Dong S.-H. Wave Equations in Higher Dimensions
Подождите немного. Документ загружается.

164 13 Dirac Equation with the Coulomb Potential
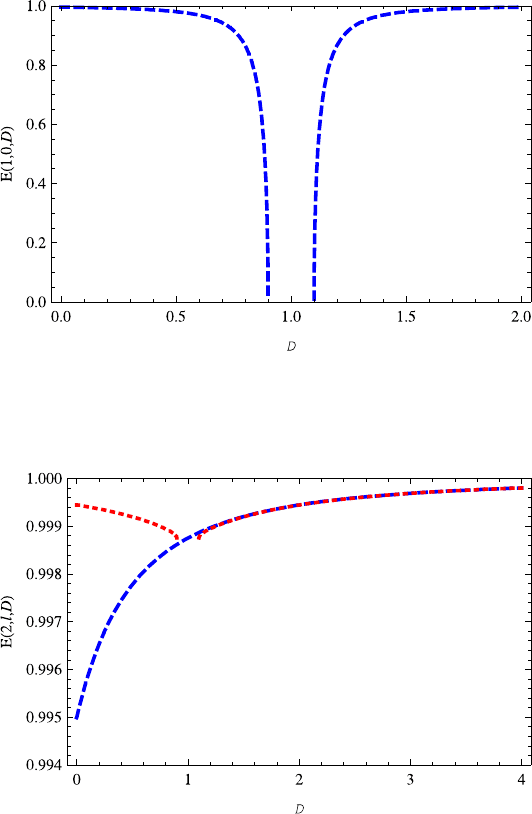
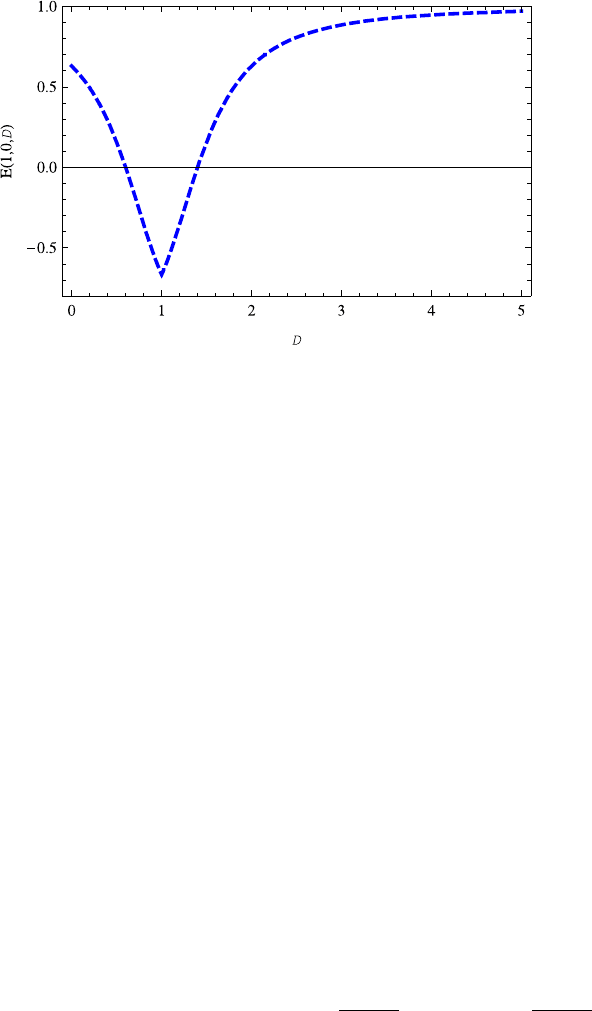
Fig. 13.4 The energy E(1, 0,D) decreases with the increasing dimension D ∈ (0, 0.9],butin-
creases with the increasing dimension D ≥ 1.1. Note that it is symmetric with respect to axis
D =1
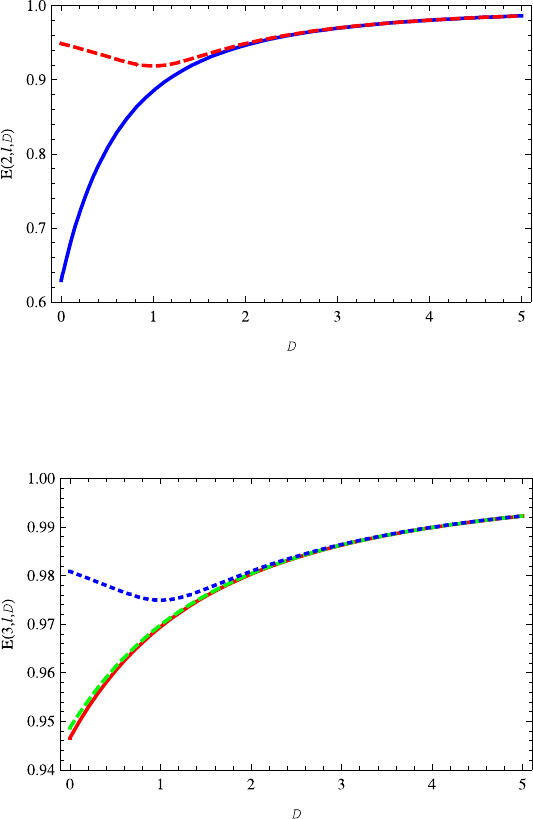
Fig. 13.5 The variation of energy E(2, 0,D) (red dotted line) on the dimension D is very similar
to E(1, 0,D). The energy E(2, 1,D) (blue dashed) increases with the increasing dimension D.
Specially, for D>1 the energy E(2, 1,D) almost overlaps E(2, 0,D)
(13.33), i.e., K
2
≥ξ
2
. This means that there are no solutions in D ∈(1−2ξ,1+2ξ).
Figure 13.5 shows the properties of E(2,l,D), in which the red dotted and blue
dashed lines correspond to l = 0, 1, respectively. The variation of the E(2, 0,D)
on D is similar to energy E(1, 0,D). But energy E(2, 1,D) increases monoton-
ically as the dimension D increases. It is found that, when D>1 the energies
E(2,l,D) (l = 0, 1) are almost overlapped. This kind of phenomenon occurs in
3 Analysis of the Eigenvalues 165
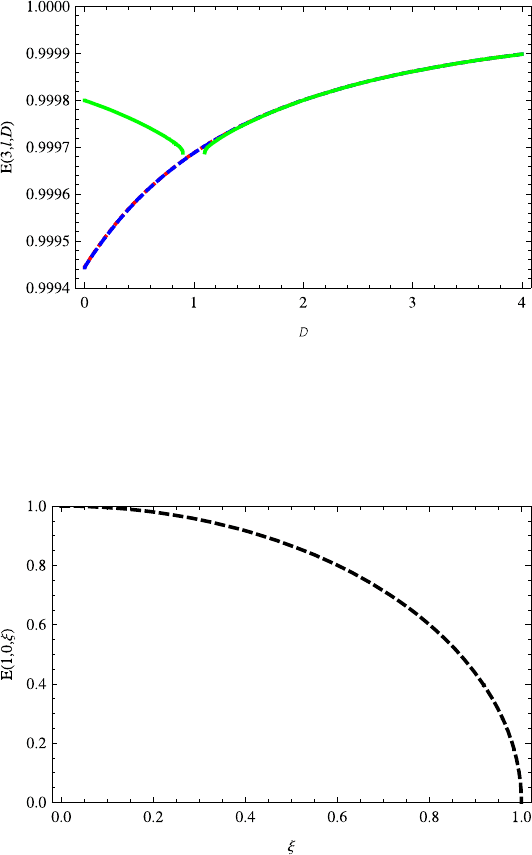
Fig. 13.6 The variations of energy levels E(3,l,D) (l = 2,1, 0) on dimension D are similar
to E(2,l,D) (l = 1, 0). The energy E(3,2,D) almost overlaps energy E(3, 1,D). This can be
explained well by Eq. (13.28). When D>1, the energies E(3,l,D) (l = 2, 1, 0) are almost
overlapped. The red dotted, blue dashed and green solid lines correspond to quantum numbers
l =2, 1, 0, respectively
Fig. 13.7 The energy E(1, 0,ξ)decreases with the increasing potential parameter ξ ≤1
n = 3(l = 0, 1, 2) as shown in Fig. 13.6. This can be explained well through the
series expansion for 1/D as given in Eq. (13.27).
Third, we exhibit the variations of energy E(n,l,ξ) on the potential strength
ξ in Figs. 13.7, 13.8, 13.9. It is found from Fig. 13.7 that the energy E(1, 0,ξ)
decreases with ξ ≤1. This can be explained by Eq. (13.26). That is to say, ξ ≤l +1.
Figures 13.8 and 13.9 show the variations of the E(2,l,ξ) and E(3,l,ξ) on the
166 13 Dirac Equation with the Coulomb Potential
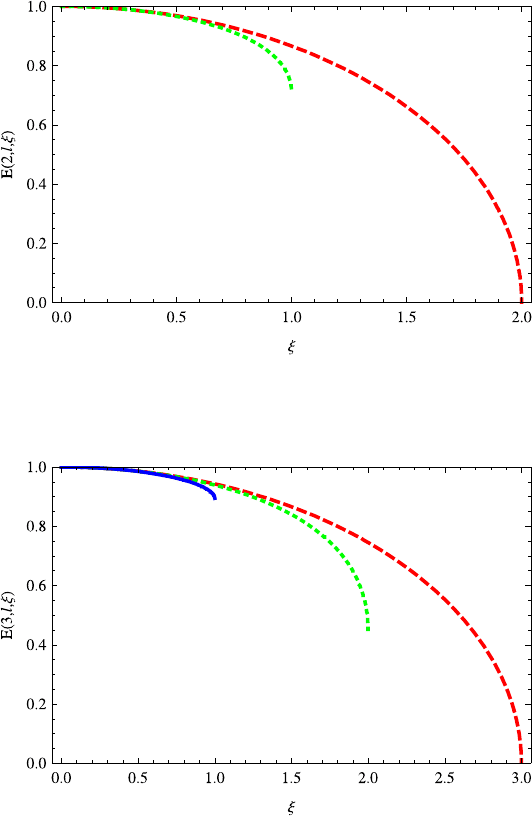
Fig. 13.8 The energies E(2,l,ξ) (l = 0, 1) decrease with the increasing potential parameter ξ.
Notice that ξ ≤1forl =0(green dotted line), while ξ ≤2forl = 1(red dashed line)
Fig. 13.9 The energies E(3,l,ξ)(l =0, 1, 2) decrease with the increasing potential parameter ξ .
Note that ξ ≤ 1forl = 0(blue solid line), while ξ ≤2forl = 1(green dotted line)andξ ≤ 3for
l =2(red dashed line). Generally, the energies E(n,l,ξ) decrease with the potential parameter
ξ ≤l +1foragivenl
potential parameter ξ . The energies E(n,l,ξ) decrease with potential parameter
ξ ≤ l + 1. In Fig. 13.8, the green dotted and red dashed lines correspond to l =
0, 1, respectively. In Fig. 13.9, the blue solid, green dotted and red dashed lines
correspond to l = 0, 1, 2, respectively. Generally, the energies E(n,l,ξ) decrease
with the potential parameter ξ ≤l +1 for a given l.

4 Generalization to the Dirac Equation 167
Fourth, it is worth pointing out the interest of the singularity at |K|=ξ , i.e.,
l +(D −1)/2 = ξ =Zα. This is a famous phenomenon to make the energy formula
broken down for potential strength with Zα ≥ 1 in three dimensions. When the
Dirac equation with the 1/r potential is generalized to the (D + 1)-dimensional
case, however, the singularity survives and moves to big values for Zα, e.g., Zα =
3/2forD =4. Such a kind of phenomenon has been addressed in Ref. [445].
Finally, we want to briefly address the one-dimensional hydrogen atom as dis-
cussed by Moss [189]. The interest arises from its relevance to the behavior of
hydrogen-like atoms in the strong magnetic fields and to hydrogenic impurities con-
fined in the quantum-well wire structures [188]. It is found that when D = 1 and
Z = 1, the energy spectrum (13.26) does not exist at all since the factor
K
2
−ξ
2
becomes imaginary. This is because from the definition |K|=l +(D −1)/2, K is
equal to zero regardless of the value of potential parameter ξ if D =1 and l = 0.
This conclusion agrees well with that of Ref. [189].
4 Generalization to the Dirac Equation with a Coulomb
Potential Plus a Scalar Potential
4.1 Introduction
The Dirac equation with a Coulomb potential has been discussed above. Recently,
the bound states and the S-matrix in the quantum scattering theory of the Dirac
equation with a Coulomb plus a scalar potential have been investigated in 3 + 1
dimensions [446–448]. Apart from this, the bound states of the Dirac equation with
the Coulomb plus scalar potential have been carried out both in two dimensions and
in D dimensions [105, 449]. In this section we are going to study the Dirac equation
with a Coulomb potential plus a scalar potential.
4.2 Exact Solutions
We now consider the Dirac equation with a mixed potential including a Coulomb
potential and a scalar potential. The Coulomb potential is taken as
V
c
=−
v
r
. (13.35)
Also, the scalar potential is chosen as
V
s
=−
s
r
, (13.36)
which is added to the mass term of the Dirac equation. The v and s denote the
electrostatic and scalar coupling constants, respectively.
168 13 Dirac Equation with the Coulomb Potential
After some algebraic manipulations, we observe that the radial components
F
KE
(r) and G
KE
(r) satisfy the following first-order differential equations [105]
d
dr
G
KE
(r) +
K
r
G
KE
(r) =
E −M +
v +s
r
F
KE
(r),
−
d
dr
F
KE
(r) +
K
r
F
KE
(r) =
E +M +
v −s
r
G
KE
(r).
(13.37)
Because this equation keeps invariant by interchanging F
KE
↔ G
−K−E
and
V
c
(r) ↔−V
c
(r), we only discuss the attractive Coulomb potential case V
c
(r)
(v>0). The solutions for the repulsive Coulomb potential V
c
(r) (v<0) can be
obtained from the former by interchanging F
KE
↔ G
−K−E
. The scalar potential
V
s
, however, has to be discussed by two different cases s>0 and s<0.
Likewise, we introduce ρ = 2r
√
M
2
−E
2
for the bound states |E| <M. Thus,
Eq. (13.37) becomes
d
dr
G
KE
(ρ) +
K
ρ
G
KE
(ρ) =
−
1
2
M −E
M +E
+
v +s
ρ
F
KE
(ρ),
d
dr
F
KE
(ρ) −
K
ρ
F
KE
(ρ) =
−
1
2
M +E
M −E
−
v −s
ρ
G
KE
(ρ).
(13.38)
Similarly, define the wavefunction
±
(ρ) with the forms
G
KE
(ρ) =
√
M −E [
+
(ρ) +
−
(ρ)],
F
KE
(ρ) =
√
M +E [
+
(ρ) −
−
(ρ)].
(13.39)
Substitution of them into Eq. (13.38) leads to
d
dρ
+
(ρ) ±
d
dρ
−
(ρ) ±
K
ρ
[
+
(ρ) ±
−
(ρ)]
=
−
1
2
±
v ±s
ρ
M ±E
M ∓E
[
+
(ρ) ∓
−
(ρ)]. (13.40)
Their addition and subtraction allow us to obtain
d
dρ
±
(ρ) ∓
vE +sM
ρ
√
M
2
−E
2
−
1
2
±
(ρ)
=−
K
ρ
±
vM +sE
ρ
√
M
2
−E
2
∓
(ρ). (13.41)
Take the following conventions
τ =
vE +sM
√
M
2
−E
2
,τ
=
vM +sE
√
M
2
−E
2
. (13.42)
Then, Eq. (13.41) is simplified to
d
dρ
±
(ρ) ∓
τ
ρ
−
1
2
±
(ρ) =∓
τ
±K
ρ
∓
(ρ). (13.43)
4 Generalization to the Dirac Equation 169
Based on this relation, we are able to obtain the following important second-order
differential equations
1
d
2
dρ
2
+
1
ρ
d
dρ
+
−
1
4
+
τ ±1/2
ρ
−
λ
2
ρ
2
±
(ρ) =0, (13.44)
where
λ
2
=K
2
−v
2
+s
2
. (13.45)
For a weak Coulomb potential, we take
λ =
K
2
−v
2
+s
2
> 0. (13.46)
From the behaviors of the wavefunction at the origin and at infinity, we define
±
(ρ) =ρ
λ
e
−ρ/2
R
±
(ρ). (13.47)
Substitution of this into (13.44) yields
d
2
dρ
2
R
±
(ρ) +
−1 +
1 +2λ
ρ
d
dρ
R
±
(ρ)
+
τ −λ −1/2 ±1/2
ρ
R
±
(ρ) =0, (13.48)
whose solutions are the confluent hypergeometric functions
R
+
(ρ) =a
01
F
1
(λ −τ ;2λ +1;ρ),
R
−
(ρ) =b
01
F
1
(1 +λ −τ ;2λ +1;ρ).
(13.49)
Let us study the relation between a
0
and b
0
. With the same technique as above,
we have
λ −τ
ρ
a
0
+
τ
+K
ρ
b
0
1
F
1
(1 +λ −τ ;2λ +1;ρ) =0. (13.50)
Since both a
0
and b
0
are not equal to zero, we obtain
b
0
=
τ −λ
τ
+K
a
0
. (13.51)
From Eq. (13.39) one has
G
KE
(ρ) = N
KE
√
M −Eρ
λ
e
−ρ/2
×[(τ
+K)
1
F
1
(λ −τ ;2λ +1;ρ)
+(τ −λ)
1
F
1
(1 +λ −τ ;2λ +1;ρ)],
F
KE
(ρ) = N
KE
√
M +Eρ
λ
e
−ρ/2
×[(τ
+K)
1
F
1
(λ −τ ;2λ +1;ρ)
−(τ −λ)
1
F
1
(1 +λ −τ ;2λ +1;ρ)],
(13.52)
1
Notice that Eq. (13.44) is a special case of the Tricomi equation as given in Eq. (13.14).
170 13 Dirac Equation with the Coulomb Potential
where N
KE
=a
0
(τ
+K)
−1
(2
√
M
2
−E
2
)
−1/2
.
We now study the eigenvalues of this quantum system. The quantum condition is
given by
τ −λ =n
=0, 1, 2,.... (13.53)
When n
=0, λ =τ , and
K
2
=τ
2
+v
2
−s
2
=(τ
)
2
. (13.54)
Therefore, K has to be positive to avoid a trivial solution.
Introduce a principal quantum number
n =|K|−(D −3)/2 +n
=|K|−(D −3)/2 +τ −λ
=l +1 +n
=1, 2,.... (13.55)
BasedonEqs.(13.42) and (13.53), we have
Ev +Ms
√
M
2
−E
2
=n −|K|+
D −3
2
+λ =n
+λ =n −l −1 +λ ≡κ. (13.56)
The energy E can be solved from Eq. (13.56)
E(n,l,D) =M
−
vs
v
2
+κ
2
±
vs
v
2
+κ
2
2
−
s
2
−κ
2
v
2
+κ
2
1/2
. (13.57)
We now consider a few special cases. First, if v =0, then λ =
√
K
2
+s
2
. Thus,
the energy becomes
E(n,l,D) =±M
1 −
s
2
(n −l −1 +
√
K
2
+s
2
)
2
1/2
. (13.58)
It implies that there are two branches of symmetric solutions for the positive and
negative energies.
For a large D,wehave
E(n,D) ±M[1 −2s
2
D
−2
+4s
2
(2n −3)D
−3
−···], (13.59)
which implies that the energy is independent of l for a large D.
For a small s,wehave
E(n,l,D) ±M
1 −
s
2
2[n +(D −3)/2]
2
+
s
4
2[n +(D −3)/2]
4
2n +D −3
2l +D −1
−
1
4
, (13.60)
where the first term on the right hand side is the rest energy M (c
2
=1), the second
one is from the solutions of the Schrödinger equation, and the third one is the fine
structure energy, which removes the degeneracy between the states with the same n.
On the other hand, it is shown from λ given in (13.46) that the scalar potential
parameter s can be taken arbitrarily for the vector potential parameter v =0.
4 Generalization to the Dirac Equation 171
Second, if s =0, then λ =
√
K
2
−v
2
. It is found from Eq. (13.46) that E has
thesamesignasv when K
2
>v
2
. For the attractive Coulomb potential (v>0) we
have the positive energy E(n,l,D)
E(n,l,D) =M
1 +
v
2
(n −l −1 +
√
K
2
−v
2
)
2
−1/2
. (13.61)
This coincides with the conclusion from the Sturm-Liouville theorem for a weak
attractive potential [184, 247, 248]. For a large D we have the similar result as
Eq. (13.59)(s is replaced by v).
For a small v,wehave
E(n,l,D) M
v
|v|
1 −
v
2
2[n +(D −3)/2]
2
−
v
4
2[n +(D −3)/2]
4
2n +D −3
2l +D −1
−
3
4
. (13.62)
Similarly, the physical meanings of three terms are similar to those of Eq. (13.60)
except for the different expansion coefficients. This case has been studied in our
recent work [104, 450], to which the reader can refer for more information.
Third, if v = s, from Eq. (13.56) both v and s have to be positive, λ =|K|, and
the positive κ is given by
κ =n −l −1 +|K|=
n +
D−3
2
, when K ≥0,
n −2l −
1+D
2
, when K<0.
(13.63)
The energy is written as
E(n,l,D) =
⎧
⎪
⎨
⎪
⎩
M{1 −
2v
2
v
2
+(n+
D−3
2
)
2
}, for K ≥0,
M{1 −
2v
2
v
2
+(n−2l−
1+D
2
)
2
}, for K<0,
(13.64)
which implies that the energy is independent of the angular momentum quantum
number l for K ≥0, but for K<0 it depends upon l. If we choose the negative sign
in the result, we have E =−M, which is a singular solution of Eq. (13.56).
We now determine the normalization factor N
KE
from the normalization condi-
tion
†
KE
KE
dV =1. (13.65)
Similarly, through a direct calculation we get
N
KE
=
(M
2
−E
2
)
1/4
(2λ +1)
(τ +λ +1)
2Mτ
(K +τ
)(τ −λ)!
1/2
. (13.66)
172 13 Dirac Equation with the Coulomb Potential
Fig. 13.10 The energy E(1,0,D) decreases with the increasing dimension D ∈ (0, 1),butin-
creases with the increasing dimension D ≥1. The parameters v =0.2ands =0.3aretakenhere
andalsoinFigs.13.11 and 13.12
4.3 Analysis of the Energy Level
We now analyze the variation of the energy E(n,l,D) on the dimension D.Itis
shown from Eqs. (13.46) and (13.56) that the energy E(n,l,D) giveninEq.(13.57)
is closely related with the following two conditions,
s
2
+K
2
>v
2
,Ev+Ms > 0, (13.67)
from which we can determine whether the energy is positive or negative. Recall that
we only consider the case with positive v. For the case of negative v, the energy
E(n,l,D) changes its sign. For simplicity, we take sign “+” for the second term
in Eq. (13.57). In fact, the energy levels are also valid if we take sign “−”forthe
second term, while the variations shall change with the different choice of the signs.
First, we consider the case s>0. We discuss the energy E(n,l,D) in two cases
s>vand s<v. For the first case s>v, due to Eq. (13.67) the bound states with
0 <E<M are physically acceptable. We take v =0.2 and s =0.3 for the weak at-
tractive Coulomb potential. It is shown in Figs. 13.10, 13.11, 13.12 that the energies
E(n,0,D) first decrease for D ∈ (0, 1) and then increase with the increasing di-
mension D ≥1, while the energies E(n,l,D) (l =0) are almost independent of the
quantum number l. Also, the energies E(n,l,D) are almost overlapped for a large
D. It is shown that the energies E(n,0,D) are symmetric with respect to D = 1
for D ∈ (0, 2). For the second case s<v,wetake
v = 0.3 and s =0.2. It is found
that there does not permit bound state for l = 0 and D ∈ (0.55, 1.45). This can
be explained well from Eq. (13.46), i.e., ((D −1)/2)
2
+s
2
≥v
2
for l = 0. Conse-
quently, there exist the bound states for D ≥1+2
√
v
2
−s
2
and D ≤1−2
√
v
2
−s
2
.
The corresponding variations of energies E(n,l,D) on the dimension D are il-
lustrated in Figs. 13.13, 13.14, 13.15, 13.16. It is shown in Fig. 13.13 that the
4 Generalization to the Dirac Equation 173
Fig. 13.11 The variation of energy E(2, 0,D) (red dashed line) on the dimension D is similar to
E(1, 0,D). The energy E(2, 1,D)(blue solid line) increases with the dimension D.ForD>2the
energy E(2, 1,D) almost overlaps E(2, 0,D)
Fig. 13.12 The variations of energy levels E(3,l,D) (l = 2, 1, 0) on dimension D are similar
to E(2,l,D) (l =1, 0). The energy E(3, 2,D) almost overlaps energy E(3, 1,D).ForD>2, the
energies E(3,l,D)(l =2, 1, 0) are almost overlapped. The blue dotted, green dashed and red solid
lines correspond to l =0, 1, 2, respectively
energy E(1, 0,D) decreases with the increasing dimension D ∈ (0, 0.55],butin-
creases with the increasing dimension D ≥ 1.45. There does not exist bound state
for D ∈ (0.55, 1.45). It is shown in Figs. 13.15 and 13.16 that the variations of en-
ergies E(n,0,D)(n =2, 3) are very similar to E(1, 0,D). The energies E(n,1,D)
(n = 2, 3) increase with the dimension D. Note that the energies E(n,l,D) are al-
