system has gained energy. This energy was extracted from its environment. This sign
convention is not universally followed, but it leads to equations that represent physical
processes in an intuitively satisfying way.
The expansion work associated with a finite change in volume is calculated by integrating
equation (1.19). Except for the special case of isobaric processes, volume and pressure are
not independent variables. In order to integrate equation (1.19), then, we need a function
that relates volume with pressure. One such function is the bulk modulus, K, which is a
material property, i.e. a parameter that has a well-defined value for every substance and is
independent of system size:
K =−V
∂P
∂V
. (1.20)
