Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

198 The Second Law of Thermodynamics
Box 4.1
Continued
state i. Obviously,
i
n
i
=N. We subdivide the set of N objects into i subsets of n
i
objects. Two microstates
are said to be equivalent if n
i
is the same for all i. If two microstates fulfill this requirement but, additionally,
each of the individual objects in each subset n
i
are the same except that they are arranged in a different
order, then they are the same microstate, rather than equivalent microstates. By the same argument that we
used for N, each subset of n
i
objects can be arranged in n
i
! different ways. This is true for all n
i
, so the product
(n
i
!) is the total number of repeated (i.e. identical) microstates. The product of the number of equivalent
microstates, which we symbolize with O, times the number of repeated microstates,
(n
i
!) must be equal
to the total number of possible arrangements of the N objects, N!, as there is no other possibility that we
have not considered. The number of equivalent microstates is therefore given by:
O =
N!
n
i
!
, (4.1.1)
which in the case of i = 2 (that we will use frequently), simplifies to:
O =
N!
n!
(
N −n
)
!
, (4.1.2)
where n objects are in state 1, and (N −n) objects in state 2.
Consider again the example of 5 coins. The number of microstates defined by 3 heads and 2 tails is 5!/(3!
2!) = 10. All of these microstates are equivalent and correspond to the same macrostate. In contrast, the
macrostate corresponding to 4 heads and 1 tail has 5!/(4! 1!) = 5 microstates.
The numbers of objects (molecules, atoms, ions, crystalline sites, etc.) in systems of physical interest
are much larger than this, typically of order 10
23
. The factorial of 10
23
is a very large number, beyond
the computational range of everyday calculators and computers. Luckily, however, what we are interested
in is the logarithm of O, rather than in O itself, and this we can easily calculate by means of Stirling’s
approximation, which states that, for N very large:
lnN!≈N lnN −N. (4.1.3)
Where does this come from? First, we can write ln N! as a sum of logarithms:
lnN!=
i=N
i=1
lni. (4.1.4)
Although the factorial function is defined only for integers, which are discrete variables, when N becomes
very large we can approximate the right-hand side of (4.1.4) as an integral, i.e.
i=N
i=1
lni ≈
N
1
lnidi = N lnN −N +1 ≈ N lnN −N, (4.1.5)
which is (4.1.3).
A given macrostate can arise from more than one microstate. We shall say that these
microstates are equivalent. We will also postulate that: (i) All individual microstates are
equally probable and (ii) the observed macrostate, which we will call the equilibrium
199 4.6 A microscopic view of entropy
V
f
=V
A
+V
B
T
f
=T
i
V
A
V
B
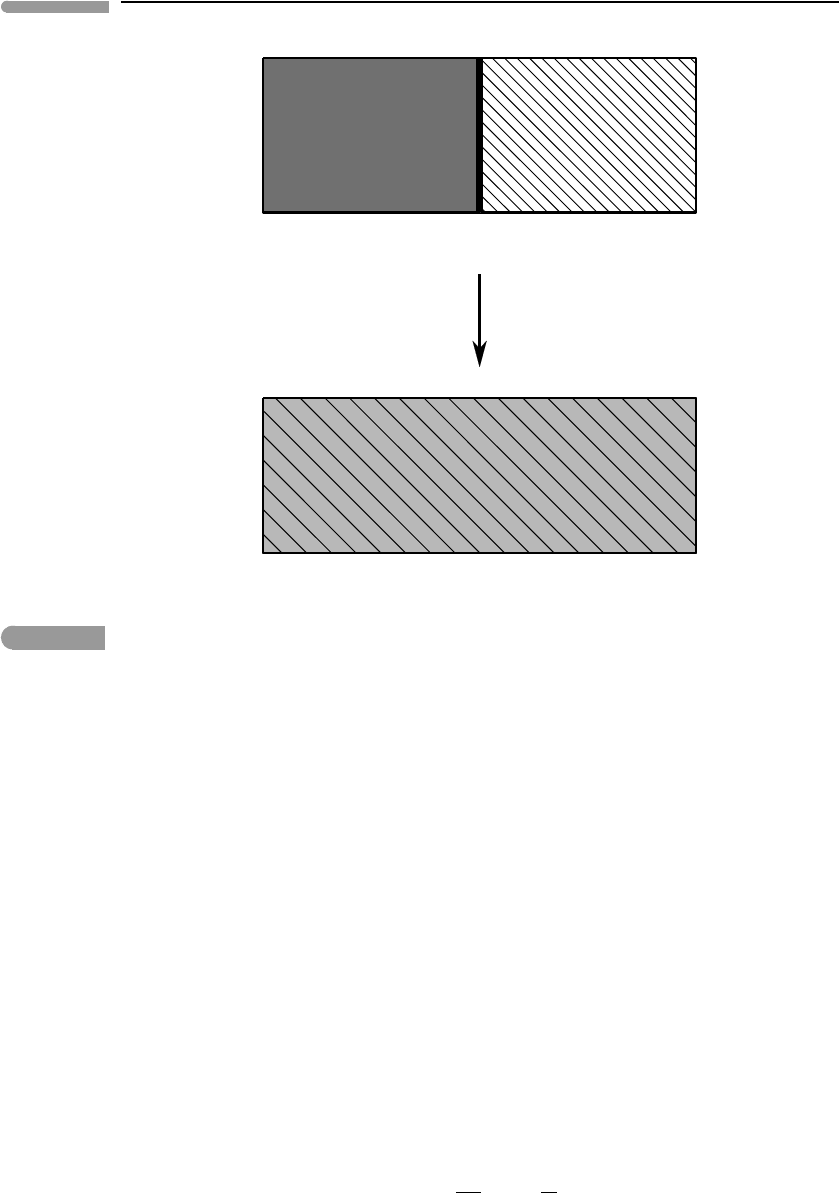
Fig. 4.7
Mixing of two different ideal gases in an isolated container. Each of the gases can be thought of as undergoing a free
expansion from its initial volume to the same final volume, V
f
.
macrostate, is the one that corresponds to the greatest number of equivalent microstates.
An example will clarify the meaning and implications of these statements and show their
plausibility, although it must be borne in mind that their acceptance as a valid description
of nature relies on the much larger theoretical framework of statistical mechanics, and on
an enormous amount of observations.
Let us consider the process of spontaneous mixing of two different ideal gases at uniform
and constant temperature and pressure. Suppose that we have two containers separated
by a wall, with volumes V
A
and V
B
, as shown in Fig. 4.7. Container A is filled with a
moles of ideal gas A, and container B is filled with b moles of ideal gas B. When the
partition is removed the gases mix by diffusion, so that after some time the entire volume,
V
f
=V
A
+V
B
is filled with a homogeneous mixture in which the mol fractions of the two
gases are X
A
=a/(a +b) =V
A
/V
f
and X
B
=b/(a +b) = V
B
/V
f
(use the ideal gas EOS
to prove these identities). Pressure and temperature were the same for the two gases before
mixing and remain unchanged during mixing.
Diffusive homogenization of the two gases in an isolated system is a spontaneous process,
so it must be accompanied by an increase in entropy, which we calculate as follows. From
equation (4.12) we write the entropy change S from an initial state i to a final state f as
follows:
S =
f
i
dE
T
+
f
i
P
T
dV . (4.45)

200 The Second Law of Thermodynamics
Using the ideal gas equation of state and the fact that internal energy of an ideal gas is a
function of temperature only (Section 1.14), we re-write equation (4.45) for an ideal gas as
follows:
S =
f
i
C
V
T
dT +R
f
i
dV
V
=C
V
ln
T
f
T
i
+R ln
V
f
V
i
. (4.46)
Diffusive homogenization of ideal gases is an isothermal process, because ideal gases are
made up of non-interacting particles (i.e. there are no forces between the particles, and hence
no work is performed when particles of different gases mix). However, the volume occupied
by each of the gases increases during mixing, from the volume of the respective initial
container to the total final volume, V
f
. The entropy of each gas therefore increase during
mixing by the same amount that it would increase if it was undergoing a free expansion
(compare equation (4.22)). Because entropy is additive (Section 4.2), the total entropy
increase for the mixing process is given by:
S
mixing
=aS
A
+bS
B
=aRln
V
f
V
a
+bR ln
V
f
V
b
=−aR ln
(
X
A
)
−bR ln
(
X
B
)
> 0. (4.47)
As mol fractions in a mixture are always less than 1, spontaneous mixing of gases is always
accompanied by an increase in entropy. I never cease to marvel at this result. We defined
entropy on the basis of heat transfer (equation (4.6)), and yet it can correctly predict the
behavior of a system in which there is no heat transfer involved! As you will soon see,
the importance of equation (4.47) goes beyond merely telling us that ideal gases will mix
spontaneously.
Let us look at this same process microscopically. The two vessels initially contain aN
and bN molecules of different ideal gases, where N ≈ 6.02 ×10
23
is Avogadro’s number.
We will now count microstates, as described in Box 4.1. You can think of the “objects” that
I refer to in Box 4.1 as being little boxes into which the containers are subdivided, such
that there is exactly one box per molecule. Averaging over a sufficiently long time, there is
always exactly one molecule inside each box, and there are no empty boxes. The boxes can
be in two different states: occupied by either a molecule of gas A or a molecule of gas B.
Before the partition is removed all boxes in side A are filled with molecules of A, and all
boxes in side B are filled with molecules of B. According to equation (4.1.2), the number
of microstates in each of the two vessels, O
A
and O
B
, is:
O
A
=
(
aN
)
!
(
aN
)
!0!
=
(
bN
)
!
(
bN
)
!0!
=O
B
=1. (4.48)
The total number of microstates for the system before the gases are allowed to mix, O
i
,
is the product of these two numbers, as any microstate in A can be combined with any
microstate in B, i.e.:
O
i
=O
A
O
B
=1. (4.49)
When the gases are allowed to mix there are (a +b)N boxes in a single vessel of volume
V
F
, of which aN are in one state (occupied by an A molecule) and bN in the other (occupied
by a B molecule). Using equation (4.1.2), the number of microstates is given by O
f
,as
201 4.6 A microscopic view of entropy
follows:
O
f
=
[
(
a +b
)
N
]
!
(
aN
)
!
(
bN
)
!
. (4.50)
We now use Stirling’s approximation (equation (4.1.3)):
ln O
f
=
(
a +b
)
N ln
[
(
a +b
)
N
]
−
(
a +b
)
N
−
[
(
aN
)
ln
(
aN
)
−
(
aN
)
]
−
[
(
bN
)
ln
(
bN
)
−
(
bN
)
]
, (4.51)
which simplifies to:
ln O
f
=aN ln
a +b
a
+bN ln
a +b
b
(4.52)
or:
ln O
f
=−aN ln
(
X
A
)
−bN ln
(
X
B
)
. (4.53)
Consider these results in the light of the two postulates about microstates and macrostates.
According to postulate (i) all microstates are equally probable. Equation (4.49) says that
there is only one microstate that corresponds to the initial macrostate: all boxes on each side
filled with only one kind of molecule. We know that, if the partition is not there, this is not
the equilibrium macrostate. Rather, the equilibrium macrostate is one in which the gases are
mixed uniformly over both containers. According to equation (4.53), for a system size of
order 1 mol this macrostate corresponds to ∼e
N
equivalent microstates (N ≈6.02×10
23
).
By postulate (i) each of these equivalent microstates has the same probability of occurring
as the single microstate that describes the un-mixed gases. There are, however, e
N
times
more equivalent microstates than in the first case, so it is e
N
times more likely that we will
observe a homogeneous mixture than separate gases (as long as the partition is not there).
This is what postulate (ii) says, that the equilibrium macrostate, i.e. the final state that the
system will spontaneously evolve to, is the one that corresponds to the most microstates.
Note that we have not proved that equation (4.53) is indeed the maximum, only that it
is (much) larger than (4.49). It happens to be the maximum, though (see, for example,
Glazer & Wark, 2001). The probability that the two gases will spontaneously un-mix and
return to the state they were in before the partition was removed is not zero (this is what
postulate (i) says), but it is so vanishingly small that we can be certain that we will not
observe this phenomenon in the lifetime of the Solar System (quite a bit longer than that,
actually). There is an additional point in this argument. This is the fact that fluctuations
take place in all natural systems, during which the system visits microstates that are very
close, but not identical, to an equilibrium microstate. These fluctuations are so small and
swift that they are not reflected in the macrostate of the system, except when a system is
close to a critical phase transition (more on this later). In principle, another exception could
occur when the system of interest is so small that 1/e
N
becomes an “observable” number.
In Chapter 14 we will see that this may be a limiting factor for the minimum size of living
organisms.
202 The Second Law of Thermodynamics
4.6.2 Boltzmann’s postulate
The attentive reader might have noticed a striking similarity between equations (4.47) and
(4.53). The two equations are identical, but for a constant multiplicative factor.They suggest,
but do not prove, the following relationship between entropy and number of microstates:
S = k
B
ln O, (4.54)
where k
B
= R/N = 1.38 ×10
−23
JK
−1
molecule
−1
is known as Boltzmann’s constant.
Equation (4.54), due to Ludwig Boltzmann and reputedly inscribed on his tombstone, is
known as Boltzmann’s postulate.As with the laws of thermodynamics, it remains a postulate
because it cannot be shown to be valid from simpler statements or observations. We accept
its validity a posteriori, on the basis of the agreement of the theory built upon it with the
observed behavior of nature. Boltzmann’s postulate provides a microscopic interpretation
of entropy that is intuitively satisfying. It says that the highest entropy macrostate is the one
that corresponds to the greatest number of equivalent microstates. The increase in entropy
that accompanies all spontaneous processes in an isolated system is simply a reflection of
the system “falling onto” the most likely set of equivalent microstates. The impossibility
of entropy decreasing in an isolated system should actually be seen as a vanishingly small
probability – “practically impossible” for any conceivable macroscopic system. As to the
relationship between entropy and disorder, which obsesses philosophers, social scientists,
post-modernist writers and other commentators, we shall have more to say about it shortly.
Let us go back to the relationship between (4.53) and (4.47). Using (4.54), we can write
the following equation:
S
configurational
=k
B
ln O
f
−lnO
i
=k
B
ln
O
f
O
i
. (4.55)
The increase in entropy that accompanies mixing of the gases is a function only of the ratio
between the number of microstates in the final and initial states. This increase in entropy is
called the configurational entropy, as it reflects a change in the microscopic configuration
of the system. It is important to realize that equation (4.54) gives the functional relationship
between S and O, but it says nothing about the factors that enter into the definition of the
microstates that are counted by O. The number of microstates in our example was defined
solely on the basis of the identity of the molecules, but in reality other parameters must
be considered too, most importantly temperature. As the temperature increases so does the
number of quantized energy levels available for atomic vibrations, and therefore the number
of accessible microstates and, by (4.54), the entropy. Because we consider an isothermal
transformation the effect of temperature on the value of O cancels out in equation (4.55),
leaving the change in configuration as the only contribution to the entropy of mixing.
There is an important question here: how does the microscopic interpretation of entropy
relate to the concept of energy dissipation and the fact that an entropy increase reflects an
irreversibly lost opportunity to perform mechanical work? The following is a possible way
of looking at this, which traces its origin to James Clerk Maxwell (who was perhaps the
greatest scientist of the nineteenth century). Suppose that rather than a removable partition
the two vessels with different gases in Fig. 4.7 are separated by a fixed wall with a small
opening covered by a revolving trapdoor that will turn only when it is simultaneously hit by a
pair of different molecules coming from opposite directions. The door is just large enough to
allow a single molecule through in each direction at any one time (Maxwell used imaginary
demons for this sort of thought experiment, and his demons were actually asked to perform

203 4.6 A microscopic view of entropy
a task opposite to the one we describe here, see, for example, Baylin, 1994). The door is
connected to an external reservoir of mechanical energy, such as a spring or a weight, so that
rotation in one direction compresses the spring (or raises the weight) by a small amount,
and rotation in the opposite direction releases the spring by the same amount. As long as the
door keeps revolving in the same direction there is net storage of mechanical energy, but if
the door flip-flops then there is no net storage nor release of mechanical energy. When we
first free the revolving door with different gases in each container it will only be hit by A
molecules coming from the left and B molecules coming from the right, so it will spin in only
one direction and store mechanical energy.As the gases mix the door may start flip-flopping,
but as long as there is a gradient in composition across the partition there will be a net storage
of mechanical energy, because the door will rotate more often in one direction than in the
other. When the gas compositions become identical on both sides there will be a net amount
of mechanical energy stored in the spring or weight, which came exclusively from diffusive
mixing of the two gases. If we use a removable partition rather than our little trapdoor this
work potential is lost, and entropy is generated, or equivalently, energy is dissipated.
But the gases were allowed to mix anyway, even if they perform work, so didn’t their
entropy increase, as calculated by (4.47)or(4.53)? No, as one must still abide by the First
Law. The mechanical energy stored in the spring came from somewhere: it must have come
from the kinetic energy of the molecules. If the container is adiabatic then the gas cooled
down. Equation (4.53) is now not a complete description of the number of microstates in
the final state, as it does not account for the fact that, as temperature decreases, the number
of accessible energy levels of the molecules decreases too, and so does the number of
microstates (see Glazer & Wark, 2001). Does the trapdoor example carry over to entropy
generation by diffusive heat flow? Yes, but it is less obvious how. The key is that O changes
with temperature, so we need to imagine some contraption that is able to store mechanical
energy by intercepting packages of molecular kinetic energy exchanged between bodies at
different temperatures.
Worked Example 4.3 Configurational entropy in crystals
Configurational entropy plays an important role in the thermodynamic characterization of
minerals and melts, that we will study in later chapters. Here we focus on the configurational
entropy of minerals of constant composition and variable crystalline structure. Consider the
case of potassium feldspar. A unit cell of potassium feldspar consists of four formula units,
so that it has the composition: K
4
Al
4
Si
12
O
32
. Silicon and aluminum occupy tetrahedral
sites in the crystalline structure. There are a total of sixteen tetrahedral sites in a potassium
feldspar unit cell. In all potassium feldspar polymorphs it is possible to distinguish between
two types of tetrahedral sites, on the basis of their sizes and symmetries, which we can call
T1 and T2 sites, and such that there are eight T1 sites and eight T2 sites. In addition, in
some of the crystal forms it is also possible to discriminate between two subsets of the T1
sites, which we will label T1a and T1b. The four Al and twelve Si atoms can occupy the
sixteen tetrahedral sites in a number of different ways, giving rise to minerals with different
crystal symmetries and different amounts of configurational entropy.
Consider microcline first. In this mineral the T1a and T1b sites are distinguishable. The
four T1a sites are occupied by Al, whereas the four T1b sites and the eight T2 sites are
occupied by Si. This regular distribution of Si and Al atoms is said to constitute a structure
with long-range order. It is also a structure with a high information content: we have total
certainty of what we are going to find in each type of site. In sanidine, on the other hand,

204 The Second Law of Thermodynamics
Al and Si distribute themselves randomly over all sixteen tetrahedral sites, such that, one
average (i.e. in a macrsocopic crystal with ∼ N atoms in it) there are six Si and two Al
atoms in the T1 sites and six Si and two Al atoms in the T2 sites. This structure lacks long-
range order, and has a low information content: all we know is that we have a one in three
probability of finding an Al atom in any one tetrahedral site, compared to the certainty that
we had in the case of the ordered structure of microcline.
We will now count the number of equivalent microstates that give rise to each of the two
macrostates, microcline and sanidine. One “unit cell mol” of potassium feldspar contains
N unit cells, i.e. 4N Al atoms and 12N Si atoms. The objects that we will count are
crystalline sites, each of which can be in two possible states: filled with Si or filled with Al.
In the following equations, the numerator is the number of crystalline sites, the first term in
the denominator is the number of those sites occupied by Si, and the second term in the
denominator is the number of sites occupied by Al. For microcline we must consider the
number of microstates of each of the three types of distinguishable sites, so that we have:
O
T1a
=
(
4N
)
!
0!
(
4N
)
!
O
T1b
=
(
4N
)
!
(
4N
)
!0!
O
T2
=
(
8N
)
!
(
8N
)
!0!
.
(4.56)
The total number of microstates for the microcline macrostate is:
O
microcline
=O
T1a
O
T1b
O
T2
=1 (4.57)
or:
ln O
microcline
=0. (4.58)
For sanidine:
O
T1
=
(
8N
)
!
(
6N
)
!
(
2N
)
!
O
T2
=
(
8N
)
!
(
6N
)
!
(
2N
)
!
,
(4.59)
using Stirling’s approximation we find:
ln O
T1
=ln O
T2
=6N ln
4
3
+2N ln 4 (4.60)
and:
ln O
sanidine
=ln O
T1
+lnO
T2
=2N
6ln
4
3
+2ln4
. (4.61)
The sanidine macrostate corresponds to a (much) greater number of microstates than the
microcline macrostate, so by Boltzmann’s postulate it must have a higher entropy. This
configurational entropy does not reflect a change in the composition of the phase, as in
the example of the mixing gases, but rather a change in the microscopic configuration of

205 4.6 A microscopic view of entropy
the crystal. We use Bolztmann’s equation, (4.54), to calculate the configurational entropies
of the two polymorphs. Noting that equations (4.58) and (4.61) are based on four formula
units of potassium feldspar, the molar configurational entropies (i.e. per mol of KAlSi
3
O
8
)
are:
S
configurational, microcline
=
1
4
k
B
ln O
microcline
=0 (4.62)
and:
S
configurational, sanidine
=
1
4
k
B
ln O
sanidine
=R
3ln
4
3
+ln4
=R ln
256
27
≈18.7J K
−1
mol
−1
(4.63)
The total entropy of a crystal, S
total
, is in general made up of two contributions:
S
total
=S
thermal
+S
configurational
. (4.64)
One contribution, S
thermal
, arises from the distribution of (quantized) energy levels of the
constituent atoms, and is called the thermal or vibrational component of entropy. Thermal
entropy increases with temperature, as the vibrational energy of the atoms gets “dispersed”
over a greater number of possible energy levels, which increases the number of accessible
microstates. Configurational entropy, S
configurational
, arises from the distribution of atoms in
the crystalline structure and remains constant with temperature as long as the distribution of
atoms between different crystalline sites does not change (e.g. as long as sanidine does not
invert to microcline). In a crystal with perfect long-range order there is no configurational
entropy, but at all finite temperatures the ordered crystal still has thermal entropy (see
Section 4.7).
The lower “information content” of the crystalline structure with greater configurational
entropy (sanidine in this example) is at the core of the relationship between “entropy and
disorder”: greater order implies greater certainty of what kind of atom is in what kind of
crystalline site, and therefore a lower number of microstates and lower configurational
entropy. It is a tragedy that this concept, that has a strict and unambiguous meaning in the
context of the microscopic structure of matter, has been misunderstood and misappropriated
by non-scientists, who then proceed to apply it erroneously in a wide range of contexts where
it does not belong. Entropy as a measure of disorder is a strictly microscopic concept and is
meaningless as a description of “macroscopic order”. Perhaps the most egregious example
of the misuse of the concept of entropy is its erroneous application to support the spurious
claim that biological evolution, and indeed the existence of life itself, requires a supernatural
explanation. I will dispel these detestable notions in Chapter 14.
There is one final point that you may be wondering about. If sanidine has higher entropy
than microcline (i.e. it is a macrostate that is more likely because it corresponds to a larger
number of equivalent microstates), then why does microcline exist at all? Shouldn’t it
spontaneously invert to sanidine? If the crystal were an isolated system (constant internal
energy and volume) then it would, subject to the removal of constraints (interatomic forces,
which would be the equivalent of the partition in Fig. 4.7). But the direction of a spontaneous
change in a crystal that is not an isolated system (which is the common situation in nature)
is not necessarily determined by an increase in entropy, but rather by a decrease in the
thermodynamic potential appropriate to the constraints on the system. We return to this in
Sections 4.8 and 4.9.
206 The Second Law of Thermodynamics
4.7 The Third Law of Thermodynamics
4.7.1 Statement of the Third Law of Thermodynamics
There is an additional principle, called the Third Law of Thermodynamics, that is inde-
pendent of the First and Second Laws. It is essential in the development of chemical
thermodynamics, although much of classical thermodynamics and its applications to heat
engines and other engineering processes do not require it. We introduce the Third Law
by stating that experimental evidence shows that, as temperature approaches 0 K, heat
capacities (C
P
and C
V
) approach zero faster than temperature, i.e.:
lim
T →0
C
P
T
=0. (4.65)
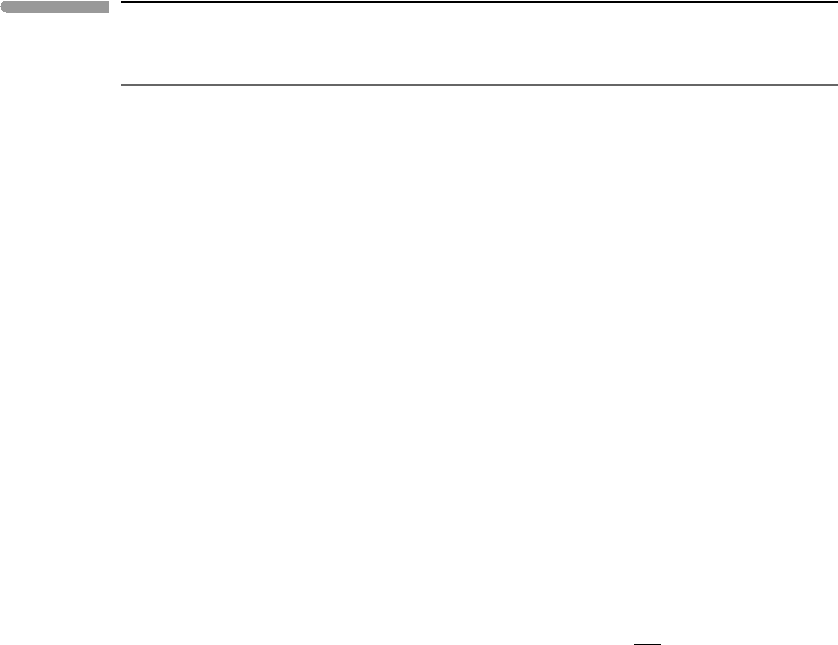
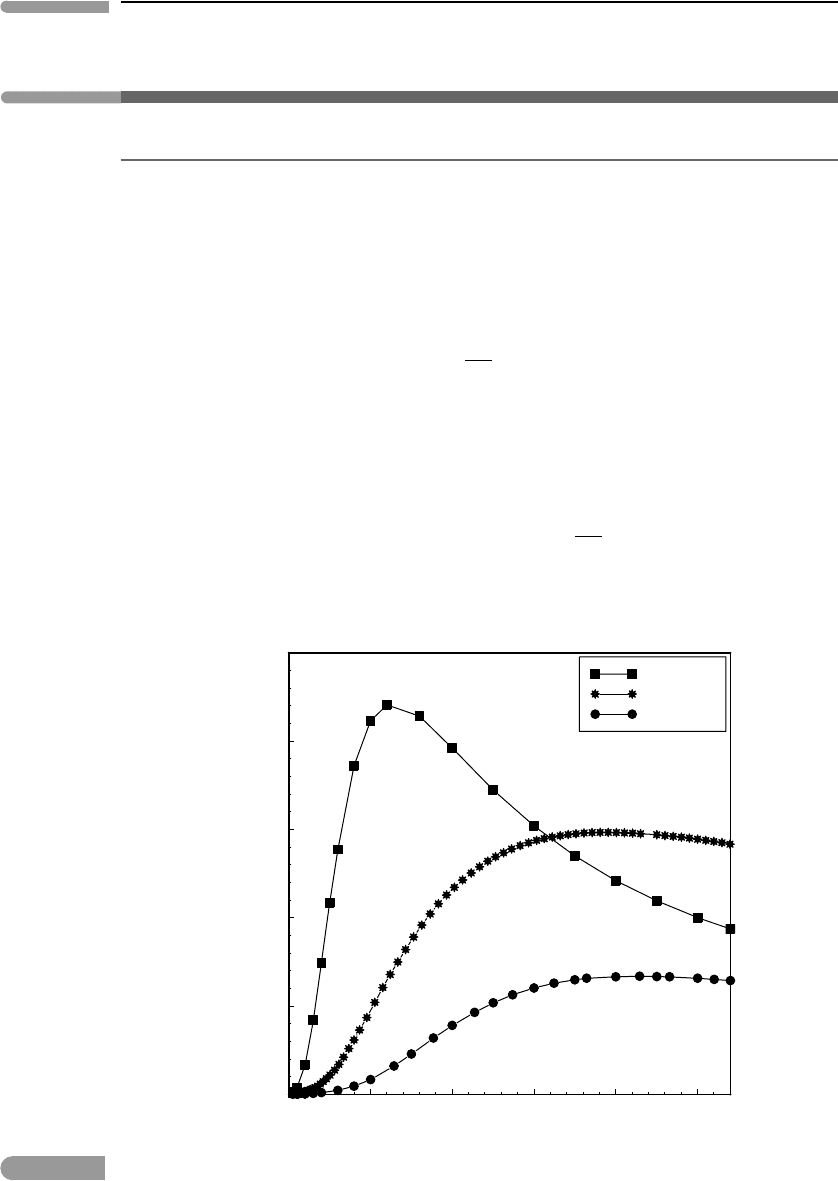
Afew examples are shown in Fig. 4.8. A consequence of (4.65) is that the entropy difference
of a substance between 0 K and any other temperature, T, is a finite value. Assuming that
heating takes place at constant pressure, and that there are no phase transitions between 0
and T, then dQ =C
p
dT , and we have:
S
(
T
)
−S
(
0
)
=
T
0
dS =
T
0
C
P
T
dT (4.66)
with condition (4.65) guaranteeing that the integral does not blow up. An unknown
integration constant remains, however, which is the entropy at 0 K.
0 50 100 150 200 250
0
0.1
0.2
0.3
0.4
0.5
T (K)
C
P
/T(JK
–2
mol
–1
)
KCl
Enstatite
MgO
Fig. 4.8
Low temperature behaviors of C
P
/T for an ionic crystal (KCl, data from Berg & Morrison, 1957), a crystalline oxide
(MgO, data from Barron et al., 1959) and a crystalline silicate (enstatite, data from Krupka et al., 1985).

207 4.7 The Third Law of Thermodynamics
It is found that the entropy change associated with any transition between equilib-
rium states of perfect crystalline substances vanishes as well as T → 0. The meaning of
“perfect crystalline substance” is that the crystal has no configurational entropy, i.e. that its
atoms are perfectly ordered in the sense that we discussed in Worked Example 4.3. Consider
a transformation A → B between equilibrium states of perfect crystals. This could be, for
instance, a chemical reaction. Using the notation for changes in state variables that we laid
out in Section 1.13.1, we have:
r
S
P ,T
=
S
P ,T
B
−
S
P ,T
A
=
S
P ,0
B
−
S
P ,0
A
+
T
0
C
P
T
B
dT −
T
0
C
P
T
A
dT (4.67)
and
r
S
P ,T
→0 when T → 0. The low-temperature behavior of heat capacities, equation
(4.65), means that the two integrals in (4.67) also vanish as T → 0. One must therefore
conclude that:
lim
T →0
S
P ,0
B
−
S
P ,0
A
=0. (4.68)
The integration constant in (4.66), which is the entropy at absolute zero, is therefore the
same for all perfect crystalline substances. This was the original statement of the Third Law,
put forward by the physical chemist W. Nernst in 1906. Note that (4.68) does not fix the
absolute value of S at 0 K, only that the value is the same for all crystalline substances.
Max Planck gave a stronger statement of the Third Law in 1916, by postulating that entropy
vanishes at 0 K. Planck’s statement of the Third Law, which is the one that is widely accepted
today (and which subsumes Nernst’s original statement) is that the entropy of all perfect
crystalline substances vanishes at 0 K. The emphasis on “perfect crystalline substance” is
important. For example, Planck’s statement of the Third Law implies that the entropy of
microcline vanishes at 0 K. The entropy of sanidine, however, does not vanish, because its
configurational entropy remains unchanged with temperature (as long as the atoms don’t
order themselves spontaneously, in which case it is no longer sanidine).
The Third Law remains a postulate (just as the First and Second Laws), but its plausibility
can be demonstrated from quantum mechanical arguments. There is another statement of
the Third Law, which can be shown to be equivalent to Planck’s statement, and which is
also due to Nernst. This is that it is impossible to reach T =0 in a finite number of reversible
steps. We will not be concerned with this statement in this book, but the interested reader
can see Baylin (1994) for a derivation.
4.7.2 An absolute entropy scale
The Third Law of Thermodynamics makes it possible to define absolute values of entropy
for all substances. This is in contrast to energy functions, such as enthalpy. Recall that in
Section 1.13.1 we constructed a scale for enthalpy by setting to zero the enthalpies of all
pure elements in their stable forms at 298.15 K and 1 bar, and then defining enthalpies of
formation for compounds relative to this arbitrary zero. The Third Law says that we cannot
do this for entropy. From equation (4.66) it follows that the entropy of all substances,
including pure elements, at 298.15 K and 1 bar is not zero. Using Planck’s postulate, we
define the reference state entropy, also called the reference state Third Law entropy, and
