Dionne G.F. Magnetic Oxides
Подождите немного. Документ загружается.


70 2 Magnetic Ions in Oxides
K
d
xz
;d
yz
D
˝
d
xz
.1/
d
yz
.2/
ˇ
ˇ
1
r
12
ˇ
ˇ
d
xz
.1/ d
yz
.2/
˛
D F
0
2F
2
4F
4
.Slater integrals/ (2.31a)
D A 2B C C.Racah parameters/;
J
d
xz
;d
yz
D
˝
d
xz
.1/
d
yz
.2/
ˇ
ˇ
1
r
12
ˇ
ˇ
d
yz
.1/ d
xz
.2/
˛
D 3F
2
C 20F
4
.Slater integrals/ (2.31b)
D 3B C C.Racah parameters/:
From combinations of these integrals together with the help of group theory, the
“promotional” energies are computed and added to the octahedral energies already
determined from the respective electron distribution of the particular state to com-
plete the energy term diagram. The F
n
parameters are the Slater integrals of the
various d orbital functions expressed in their basic spherical harmonics. Their iden-
tities have been documented in the original work [26]. Alternatively, Racah [27]
defined parameters A, B,andC that can be reduced to the Slater integrals. In tab-
ular form, these interaction energies and related term information of the d
2
case
are listed in Table2.8 for the strong-field method [7]. The evolution from the term
diagram to the weak field result is sketched in Fig. 2.16. For additional comparison
with the listings in Table 2.8, the term energies from the weak field approach are
presented in Table 2.9.
The strong field approach to crystal field theory will be revisited in our discussion
of S-state ion magnetoelastic properties in Chap. 5 and in the general discussion of
electric dipole optical transitions for Faraday rotation in the magnetic garnets in
Chap. 7. For future reference, the Racah parameter relations for the lowest five d
n
terms listed in Table 2.4 are recorded in Table 2.10. In most situations, however, a
simpler approximation based on the ground state stabilization energies is all that is
necessary to sort out the causes of the various magnetic properties.
Table 2.8 d
2
Term splittings for strong field octahedral coordination
Free-ion term E
rep
Crystal-field term E
cf
1
S A C 14B C 7C
1
A
1g
2"
0
1
G A C 4B C 2C
1
A
1g
2"
0
C 4Dq
1
E
g
2"
0
C 4=7Dq
1
T
1g
2"
0
C 2Dq
1
T
2g
2"
0
– 26=7Dq
3
P A C 7B
3
T
1g
2"
0
1
D A – 3B C 2C
1
E
g
2"
0
C 24=7Dq
1
T
2g
2"
0
– 16=7Dq
3
F A – 8B
3
A
2g
2"
0
C 12Dq
3
T
2g
2"
0
C 2Dq
3
T
1g
2"
0
6Dq
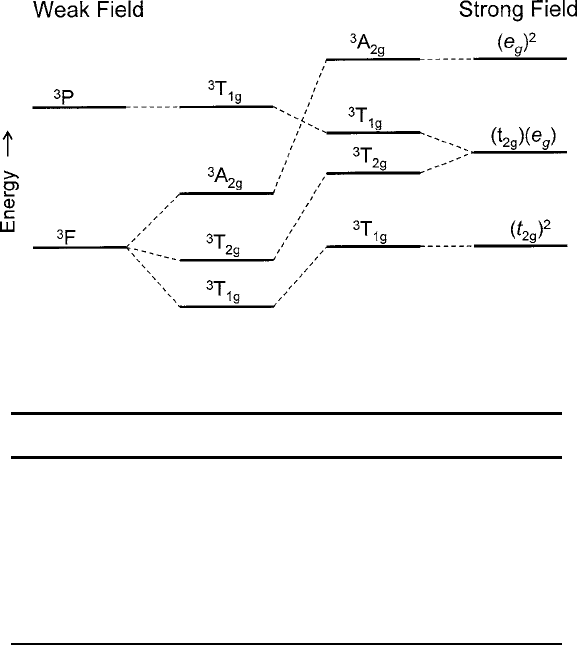
2.3 Crystal Electric Fields 71
Fig. 2.16 Schematic of energy levels of a d
2
ion in an octahedral crystal field of increasing
strength
Table 2.9 d
2
Term energies for weak field octahedral coordination
Crystal-field
configuration
E
cryst
Crystal-field term E
term
e
g
2
2"
0
C12Dq
1
A
1g
A C 8B C 4C
1
E
g
A C 2C
3
A
2g
A – 8B
t
2g
1
e
g
1
2"
0
C2Dq
1
T
1g
A C 4B C 2C
1
T
2g
A C 2C
3
T
1g
A C 4B
3
T
2g
A – 8B
t
2g
2
2"
0
–8Dq
3
T
1g
2"
0
2.3.7 Rare-Earth Ion Solutions
Crystal-field stabilizations of the orbital angular momentum must be treated
differently in the rare-earth 4f
n
series because H
LS
>>V
cf
, which means that
J is the quantum number that defines the angular momentum. Because of the
stronger œLS energy, the terms are first split into a multiplet of states with J
values running from
j
L S
j
to
j
L C S
j
in the standard notation of atomic spec-
tra. As a consequence the term splittings caused by the crystal field are labeled
according to the J instead of the L degeneracies (although in the physical reality
only L is quenched). Nonetheless, effects of the crystal field are significant in
magnetic properties of oxides, and the various term splittings established with the
aid of group theory and operator equivalents as determined by Lea et al. [28]are
summarized in Table 2.11. These eigenstates of the crystal field represent raising
of the J degeneracy and therefore can cause a decrease in the effective J value as
72 2 Magnetic Ions in Oxides
Table 2.10 Racah parameter energy relations for d
n
and d
10n
with
n D 2,3,4, and 5
d
2
d
3
1
S D A C14B C 7C
2
F D 3A C 9B C 3C
1
D D A – B C 2C
2
G D 3A – 11B C 3C
1
G D A C 4B C 2C
2
H D
2
P D 3A – 6B C 3C
3
P D A C7B
4
P D 3A
3
F D A – 8B
4
F D 3A – 15B
d
4
d
5
a
3
D D 6A – 5B C 4C
4
F D 10A – 13B C 7C
3
F D 6A – 5B C
.
11=2
/
C
4
D D 10A – 18B C 5C
˙
.
3=2
/
68B
2
C 4BC C C
2
1=2
3
G D 6A – 12B C 4C
4
P D 10A – 28B C 7C
3
H D 6A – 17B C 4C
4
G D 10A – 25B C 5C
5
D D 6A21B
6
S D 10A – 35B
The derivation of these relations can be found in [5], Sect. 4.6
a
These terms are listed differently from that anticipated by Hund’s
rule in that the
4
Pand
4
F terms are exchanged in position on the
energy ladder. This is an artifact of this configuration and results for
the relative values of B and C , which are found to occur in the ratio of
C=B4:5. There are other departures from the Hund’s rule norm that
will be fall beyond the scope of this discussion
Table 2.11 4f
n
(Rare-earth
group) term splittings in an
oxygen coordination of O
h
symmetry
J Term representations
1/2 E
1=2g
1 T
1g
3/2 G
g
2 E C T
2g
5/2 E
5=2g
C G
g
3 A
2g
C T
1g
C T
2g
7/2 E
1=2g
C E
5=2g
C G
g
4 A
1g
C E
g
C T
1g
CT
2g
9/2 E
1=2g
C 2G
g
5 E
g
C 2T
1g
C T
2g
11/2 E
1=2g
C E
5=2g
C 2G
g
6 A
1g
C A
2g
C E
g
C T
1g
C2T
2g
13/2 E
1=2g
C 2E
5=2g
C 2G
g
7 A
2g
C E
g
C 2T
1g
C 2T
2g
15/2 E
1=2g
C E
5=2g
C 3G
g
8 A
1g
C 2E
g
C 2T
1g
C 2T
2g
The contents of this table are included
here because they cover the full scope of
the 4f
n
series that is important in the
context of this book. In addition, these
entries could also the first complete listing
available in the public domain
2.4 Orbital Energy Stabilization 73
measured magnetically, just as in the case of orbital angular momentum quenching
of L in the d
n
series.
The situation regarding the rare-earth ions becomes more complex not only be-
cause the high J multiplicities render analytical solutions more laborious, but also
because magnetic exchange fields interact only with the spin component of J .More-
over, since the crystal field is about two orders of magnitude smaller than that of
the d
n
series, the degree of J quenching can been influenced by externally ap-
plied magnetic fields of magnitudes (>10T) that are now attainable with modern
magnet technology, for example, superconducting magnets. These effects of com-
peting crystal fields, exchange fields, and applied magnetic fields are examined in
the context of the magnetic properties of the rare-earth iron garnets in Sect. 4.3.3,
and later in relation to electron spin resonance in Chap. 6.
2.4 Orbital Energy Stabilization
When cations are placed in a lattice, the crystal field from the anion charges is
derived from the ionic part of the chemical bonding. As a consequence, the spin
occupancies pictured in Fig. 2.3 for free ions will undergo modifications. Within
the point charge approximation, the resultant electrostatic potential between neigh-
boring ions (often approximated by a lattice energy calculation) and the ionization
potentials of the various ionic species determine the electronic energies of the
outermost electrons. For the 3d
n
-electron series, the local crystal field of the imme-
diate anion neighbors provides additional binding energy by lowering the electronic
ground state energy as part of the splitting of the orbital degeneracy that was re-
viewed in the previous section. The additional electronic stabilization is therefore
the result of a perturbation by the point-charge field, which may be further enhanced
by a covalent interaction.
2.4.1 One-Electron Model
In the weak-field regime, a one-electron model can be constructed for the 3d
n
series
as a general qualitative approximation to provide an occupancy map of the ground
state electronic structures sketched. Instead of computing the electron interaction
energies in terms of the Slater integrals or Racah parameters, the ground state is first
pictured as a distribution of electrons among the cubic field terms [29]. The energy
levels of the T
2g
and E
g
terms from the
2
Dand
5
D cases of Fig. 2.12 for single-d
electron are used as a “floor plan” to keep track of the likely d -electron spin dis-
tributions in the ground states, as diagrammed in Figs. 2.17 and 2.18 for octahedral
and tetrahedral coordinations, respectively, including the low-spin states that can
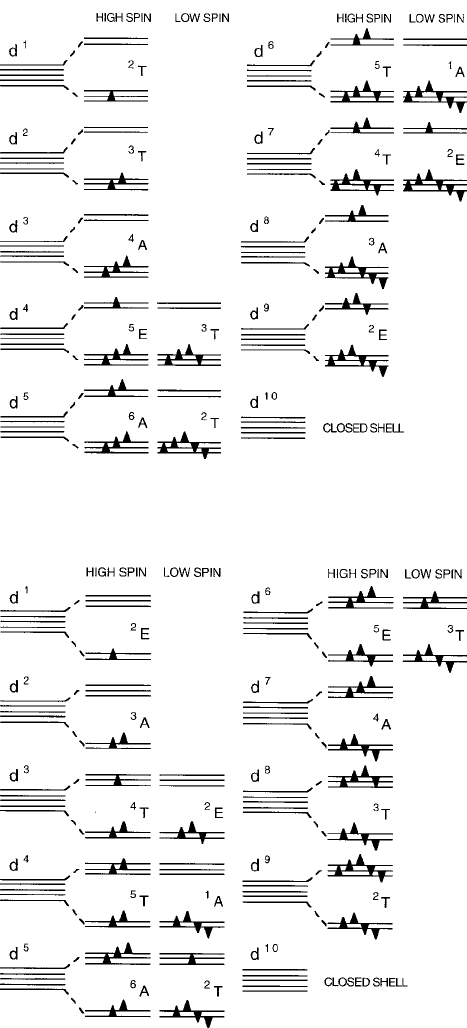
74 2 Magnetic Ions in Oxides
Fig. 2.17 One-electron d -orbital occupancy diagrams: octahedral site
Fig. 2.18 One-electron d -orbital occupancy diagrams: tetrahedral site

2.4 Orbital Energy Stabilization 75
occur in strong crystal fields (violation of Hund’s rule).
2
In this abbreviation, the
“Aufbau method” is applied by adding d electrons sequentially beginning with the
orbital state of lowest energy, while observing Hund’s rule of spin polarization for
the first (’)halfofthed shell and the Pauli principle of spin pairing as spins are
added to the second (“)half.
3
Although not shown explicitly, the crystal-field stabilization energy of each
configuration is offset by intraorbital electron repulsion energy e
2
=r
ij
,whichis
manifested by the particular ionization potential (IP) in Fig. 2.19a (replotted from
Fig. 2.8 for 2Cand 3C) as a function of n across the transition series.
4
Note that the
destabilization energy (characterized by the parameter U
ex
)of“ spins relative to ’
spins in the same orbital is on the order of 2–3 eV, which exceeds the 10Dq values
of many oxide sites.
It is appropriate here to point out that the Aufbau approximation can apply with
rigor to only the d
1
or d
9
cases. For that reason, the model is often called the “one-
electron approximation.” Where the effects of intraelectron repulsion must be taken
into account, multielectron solutions with their attendant complexities in dealing
with other important perturbations such as spin–orbit coupling, magnetic exchange,
and Zeeman effects in an external magnetic field can be considered.
2.4.2 High- and Low-Spin States
Where H
Hund
exceeds H
cf
, this procedure establishes a ground state with the max-
imum available spin number. The electrons are distributed among the orbital states
with Pauli spin pairing permitted only after each of the five orbitals are half-filled
(Hund’s rule). Since the energy distribution also changes with the assignment of
electrons, one of the main insights gained from this approximation is the relative
magnitudes of the cation site stabilization energies. In Figs. 2.17 and 2.18, the one-
electron ground-state configurations for the octahedral and tetrahedral sites include
2
The Aufbau concept can be used here directly because all of the electrons occupy orthogonal
crystal-field states of the same orbital term under the influence of the same nuclear charge. When
applied to molecular bonding that involves Coulomb fields of multiple nuclei, the applicability is
limited by the covalent sharing of orbital states that are not fully orthogonal.
3
The reader is cautioned that these diagrams are used to sort out the electron occupancies of the
orbital ground state in order to anticipate the quantum designation of the ground state. The virtue of
the one-electron models is the ready insight that they can provide without the necessity of complex
mathematical analysis and computation.
4
In collective-electron band theory that was introduced by Stoner [30], the Fermi level is used
as the reference energy for electrical properties, and it has been found phenomenologically con-
venient to separate the spin populations into up (majority ’) and down (minority “) spin bands
based on the difference in energy between the upper and lower parts of the d -shell spin ladders
depicted in Fig. 2.3. This model is then used to explain the net collective moment in the manner of
a ferrimagnetic spin system.
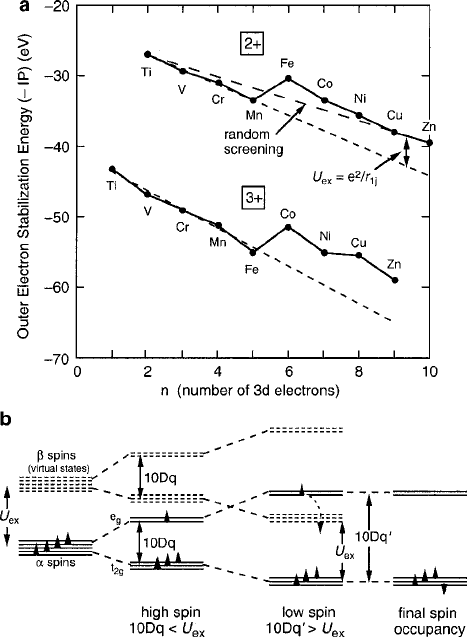
76 2 Magnetic Ions in Oxides
Fig. 2.19 Origin of the low-spin state of d
4
shown in (b), where the upper “ half is depicted as
a virtual shell of energy U
ex
.(a) is extracted from Fig. 2.8 to serve as an energy reference. For
10Dq < U
ex
, all four spins are aligned parallel, producing S D 2 in the ’ states. When the
crystal-field splitting increases to 10Dq
0
>U
ex
, the single e
g
’ spin will flip as it can now stabilize
in the t
2g
states of the “ shell, both satisfying the Pauli principle and creating a low-spin value of
S D 1. Note that in the one-electron approximation, the IP energy information from (a) associated
with the U
ex
D e
2
=r
ij
correlation energy has been discarded
the “low-spin” states that occur in strong crystal fields (in violation of Hund’s
rule when the destabilization from the 10Dq splitting energy overrides the e
2
=r
ij
spin polarization energy that we designate U
ex
).
In the weak-field limit, the spins are polarized to the maximum extent and the re-
sult is logically called the “high-spin” state. Although these spin alignments are the
usual situations in the 3d
n
series, low-spin occupancies may also occur in selected
instances where 10Dq>U
ex
. When this occurs, the lower half of the 3d
n
shell be-
gins to fill before the upper one is half-filled, as illustrated by the example of 3d
4
in Fig. 2.19b. In most situations, stable low-spin condensations are not expected un-
less cation valences exceed 3C in octahedral sites. The example chosen here (3d
4
),
2.4 Orbital Energy Stabilization 77
the Jahn–Teller case to be discussed in the next section, is not usually expected to
condense into a low-spin configuration unless the valence increases to 4C,forex-
ample, the infrequently encountered Fe
4C
ion. A more likely situation where U
ex
can exceed 10Dq would be found with n D 6 in an octahedral site. Co
3C
3d
6
in a
perovskite lattice is analyzed for high, low, and intermediate spin configurations by
Ibarra et al [31]. A detailed diagram in the manner of Fig. 2.19b is included to illus-
trate the application of these concepts to a challenging problem. The magnetoelastic
implications of this family of ions are discussed further in Chap. 5 (see Fig. 5.7).
In general, low-spin states do not occur in tetrahedral sites because of the 4/9
reduction factor in the value of Dq, but the hypothetical configurations are included
here for completeness. The respective d -electron diagrams are included in Figs. 2.17
and 2.18, and the corresponding stabilization energies in units of Dq are listed in
Tables 2.12 and 2.13. Comparison of these estimates of site stabilization energy can
be used to decide the likely preference for ions in lattices such as spinels and garnets
Table 2.12 High- and low-spin d -electron stabilization energies in an octahedral coordination
d Electrons
High-spin
configuration
Stabilization
energy
Low-spin
configuration
Stabilization
energy
1
t
2g
1
4Dq
t
2g
1
4Dq
2
t
2g
2
8Dq
t
2g
2
8Dq
3
t
2g
3
12Dq
t
2g
3
12Dq
4
t
2g
3
e
g
1
6Dq
t
2g
4
16Dq
5
t
2g
3
e
g
2
0
2g
5
20Dq
6
t
2g
4
e
g
2
4Dq
t
2g
6
24Dq
7
t
2g
5
e
g
2
8Dq
t
2g
6
e
g
1
18Dq
8
t
2g
6
e
g
2
12Dq
t
2g
6
e
g
2
12Dq
9
t
2g
6
e
g
3
6Dq
t
2g
6
e
g
3
6Dq
10
t
2g
6
e
g
4
0
t
2g
6
e
g
4
0
Table 2.13 High- and low-spin d -electron stabilization energies in a tetrahedral coordination
d Electrons
High-spin
configuration
Stabilization
energy
Low-spin
configuration
Stabilization
energy
1
e
g
1
6Dq
e
g
1
4Dq
2
e
g
2
12Dq
e
g
2
8Dq
3
e
g
2
t
2g
1
8Dq
e
g
3
18Dq
4
e
g
2
t
2g
2
4Dq
e
g
4
24Dq
5
e
g
2
t
2g
3
0
e
g
4
t
2g
1
20Dq
6
e
g
3
t
2g
3
6Dq
e
g
4
t
2g
2
16Dq
7
e
g
4
t
2g
3
12Dq
e
g
4
t
2g
3
12Dq
8
e
g
4
t
2g
4
8Dq
e
g
4
t
2g
4
8Dq
9
e
g
4
t
2g
5
4Dq
e
g
4
t
2g
5
4Dq
10
e
g
4
t
2g
6
0
e
g
4
t
2g
6
0
78 2 Magnetic Ions in Oxides
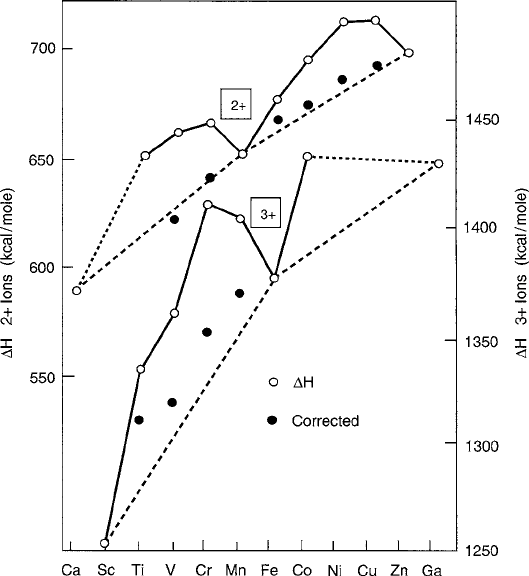
Fig. 2.20 Heats of hydration of divalent and trivalent transition metal ions as a function of atomic
number. Ligand field energies are subtracted from total values to reveal the expected smooth
monotonic increase in binding energy with increasing atomic number. Data are from Holmes and
McClure [32]
where both octahedral and tetrahedral sites are present. For the specific example of
Cr
3C
, a site preference energy of 4Dq can be deduced from the stabilization energies
listed in the tables.
The success of this simple model may be observed from the heat of hydration
data of Holmes and McClure [32] plotted Fig. 2.20, where the excess binding energy
attributed to the octahedral ligand field stabilization is seen to follow the values
predicted in Table 2.12 for the high-spin case. Of the various 3d
n
ions, the most
dramatic example of a transition from high spin to low spin is 3d
5
,i.e.,Fe
3C
or
Mn
2C
whereby the spin value can decrease from S D 5=2 to 1/2 when Dq=B>3
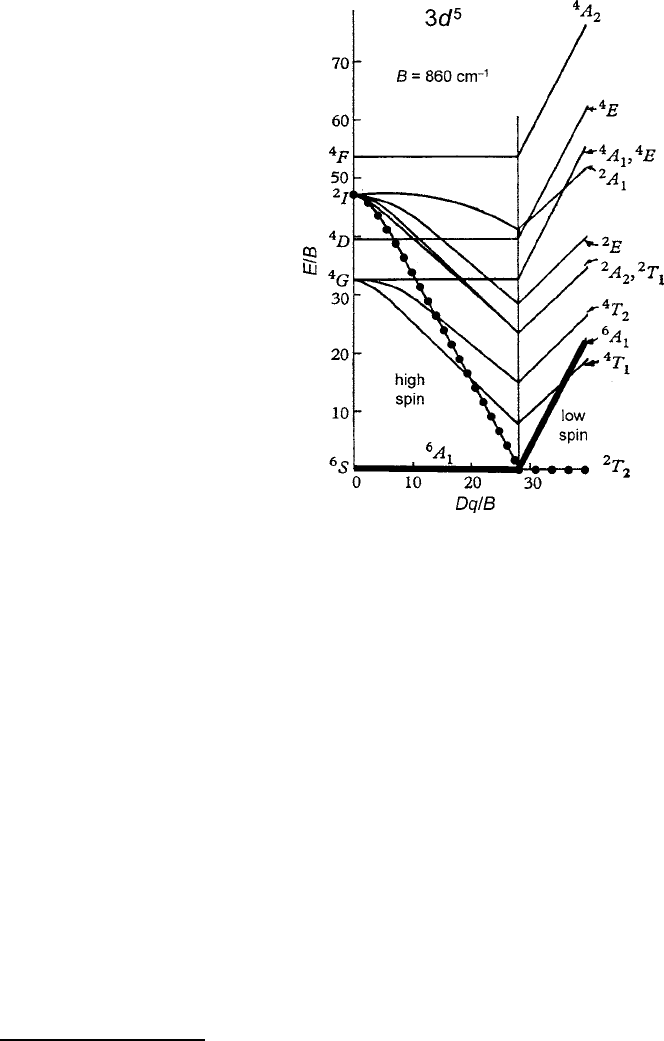
in Fig. 2.21, adapted from the calculations of Tanabe and Sugano [33]. Such a low-
spin state occurs for isolated Fe
3C
ions in K
3
Co
3C
.CN
6
/ as observed in electron
spin resonance (EPR) measurements [34]. With .CN
6
/
6
ligands, this intriguing
situation is examined in relation to spin–lattice relaxation in Sect. 6.2.Co
3C
.3d
6
/
also has a low-spin configuration in an octahedral site [35], where all six electrons
fill the t
2g
shell producing S D 0 instead of 2.
2.4 Orbital Energy Stabilization 79
Fig. 2.21 Splitting of states
of the d
5
configuration by an
octahedral field (abbreviated
and modified from Tanabe
and Sugano calculations
[33]). The Racah parameter
B
D860 cm
1
represents
the effect of the internal spin
polarization energy U
ex
of
Fig. 2.19b. Note the transition
from a high-spin orbital
singlet
6
A
1
ground term to a
low-spin orbital triplet
2
T
2
when Dq=B > 3
From the tables and figures presented so far, it is evident that the cubic fields
alone fail to remove all of the ground-state degeneracy. Further stabilization can
occur, and there are two mechanisms by which this can take place locally: (1) orbit–
lattice coupling manifested in the now-celebrated Jahn–Teller (J–T) spontaneous
distortion effects, and (2) the more conventional spin–orbit (S–O) coupling that can
override and reverse the J–T distortion in select cases. Both of these phenomena are
responsible for important magnetic and electronic behavior.
2.4.3 Orbit–Lattice Stabilization (Jahn–Teller Effects)
In Figs. 2.17 and 2.18 that were discussed in relation to the single d electron
approximations in octahedral and tetrahedral sites, it is evident that in many cases
not all of the degeneracy is removed by quenching of the orbital angular momen-
tum in cubic fields. Group theory dictates that lower symmetry arrangements of
the oxygen ligands can lift the remaining degeneracies and eliminate all vestiges of
unquenched orbital angular momentum in the ground states.
5
Except for situations
5
It should be pointed out that this lifting of degeneracies does not include those of Kramers dou-
blets, which are spin degeneracies that can be split only by magnetic fields. Kramers doublets occur
