Dionne G.F. Magnetic Oxides
Подождите немного. Документ загружается.

40 2 Magnetic Ions in Oxides
2.1.2 Iron Group 3d
n
Ions
In the periodic table of Fig. 2.1, the elements with ions that produce significant
magnetic effects in combination with oxygen are distinguished by shading. Their
common feature is an incomplete inner d
n
of f
n
shell. In general, the atoms sur-
render their outer s electrons to form ionic bonds with atoms that accept them to
complete their own unfilled s and p shells. In an analogy to electron tubes with a
cathode (emitter) and anode (plate), the metal atom that donates electrons is called
a cation, while the “anode” atom becomes an anion. Because oxygen normally has
an anion valence of 2, the cations formed from the transition metals usually have
valence charges of at least 2C, particularly in the complex oxide compounds that
produce the magnetic properties of practical interest.
One immediate observation is that once the outer 4s electrons are stripped from
the iron group elements, the partially filled 3d
n
shell is exposed to the molecular
environment, which is a crystal lattice of specific symmetry comprising electric and
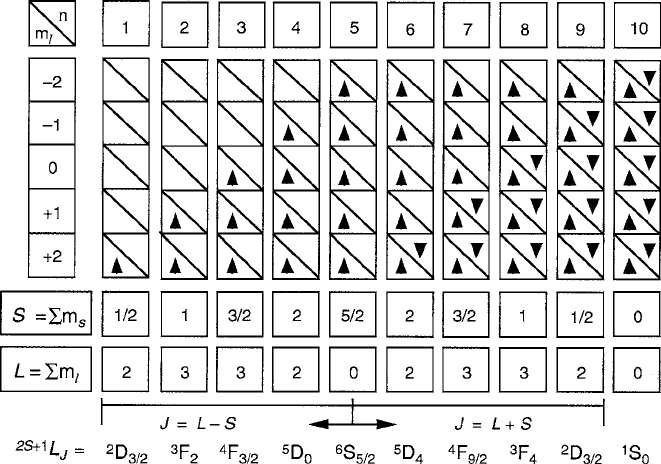
magnetic fields of its own. Figure 2.3 is a diagram of the electron occupancy of the
ten d states (two for each of the five orbitals in compliance with the Pauli exclusion
principle), indicating the formation of the multi-electron L, S,andJ quantum states
for each ground term based on Hund’s rules. The relevant parameter data for the iron
group are summarized in Table 2.1.
The ions of the lower third of the group 3d
1
, 3d
2
,and3d
3
are generally para-
magnetic and exhibit little collective properties, usually only causing perturbing
Fig. 2.3 Application of Hund’s rule in the formation of angular momentum ground terms in the
3d
n
shell. Terms are either D
.
L D 2
/
,F
.
L D 3
/
,orinthe3d
5
case, S
.
L D 0
/

2.1 The Transition Metals 41
Table 2.1 Parameters of the iron group 3d
n
ion series in oxygen sites
Ground state Radius
a
O
n
2
Electrons Ion Term LS J (
˚
A) n Remarks
3d
0
Sc
3C
1
S
0
0 0 0 0.73 6 Diamagnetic ion
3d
1
Ti
3C
2
D
3=2
2 1/2 3/2 0.67 6 Metallic with
Ti
4C
,forms
blue sapphire
in Al
2
O
3
3d
2
V
3C 3
F
2
3120.64 6 Largeion
Ti
2C
0.86 6
3d
3
Cr
3C
Mn
4C
4
F
3=2
33/23/20.61
0.54
6
6
Forms red ruby
in Al
2
O
3
3d
4
Cr
2C
Mn
3C
5
D
0
2200.82
0.65
6
6
J–T ion, magne-
tostrictive.
metallic with
Mn
4C
3d
5
Mn
2C
Fe
3C
6
S
5=2
05/25/20.82
0.64, 49
6
6, 4
S-state ion,
low-spin
S D 1=2
3d
6
Fe
2C
Co
3C
5
D
4
2240.77
0.61
6
6
Magnetostrictive,
low-spin
S D 0
3d
7
Co
2C
Ni
3C
4
F
9=2
33/29/20.73
0.60
6
6
Spin–orbit
stabilized,
highly
anisotropic,
fast-relaxing,
low-spin
S D 1=2
3d
8
Ni
2C
Cu
3C
3
F
4
3140.70
-
6
6
Magnetostrictive
low-spin
S D 0
3d
9
Cu
2C
2
D
5=2
2 1/2 5/2 0.73 6 J–T ion, magne-
tostrictive,
metallic
conductor
with S D
0
Cu
3C
b
3d
10
Cu
1C
1
S
0
0000.96 6 large
diamagnetic
ion, metallic
with Cu
2C
a
Based on radius of divalent oxygen of 1.40
˚
A
b
J.B. Goodenough, G. Demazeau, M. Pouchard, and P. Hagenm¨uller, Solid State Chem. 8, 325
(1973)
42 2 Magnetic Ions in Oxides
effects on the remainder of the series. The magnetic moments of ions from 3d
4
through 3d
9
are capable of producing strong spontaneous magnetism when in suffi-
cient densities to allow exchange coupling to the order magnetically as ferro-, ferri-,
or antiferromagnets. In all cases where the ion is chemically bonded in an anion
lattice, the combined orbital angular momentum L is uncoupled from the spin S by
the electrostatic fields of the lattice, and the spin moments dominate the magnetic
properties. Where this occurs J is no longer a meaningful quantum number. Conse-
quently, the spectroscopic g factor is approximately equal to 2 for the spin angular
momentum of a free electron in all but a couple of special situations.
2.1.3 Rare Earth 4f
n
Ions
The second most important transition group from a magnetic standpoint has an un-
filled 4f
n
shell. These elements are commonly referred to as the rare earths or
lanthanides because La is the first member of the series. The higher group with un-
filled 5f
n
shell, called the actinides also has magnetic properties. As mentioned
earlier an important distinction between the 4f
n
ions and those of the 3d
n
iron
group is the shielding of the 4f shell inside the 5s
2
and 5p
6
outer shells of the Xe
core. In other words, the magnetically active electrons are buried inside the Xe core
and are therefore shielded from electrostatic fields of the molecular environment. As
a result, the ions act largely independent of one another, even in highly concentrated
compounds, and are generally paramagnetic because the multiple lobes of the 4f
orbital wavefunctions do not extend far enough for covalent bonding and magnetic
exchange to be significant.
Inspection of Table 2.2 reveals that the J values of the ions are divided into a
lower and upper group, based on whether L is larger or smaller than S . Following
Hund’s rule, for the lower half from Ce
3C
to Eu
3C
, J DjL Sj; the upper half
from Gd
3C
to Yb
3C
features larger spin values, i.e., J DjL C Sj. It will become
evident later that the J values of the rare earths are the important angular momen-
tum parameters because of strong spin–orbit coupling energies. Consequently, the
g factors also are heavily dependent on J through the L contribution. As computed
from (1.18), the g factors listed in Table 2.2 are exclusively less than 2. Rare-earth
ions contribute a number of important effects that include the tailoring of magne-
tization vs. temperature behavior in magnetic garnets, the control of high-power
properties of microwave ferrites, and the Faraday rotation of magnetic garnets and
other compounds for optical applications.
2.1.4 4d
n
and 5d
n
Ions
The unfilled 4d and 5d shells of the other two transition series have ions that resem-
ble the 3d series in magnetic properties and can be used as alternatives for them in
certain cases. Tetravalent ruthenium
Ru
4C
with a 4d
4
configuration, for example,

2.2 Oxygen Coordinations 43
Table 2.2 Parameters of the rare earth 4f
n
ion series
Ground state Radius
a
Electrons Ion Term LS J g (
˚
A) Remarks
4f
0
La
3C
1
S
0
0 0 0 – 1.18 Diamagnetic ions
Ce
4C
0.97
4f
1
Ce
3C
2
F
5=2
3 1/2 5/2 6/7 1.14 Strong magneto-
optical
properties
Pr
4C
0.99
4f
2
Pr
3C
3
H
4
5 1 4 4/5 1.14 Strong magneto-
optical
properties
4f
3
Nd
3C
4
I
9=2
6 2 4 8/11 1.12 Strong magneto-
optical
properties
4f
4
Pm
3C
5
I
4
6 2 4 3/5 0.98 Synthetic
element
4f
5
Sm
3C
6
H
5=2
5 5/2 5/2 2/7 1.09 –
4f
6
Eu
3C
7
F
0
3 3 0 – 1.07 Diamagnetic ion
4f
7
Eu
2C
8
S
7=2
0 7/2 2 2 1.25 S-state ions
Gd
3C
1.06
4f
8
Tb
3C
7
F
6
3 3 6 3/2 1.04 Fast-relaxing ion
4f
9
Dy
3C
6
H
15=2
5 5/2 15/2 4/3 1.03 Fast-relaxing ion
4f
10
Ho
3C
5
I
8
6 2 8 5/4 1.02 Fast-relaxing ion
4f
11
Er
3C
4
I
15=2
6 3/2 15/2 6/5 1.00 Fast-relaxing ion
4f
12
Tm
3C
3
H
6
5 1 6 7/6 0.99 Fast-relaxing ion
4f
13
Yb
3C
2
F
7=2
3 1/2 7/2 8/7 0.98 Fast-relaxing ion
4f
14
Lu
3C
b
1
S
0
0 0 0 – 0.97 Fast-relaxing ion
a
Based on an oxygen coordination of 8
b
Lutiteum is included here to complete the 4f shell. It also represents the beginning of the 5d
n
series
has magnetoelastic properties similar to those of trivalent manganese
Mn
3C
with
a 3d
4
occupancy. In general, these ions have not attracted much interest for their
magnetic properties because the compounds formed from them do not exhibit strong
spontaneous magnetic properties. Moreover, several of them, such as rhodium (Rh),
palladium (Pd), osmium (Os), iridium (Ir), and platinum (Pt) are not available in
sufficient abundance to be considered for low-cost applications. Intermetallic com-
pounds of niobium (Nb), however, have found important uses as superconductors.
2.2 Oxygen Coordinations
Transition metal ions are chemically reactive and occur naturally bonded to anions
of the seventh or eighth columns in compounds that are made up of distinct crystal
structures. Such compounds can be viewed as comprising separate metal (cation)

44 2 Magnetic Ions in Oxides
and anion lattices. In the discussions to follow, emphasis is placed on the immediate
surroundings of the metal ions, specifically the disposition of the oxygen or ligand
coordinations relative to the transition ion.
2.2.1 Crystal Systems and Point Groups
The subject of crystallographic symmetry is important to the study of magnetic
oxides, and a brief review is essential for the understanding of the concepts that
determine the properties of the transition ions in oxygen coordinations. For a thor-
ough treatment of the subject the reader is referred to the more general literature and
text books [1, 2]. In this text, the focus is on the crystal field and molecular orbital
theories by which most of the magnetic-related properties are examined.
Table 2.3 lists the seven systems that comprise all the naturally occurring types of
crystalline structures. Corresponding cell sketches are presented in Fig. 2.4.These
systems are in turn composed of 32 crystallographic “point groups” or crystal
classes, which describe the basic symmetry of crystallographic building blocks or
unit cells. A point group, therefore, consists of a collection of symmetry opera-
tors that serve to define particular crystal structures. There are three such basic
operations: rotation, whereby the structure repeats itself upon rotation around a par-
ticular direction or axis, for example, fourfold meaning that it repeats the image of
its projection along the axis every 90
ı
, reflection about a plane, and inversion, mean-
ing that the crystal retains its appearance after undergoing reversal of each of the x,
Table 2.3 Crystal systems and their symmetry elements
System
Generic point
group
Unit cell Symmetry
Triclinic – a ¤ b ¤ c
˛ ¤ ˇ ¤ ¤ 90
No axes, no
planes
Monoclinic – a ¤ b ¤ c
˛ D ˇ D 90 ¤
One twofold axis
or one plane
Orthorhombic D
2h
a ¤ b ¤ c
˛ D ˇ D D 90
Three orthogonal
2-fold axes
two planes
intersecting a
2-fold axis
Tetragonal D
4h
a D b ¤ c
˛ D ˇ D D 90
One 4-fold axis
or a 4-fold
inversion axis
Rhombohedral (trigonal) D
3d
a D b D c
˛ D ˇ D ¤ 90
One 3-fold axis
Hexagonal D
6h
Three axes a in
x y plane
at ˛ D 120;
c ¤ a
One 6-fold axis
Cubic (isometric) O
h
a D b D c
˛ D ˇ D D 90
Four 3-fold axes
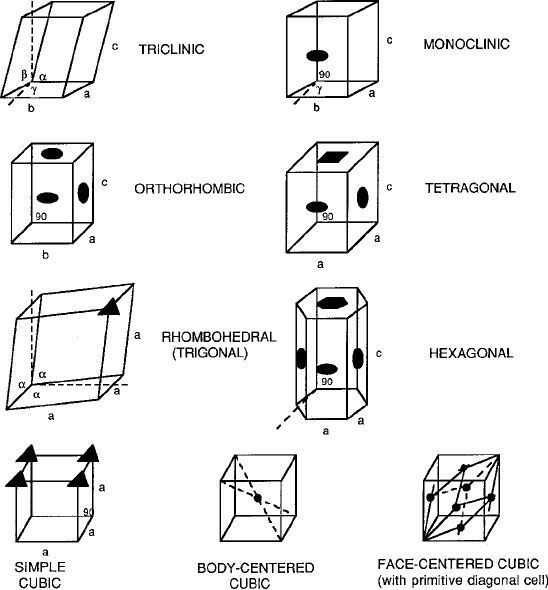
2.2 Oxygen Coordinations 45
Fig. 2.4 Crystal system diagrams
y,andz coordinates. An even further refinement categorizes the point groups into
240 “space groups,” but this level of detail will not be necessary for the purposes of
this text.
There will be no attempt to explain in detail the nomenclature of the point groups
beyond the occasional labeling of particular structures for identification purposes.
For the scope of this text, the rotation operators will be sufficient to designate the
symmetries that will be encountered.
2.2.2 Cubic Symmetry
In the magnetic oxides of interest in this text, the cations usually reside in lattice
sites comprising oxygen arrangements referred to as coordinations. The basic unit of
building block is generally of cubic (isometric) symmetry or one that is derived from
it. In Fig. 2.5 the most common situations are sketched in relation to the orthogonal
Cartesian axes and are of four types: tetrahedral, with four sides and four anions
on alternate corners as shown; octahedral with eight sides and six anions on the
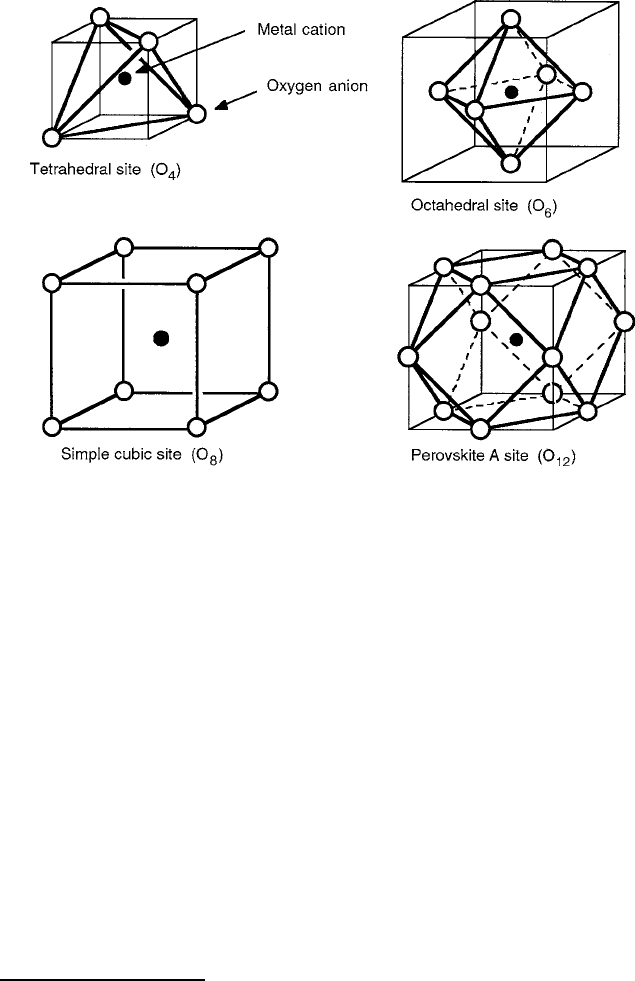
46 2 Magnetic Ions in Oxides
Fig. 2.5 Cation sites with ligand coordinations of cubic symmetry
corners of an octahedron formed with anions at the cube face centers; simple cubic
with six sides and eight anions at the cube corners; and dodecahedral, with 12 sides
and 14 anions located at the eight corners of a half-sized cube and six more at the
face centers of the full cube. This latter structure does not actually occur in the
magnetic oxides of interest here. In reality the “dodecahedral” site of the garnets
is a 12-sided cell formed from a twisted cube with only eight corner anions. Each
of these coordinations can have the required four threefold symmetry axes directed
along body diagonals, referred to as the <111> family according to the convention
of the Miller indices.
1
The corresponding indices for the three fourfold cubic axes
(along x, y,andz directions) are the <100> family and for the face diagonals, it is
the <110> family.
The most common oxygen coordination is octahedral, here labeled as O
6
for the
six anions. The octahedral site is of paramount importance in spinels, garnets, and
the various perovskite-related compounds. The tetrahedral coordination, designated
O
4
, is the alternate cation site in the spinels and garnets and is generally occupied by
smaller metal ions. Because the smaller separation between anions leads to higher
1
The Miller indices were developed to identify the various planes in a crystallographic lattice. The
system is based on the values of the three intercepts of the plane with the x, y,andz axes expressed
as the lowest integer values. The labeling convention for family of planes is fhklg and an individual
plane is (hkl). Alternatively, the normal axes to the planes are labeled < hkl > for the family and
[hkl] for an individual axis.
2.2 Oxygen Coordinations 47
mutual repulsive forces, the higher coordination numbers result in larger site vol-
umes to minimize these bonding energies. For this reason the larger ions, such as
the lanthanide rare earths, usually occupy O
12
as in the cubic perovskites, or the
dodecahedrally distorted O
8
sites in the garnets.
2.2.3 Lower Symmetries
In all but the simplest oxides, the cubic symmetry is usually reduced by lattice
distortions that are often large enough to be considered as part of a phase transi-
tion to another point group even though the effect may be local and involving only
an isolated cation complex. Even when the distortions are subtle, their effects on
the magnetic properties can be significant. Since the issue of lattice distortions and
symmetry changes will recur regularly throughout the balance of the text, it is ap-
propriate that it be introduced in an orderly manner.
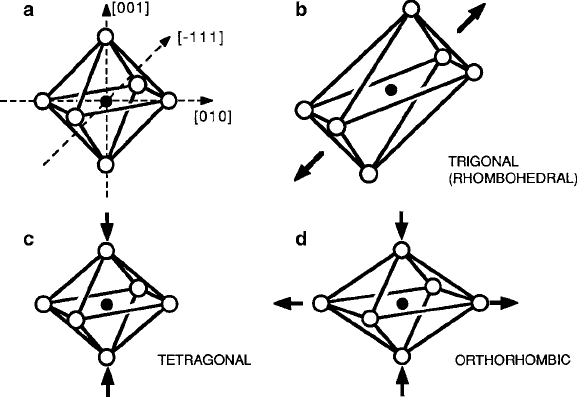
Departures from cubic symmetry vary from slight to catastrophic. However, there
is no immediate need to discuss more than the few that are depicted in Fig.2.6.
The most convenient vehicle to examine these distortions is the octahedral site,
which may undergo extensions or compressions along any of the principal axes
of symmetry. In most cases, the <111> and <100> groups shown in Fig. 2.6aare
the ones of concern. If the distortion is along a <111> axis as pictured in Fig. 2.6b,
the symmetry is reduced form cubic O
h
to trigonal or rhombohedral D
3d
or C
3v
,
and the immediate environment of the cation has one threefold symmetry axis.
Figure2.6c, d indicates the effects of a tetragonal D
4h
compression along the [001]
axis and then the addition of a second distortion, this time an extension along the
Fig. 2.6 Cubic cation sites with simple distortions
48 2 Magnetic Ions in Oxides
Fig. 2.7 Other ligand coordinations in oxides
[010] axis to create a lower orthorhombic D
2h
symmetry. Combinations of trigo-
nal and tetragonal distortions that can occur through spontaneous local distortions
called Jahn–Teller effects can reduce the symmetry even further, as will be described
in Chap. 5.
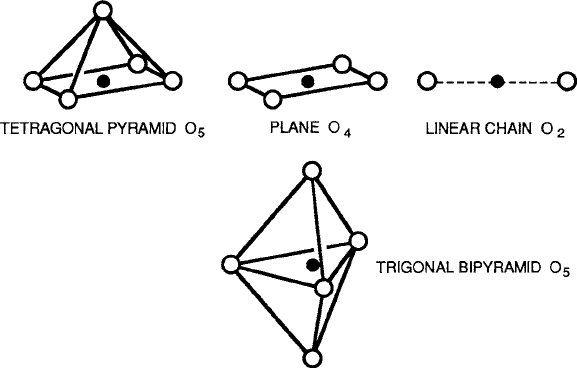
There are four other situations that are commonly encountered in magnetic ox-
ides, shown in Fig. 2.7. A tetragonal pyramid, which occurs when one of the z-axis
oxygen ions is missing, provides an O
5
coordination of C
4
symmetry (no reflec-
tion plane in this case); a square or rectangular planar configuration is formed
by the removal of both dihedral anions and provides the ultimate tetragonal or
orthorhombic D
4h
or D
2h
symmetry, and a one-dimensional chain that remains af-
ter two opposite planar oxygens are removed gives pure cylindrical symmetry. In
recent years, these abbreviated octahedra have been detected in the superconduct-
ing layered perovskite-type compounds. A fourth cation site takes the form of a
trigonal bipyramid and occurs in the hexagonal structure originally called “mag-
netoplumbite.” This site occurs as only one of 13 in the type-M hexagonal ferrite
compounds and is a strong contributor to the highly anisotropic properties of these
important compounds.
2.3 Crystal Electric Fields
For an ionic lattice, the site of the cation can be approximated by an electrostatic
trap formed by a “cage” of anion neighbors. If a “point charge” approximation is
used for both the cation and the coordination of anions, the stabilization energy of
2.3 Crystal Electric Fields 49
the cation relative to free space is determined by the strength of the resulting ionic
bond. When the electrons orbiting the cation nucleus are considered, the separate
states of a degenerate orbital term are split by the perturbation in the manner of a
Stark effect, which typically amount to about 10% of the lattice bonding energy,
depending on the relative proximities of the various wavefunction lobes to the anion
charges. This perturbation field from the anion coordination is called the crystal
field. When the orbital wavefunctions of the anions are taken into account, the point
charges are elevated to the status of ligands, and the eigenfunctions of the orbital
states are hybridized to include both cation and anion contributions. The crystal-
field model then forms the basis of ligand field theory that in turn serves as the
foundation for the molecular-orbital concepts described in later sections.
Although the bonding is principally ionic in oxide compounds, the smaller cova-
lent component is critically important for the electronic and magnetic properties of
compounds with cations of a transition series. To analyze states of an ion for which
the orbiting d electrons interact with the negative charges of the anion coordination,
the effects of point-charge crystal fields on orbital angular momentum of the cation
are examined first.
2.3.1 Angular Momentum States
To introduce the quantum mechanical effects of Stark splittings to the free ion orbital
angular momentum states, it is necessary to review the formation of the multielec-
tron orbital terms that are usually governed by Hund’s rules, which state that the
lowest energy multiplet term has the following:
1. The maximum possible combined spin value S,and
2. Within the maximum S manifold, the maximum combined L.
These rules originate from the Pauli exclusion principle and the quantum mechanical
necessity for spins to align parallel when dispersed among the set of orthogonal
orbital wavefunctions by mutual electrostatic repulsion. To visualize the “laddering”
exercise, Fig. 2.3 illustrates schematically the situation among the states of a d
n
series. The rows of stacked boxes represent an orbital angular momentum value
m
l
of operator l
z
. Each box can hold two electrons, one for each up or down spin
orientation as required by the Pauli exclusion principle. Beginning with the lower
half of the series from d
1
to d
5
, the electrons are added sequentially, obeying the
spin polarization requirement to fill the first five up spin compartments and produce
a half-filled set of orbitals with the maximum spin value of S D 5=2 when the d
5
limit is reached.
From an energy standpoint, the half-filled shell is most stable because each d
electron occupies a separate orthogonal orbital state, and the destabilizing effect of
the mutual repulsion is a minimum. This correlated spatial dispersal of the polarized
spins beyond a random distribution reduces the screening of the nucleus and stabi-
lizes the spins in proportion to their numbers (or their combined S). As the upper
