Dionne G.F. Magnetic Oxides
Подождите немного. Документ загружается.


60 2 Magnetic Ions in Oxides
Since diagonal elements remains unchanged before and after the perturbation is
applied, for the five
2
D orbital states
2."
0
C "
1
/ C 3."
0
C "
2
/ D 5"
0
; (2.16)
and it follows that, if "
0
is arbitrarily set to 0,
E
e
g
D
2
E
g
D "
1
D 6Dq;
E
t
2g
D
2
T
2g
D "
2
D4Dq: (2.17)
The value of Dq is determined semiempirically, that is, by experiment, but an ex-
pression for it can be arrived at analytically. As explained in [13],
Dq D˙
2
63
D
4
˝
r
4
˛
for D states
D
2
315
D
4
˝
r
4
˛
for F states; (2.18)
where the second signs apply to ions of the lower half of the d
n
series.
From the eigenfunctions of (2.12) that are plotted in Fig. 2.11, the ordering of
the energy levels can be determined by inspection of the relative positions of the
negatively charged lobes in relation to the negative ligand point charges. Note that
the e
g
orbitals are directed toward the ligands and therefore will assume the higher
energy states.
From these orbital sketches, the existence of an unquenched l
z
angular mo-
mentum component may be also discerned. A test for deciding whether an orbital
momentum about an axis can still be present is whether the eigenstate can be trans-
formed into another eigenstate within its degenerate manifold by a rotation about
that axis. In this case of the [001] as axis of quantization, it can be seen by visual
(or analytical) inspection that d
x
2
y
2
rotates into d
xy
by a 45
ı
rotation about z and
that the same applies to d
xz
and d
yz
. Only the latter pairs are eigenstates in a cu-
bic field, however, which means that the remaining three states have their l
z
fully
quenched, including the degenerate e
g
orbitals. This latter condition will be shown
to be significant in the discussion of the Jahn–Teller effect.
If the z axis of quantization is taken as the Œ111 direction, where threefold sym-
metry is prevalent, the appropriate basis vectors may be constructed from the pure
set of (2.6)as
e
g
D
8
<
:
1
p
3
d
2
p
2
p
3
d
1
1
p
3
d
2
C
p
2
p
3
d
1
;
t
2g
D
8
ˆ
ˆ
ˆ
<
ˆ
ˆ
ˆ
:
p
2
p
3
d
2
C
1
p
3
d
1
p
2
p
3
d
2
1
p
3
d
1
:
d
0
(2.19)
2.3 Crystal Electric Fields 61
As with the earlier eigenfunction set with the z axis along the Œ001 direction, a
straightforward application of the l
z
operator along the Œ111 direction will verify
that d
0
and the two e
g
states have zero angular momentum, while the remaining two
t
2g
states retain m
l
D˙1.
By taking linear combinations of the basis vectors in real form from (2.12), we
obtain for D
3d
or C
3v
with the z axis along a <111> direction
e D
8
<
:
1
p
3
d
x
2
y
2
C
p
2
p
3
d
xz
D e
C
1
p
3
d
xy
p
2
p
3
d
yz
D e
;
t
2
D
8
ˆ
ˆ
<
ˆ
ˆ
:
p
2
p
3
d
x
2
y
2
1
p
3
d
xz
D t
C
2
p
2
p
3
d
xy
C
1
p
3
d
yz
D t
2
:
d
z
2
D t
2
0
:
(2.20)
The functions of (2.20) are expressed in a coordinate system with z directed along
the Œ111 direction of the cube body diagonal. If an analytical problem that involved
a trigonal or rhombohedral perturbation along the Œ111 axis was to be solved with
this combination of basis vectors expressed in the regular cubic coordinate system
with x, y,andz transformed back into the x
0
, y
0
, z
0
coordinates set up coincident
with the .001/ family of axes, eigenfunctions for this purpose have been reported
by Pryce and Runciman [14] and Dionne and Palm [15].
For this set the appropriate crystal field potential energy is given by a relation
[16] analogous to (2.14).
V
0
D Y
0
4
C
q
10
=
7
Y
3
4
Y
3
4
; (2.21)
where Y
3
4
Y
3
4
D
r
9
4
r
35
4
z
x
3
3xy
2
r
4
: (2.22)
The second case to be discussed is the three electron
4
F term, which is of greater
historical importance than the one electron case because it was the basis for the
invention of the maser (microwave amplification by stimulated electron radiation).
For this situation the orbital spherical harmonics are the Y
3
group [12],
Y
3
3
D
r
7
4
r
3
8
.x iy/
3
r
3
;
Y
2
3
D
r
7
4
r
15
8
z .x iy/
2
r
3
;
Y
1
3
D
r
7
4
r
3
16
.x iy/
5z
2
r
2
r
3
;
Y
0
3
D
r
7
4
r
1
4
z
5z
2
r
2
r
3
; (2.23)
62 2 Magnetic Ions in Oxides
Y
1
3
D
r
7
4
r
3
16
.x C iy/
5z
2
r
2
r
3
;
Y
2
3
D
r
7
4
r
15
8
z .x C iy/
2
r
3
;
Y
3
3
D
r
7
4
r
5
16
.x C iy/
3
r
3
:
For the F states, the Y
6
terms of (2.8) must be included in the calculation. Solutions
of the resulting secular equation are a singlet ground state A
2g
and two higher triplets
T
1g
and T
2g
and those of the inverted case shown in Fig. 2.12, with corresponding
eigenfunctions
T
1g
8
ˆ
ˆ
<
ˆ
ˆ
:
q
3
8
Y
1
3
C
q
5
8
Y
3
3
q
3
8
Y
1
3
C
q
5
8
Y
3
3
Y
0
3
9
>
>
=
>
>
;
;
T
2g
8
ˆ
ˆ
<
ˆ
ˆ
:
q
3
8
Y
1
3
q
5
8
Y
3
3
q
3
8
Y
1
3
q
5
8
Y
3
3
1
p
2
Y
2
3
C Y
2
3
9
>
>
=
>
>
;
; (2.24)
A
2g
1
p
2
Y
2
3
Y
2
3
:
Following the reasoning leading up to (2.14), for
4
F of d
3
and
4
F of d
7
,theseterm
energies are
T
1g
D 6Dq;
T
2g
D2Dq; (2.25)
A
2g
D12Dq:
Equation (2.24) is a convenient example of the meaning of orbital angular momen-
tum quenching. The ground state is a linear combination of two spherical harmonics
of the l D 2 manifold that yield the singlet A
2g
term under the influence of the cu-
bic crystal field. As such, it has the symmetry properties of a singlet s orbital and
therefore would carry the properties of l D 0; its only contribution to the mag-
netic moment of the ion must come from the ion spin. Conversely, if the orbital
levels are inverted in energy, the triplet T
2g
becomes the ground state and the or-
bital angular momentum would have the characteristics of a degenerate p state with
l D 1. In this case, l is reduced from the d state value of 2 down to 1 and the result
is only partial quenching because not all of the ground state degeneracy has been
lifted. This residual degeneracy is an important factor in the properties of certain
transition 3d
n
, for example, Co
2C
and a number of 4f
n
rare-earth ions. Further-
more, where the crystal field splitting parameter Dq is small enough to allow the
influence of the upper terms in a subsequent perturbation calculation, appropriate

2.3 Crystal Electric Fields 63
Table 2.5 3d
n
states and energies in weak octahedral fields
d Electrons Orbital ground state
States and energies
in Dq
a
d
12
D
2
E
g
.
C6
/
2
T
2g
.
4
/
d
23
F
3
A
2g
.
C12
/
3
T
2g
.
C2
/
3
T
1g
.
6
/
d
34
F
4
T
1g
.
C6
/
4
T
2g
.
2
/
4
A
2g
.
12
/
d
45
D
5
T
2g
.
C4
/
5
E
g
.
6
/
d
56
S
6
A
1g
.
0
/
d
65
D
5
E
g
.
C6
/
5
T
2g
.
4
/
d
74
F
4
A
2g
.
C12
/
4
T
2g
.
C2
/
4
T
1g
.
6
/
d
83
F
3
T
1g
.
C6
/
3
T
2g
.
2
/
3
A
2g
.
12
/
d
92
D
2
T
2g
.
C4
/
2
E
g
.
6
/
a
For tetrahedral
.
O
4
/
coordinations, multiply Dq by 4=9; for cubic
.
O
8
/
, multiply by 8=9
orbital contributions to the magnetic moment will enter into the eigenfunctions after
spin–orbit and magnetic field perturbations are applied.
Recalling the ground state terms of the 3d
n
series listed in Table 2.4, we can now
point out that the two example solutions for
2
D and
4
F will apply equally to the
5
D and
3
F cases. Table 2.5 lists these crystal field terms for the d
n
series in units of
Dq, with their signs adjusted to take into account the sign reversal for the upper half
of the series. An insightful commentary on the correspondence and contrast among
these ions was given by Van Vleck [17].
The point charge calculation may also be approached by another powerful tech-
nique called “operator equivalents” developed by Stevens [18]. This method is based
on the replacement of the Cartesian operator functions of the V
cf
potential energy
with the equivalent L or J (whichever is applicable) angular momentum operators.
Expressed in operator equivalents, the first part of the D
4
term of the octahedral field
given by (2.7)
V
oct
cf
D D
4
x
4
C y
4
C z
4
3
5
r
4
D D
4
L
4
x
C L
4
y
C L
4
z
1
5
L.LC 1/ Œ3L .L C 1/ 1
: (2.26)
64 2 Magnetic Ions in Oxides
Since the eigenfunctions of these angular momentum operators are linear combina-
tions of the spherical harmonics, the calculation of matrix elements is straightfor-
ward. Operator equivalents can be very useful for quantitative calculations of more
complex symmetries that involve higher order terms in E
cf
and also for cases of
higher L (or J ) values. An introduction to these techniques is given in Ballhausen
[3]andLow[13] and a more comprehensive discussion including many tables of
matrix elements may be found in Hutchings [16]. To continue with this discussion,
the theory of symmetry groups will be introduced as a powerful tool for finding
crystal field solutions.
2.3.5 Group Theory and Lower Symmetry
Conventional perturbation calculations to determine crystal field states can become
arduous for more complicated systems. The solutions for the simple cases outlined
in the previous section will prove almost sufficient for our discussion of the various
magnetic properties. To cope with the frequently encountered trigonal, tetragonal,
and orthorhombic distortions of the cubic coordinations, however, the solutions are
found by a shortcut that is derived from symmetry considerations. In the point
charge calculations, diagonalization of matrices by solutions of higher order sec-
ular determinants may be accomplished by applying group theory to determine not
only the best linear combinations of wavefunctions but also the degeneracies of the
different eigenstates, for example, the A
2g
, E
g
, T
1g
,andT
2g
terms of the type de-
fined by (2.21).
Unfortunately, the scope of this text will not permit a detailed exposition of group
theory. The interested reader is directed toward any number of excellent treatments
of this subject, including those cited earlier [3–5, 19]. For the purposes at hand,
we need to recognize that the diagonalization process involves the construction of
wavefunction combinations that conform to the symmetry of the perturbation oper-
ator. Group theory provides a method for predetermining the correct eigenfunction
combinations for a particular perturbation problem and is useful in solving for the
eigenfunctions of lower symmetry fields.
There are some terminologies that should be mentioned because they will re-
cur throughout this volume. Energy levels or eigenvalues are often referred to as
irreducible representations or energy terms. Their corresponding eigenfunctions are
called basis vectors. Early development of this discipline was conducted by Bethe
[20] and Mulliken [21], and two nomenclatures of the representations have sur-
vived, although the Mulliken version seems to have gained some preference. It has
already been introduced in the designations of the spherical harmonic combinations
in (2.24). Table 2.6 lists the notations for these two systems with the corresponding
degeneracies. The results of group theory analysis of the crystal field problems have
been well documented, and the cubic field representations for the various orbital
terms are summarized in Table 2.7.
For the simple case of a descent in symmetry from cubic O
h
to tetragonal D
4h
to
orthorhombic D
2h
, the relation of the basis vector lobes to the changing locations
2.3 Crystal Electric Fields 65
Table 2.6 Comparison
of Mulliken and Bethe
representation notations
Mulliken Bethe Degeneracy
A
1
1
1
A
2
2
1
E
3
2
T
1
4
3
T
2
5
3
E
1=2
6
2
E
5=2
7
2
G
8
4
Table 2.7 Irreducible representations for cubic symmetry
Ground Term l Mulliken Bethe
S0A
1
1
P1T
1
4
D2E C T
2
3
C
5
F3A
2
C T
1
C T
2
2
C
3
C
5
G4A
1
C E C T
1
C T
2
1
C
3
C
4
C
5
H5E C 2T
1
C T
2
3
C 2
4
C
5
I6A
1
C A
2
C E C T
1
C 2T
2
1
C
2
C
3
C
4
C 2
5
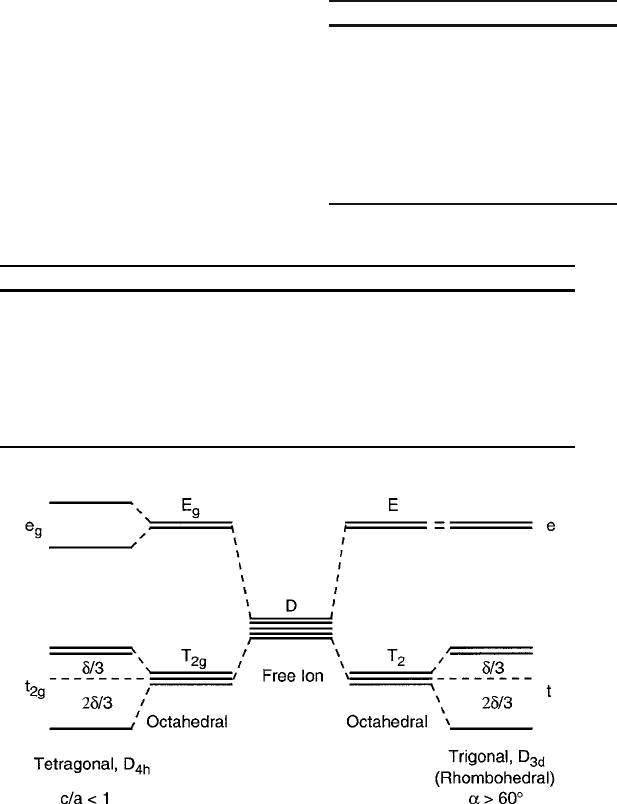
Fig. 2.13 Comparison of d -electron energy levels in crystal fields of tetragonal .c=a < 1/ and
trigonal
.
˛>60
ı
/
symmetries [15]
of the negatively charged ligands can be visualized in Fig. 2.11. For a tetragonal
distortion shown in Fig. 2.6c, T
2g
(d
xy
;d
xz
,andd
yz
/ splits in the same way as the
trigonal case, but the upper E
g
doublet is now also split because of the relation of
the d
x
2
y
2
and d
z
2
lobes to the octahedral ligands. The orthorhombic distortion D
2h
of Fig. 2.6d will remove the final degeneracy and split the d
xz
and d
yz
states. The
splittings of the orbital D term in these axially distorted cubic fields are compared
in Fig. 2.13.

66 2 Magnetic Ions in Oxides
The energy level structure for the special cases of the tetragonal or orthorhom-
bic distortions that occur with pyramidal O
5
and planar O
4
coordinations shown in
Fig. 2.7 may be inferred by extrapolating the results for the weak field solutions.
The descent in symmetry from cubic to the planar structure is particularly important
in the cuprate superconductors to be examined in Chap. 8. To obtain a quantitative
sense of the influence of a strong tetragonal field, we return briefly to the point
charge calculation and examine the effects of a tetragonal component V
T
to the oc-
tahedral crystal field energy H
cf
V
octCT
cf
D V
oct
cf
C V
T
,where[22]
V
T
D f
2
.r/ R .r/ Y
0
2
C f
0
4
.r/ R .r/ Y
0
4
; (2.27)
where f
2
.r/ and f
0
4
.r/ are radially-dependent coefficients. With the eigenfunction
set of (2.11), diagonal matrix elements may be obtained by straightforward integral
computations. Part of this process involves the defining of two additional splitting
parameters representing the integrals of the radial components of the respective ma-
trix elements over space, according to
Ds D
Z
ŒR .r/
2
3
2
f
2
.r/ d;
Dt D
Z
ŒR .r/
2
3
2
f
0
4
.r/ d: (2.28)
From this definition, it may be shown that the energy states of the tetragonal pertur-
bation follow directly from the diagonal matrix elements. The splitting of the upper
doublet E
g
of the O
h
C D
4h
group becomes
D
d
x
2
y
2
ˇ
ˇ
ˇ
V
oct
cf
C V
T
ˇ
ˇ
d
x
2
y
2
˛
D 6Dq C 2Ds Dt;
D
d
z
2
ˇ
ˇ
ˇ
V
oct
cf
C V
T
ˇ
ˇ
d
z
2
˛
D 6Dq 2Ds 6Dt: (2.29a)
and that of the lower T
2g
triplet is
˝
d
xy
ˇ
ˇ
V
oct
cf
C V
T
ˇ
ˇ
d
xy
˛
D4Dq C 2Ds Dt;
˝
d
xz;yz
ˇ
ˇ
V
oct
cf
C V
T
ˇ
ˇ
d
xz;yz
˛
D4Dq Ds C 4Dt: (2.29b)
where the lowest d
xz
and d
yz
orbitals retain their degeneracy. For Ds and Dt > 0,
the order of energy levels is shown in Fig. 2.14. As drawn, the structure is shown
with the doublet as the ground state, but this may not necessarily be the case since
the relative individual values of Ds and Dt would determine the correct order. Note
that the uppermost state is still d
z
2
and that it reaches a maximum separation of 10Dq
from the next highest state, which is now d
xy
instead of d
z
2
. The crossover point
where this upper state splitting becomes equal to 10Dq can be attained with a large
tetragonal distortion, but may not necessarily require a complete removal of the two
apical ligands along the z axis that would leave only an O
4
planar coordination. This
point is discussed further in relation to the superconductivity of cuprates in Chap. 8.
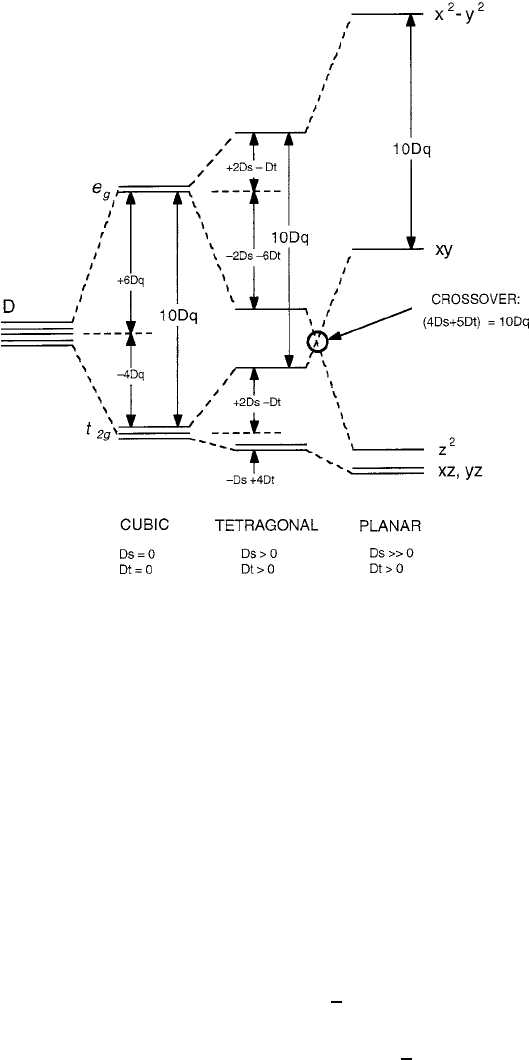
2.3 Crystal Electric Fields 67
Fig. 2.14 Details of orbital energy level splittings as a tetragonal crystal evolves from cubic to
planar, showing the 10Dq destabilization of the highest e
g
level. Diagram is based on Fig. A.47 of
[4]
Another demonstration of energy level determinations by group theory is real-
ized by the descent in symmetry from O
h
! D
3d
that is commonly encountered in
magnetic oxides. From the basis vectors for a trigonal distortion of an octahedral
site reveal that the lower triplet T
2g
is split into a doublet and a singlet, while the
degeneracy of the upper doublet E
g
is unchanged, as shown in Fig. 2.13. The fact
that the upper doublet remains degenerate will be shown to be important in a later
discussion of the Jahn–Teller effect.
For a trigonal distortion, the crystal field potential energy isV
octC£
cf
D V
oct
cf
C V
£
,
where V
is applied in a similar fashion to that of the tetragonal field component
V
T
given by (2.27), except that the set of orbital functions that it operates on are the
group of (2.20). Upon application of this perturbation, the matrix elements are
˝
e
˙
ˇ
ˇ
V
oct
cf
C V
£
ˇ
ˇ
e
˙
˛
D 6Dq C
7
3
D;
˝
t
˙
2
ˇ
ˇ
V
oct
cf
C V
ˇ
ˇ
t
˙
2
˛
D4Dq C D C
2
3
D;

68 2 Magnetic Ions in Oxides
h
t
o
2
j
V
oct
cf
C V
£
j
t
o
2
i
D4Dq 2D 6D; (2.30)
˝
t
˙
2
ˇ
ˇ
V
oct
cf
C V
£
ˇ
ˇ
e
˙
˛
D
p
2D
5
p
2
3
D:
where D and D are defined analogously to Ds and Dt of (2.28).
At this point it is instructive to compare (2.29)and(2.30). The tetragonal and
trigonal cases are similar in that the T
2g
(and T
2
) group is split into a singlet and
doublet, but as illustrated in Fig. 2.13,theE
g
(and E) term remains degenerate in
the trigonal field. Moreover, we now see that the t
˙
2
and e
˙
states mix under the V
£
perturbation. Pryce and Runciman [14] have studied this question in detail, but for
our purposes, we assume that D.5=3/ D and that the off-diagonalelements are
negligible, so that the matrix may be approximated as diagonal in later discussions.
2.3.6 Strong Field Solutions and Term Diagrams
If the crystal field is strong enough to compete with the mutually repulsive inter-
actions among orbiting electrons in ions with multiple d electrons, or if the crystal
field influence on the excited terms is important, all of the terms listed in Table 2.4
must be included as part of any thorough perturbation calculation. This situation is
encountered in cases where the crystal-field splitting is larger, for example, for cer-
tain ligands such as the cyanide radical CN
6
, or with the larger radii 4d
n
and 5d
n
ions. The most common need for the full energy term diagrams occurs in the inter-
pretation of optical transitions from the ground state, as discussed in Chap. 7.Al-
though the ground term is usually the only part of the free-ion energy level structure
that is needed to explain the properties of magnetic oxides, the reader should appre-
ciate the meaning of the term diagrams and the basis of their theoretical origins.
If the procedure outlined for the weak field is extended to include the upper terms
in the conventional way, large matrices result and solutions to the complete term
picture must be worked out by solution of the corresponding equations, simplified
wherever possible by the use of group theory and any other methods for reducing
the complexity of the matrices. To this end, Orgel [23] reported matrices and com-
putations for the d
n
series expressed in terms of the single parameter Dq. His results
for the important d
5
case of Fe
3C
or Mn
2C
(symmetric in sign for any of the cubic
coordinations in this particular instance) are shown in Fig. 2.15.
An alternative approach to the strong field problem is to consider the effects of
the ligands on the orbital electrons prior to the energy of their mutual interaction
E
term
that determines the free-ion term splittings, that is, the intra-atomic e
2
=r
12
repulsive energies. It is then assumed that the distributions of electrons among the
d orbital states are determined first by the repulsive forces of the ligands, with the
mutual interactions among the electrons treated as the perturbation. In this situation,
the starting energy states are no longer influenced by Hund’s rule of orbital ordering,
but rather by the various electron distributions among the t
2g
and e
g
orbital states as
dictated by the crystal field, in this case anticipated as octahedral.
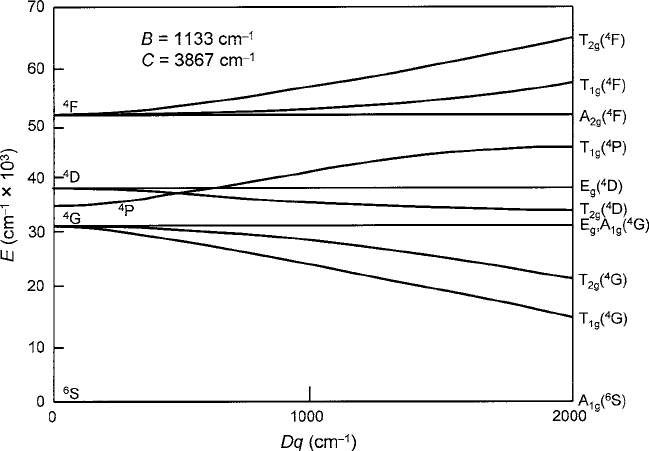
2.3 Crystal Electric Fields 69
Fig. 2.15 Multielectron energy level term diagram for a 3d
5
Mn
2C
ion in an octahedral crystal
field of O
2
ligands. Author’s computations were based on the model of Orgel [24]
In the strong field limit, it is assumed that the mutual repulsion among the elec-
trons in the ligands dominates the distribution of electrons among the d orbital
states. As a consequence, the system of free-ion terms is broken down, and the
energy states are selected according to occupation numbers of electrons in the t
2g
and e
g
shells, first by filling the lower t
2g
levels and then the less stable e
g
states.
For the simplest multiple electron case of two d electrons, there are three possible
orbital configurations within the octahedral Dq separation,
t
2g
2
,
t
2g
1
e
g
1
,and
e
g
2
. Within each of these distributions are many possible combinations that are
set by number of electrons and the number of individual states [24]. The respec-
tive energies of these groups of states is given to a first approximation by assigning
4Dqto each electron in a t
2g
level and C6Dq to the remaining e
g
electrons. To
complete the calculation, the mutual repulsion energies, which separate the free-ion
term energies through the E
rep
perturbation, are recalculated based on mixtures of
the specific octahedral t
2g
and e
g
wave functions of (2.12) and then added as com-
binations of K (Coulomb) and J (exchange) determinant element integrals in the
manner of those introduced previously in relation to interatomic electron repulsion
in Sect. 1.3.3. As an example calculation, consider the interaction between the d
xz
and d
yz
orbitals of (2.12), for which the elements (of the Slater determinant) are
simply stated from the definition in Ballhausen [25]:
