Dionne G.F. Magnetic Oxides
Подождите немного. Документ загружается.

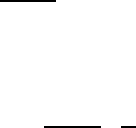
1.3 Spontaneous Magnetism 19
At temperatures near 0 K, a relation that has its origins in spin-wave theory has
proven to be the more effective at fitting data than the Brillouin function. It is com-
monly referred to as the Bloch T
3=2
law and is given by
M.T/
M.0/
D 1 AT
3=2
; (1.51)
where the exchange constant J enters the relation through the parameter
A D
0:1174
f
k
J
3=2
and f D 1, 2, or 4 for the simple, body-centered, or face-centered cubic lattice,
respectively.
The values of B as a function of T can also be computed [6] by means of a
convergent iterative procedure that is described in Chap. 4 in relation to multiple
sublattice ferrites. The importance of the Brillouin function will revisited in the
discussions of antiferromagnetism. In Chap. 7, an external field H is included as
an active variable in combination with the molecular field to illustrate the origin of
magnetoresistance properties of magnetic oxides.
1.3.3 Quantum Origins of the Molecular Field
The origin of the Weiss molecular field was first proposed by Heisenberg [7], who
postulated that spontaneous spin alignments are determined by short-range interac-
tions between adjacent spins that are made possible by the coupling of orbital wave
functions as part of the chemical bonding. The effect is electrostatic and arises from
electron exchange between bonding atoms. In a quantum mechanical format, the
Heisenberg exchange energy is expressed as a Hamiltonian function with adjacent
spins at sites i and j related by
H
ex
D2
X
i>j
J
ij
S
i
S
j
; (1.52)
where J
ij
is the exchange constant. Phenomenologically, the sign of J
ij
determines
the type of spin alignment; intuitively, it is seen that J
ij
> 0 will cause ferromag-
netism and J
ij
< 0 will create antiferromagnetism depending on the cosine of the
angle between S
i
and S
j
.
The orbital interactions the determine J
ij
are analyzed through the use of the
one-electron Hartree–Fock approximation to the wave functions of a many elec-
tron system, with modifications required to account for the indistinguishability of

20 1 Introductory Magnetism
fermions and satisfy the Pauli exclusion principle [8]. An example that can illustrate
the quantum mechanical origin of the effect is the case of two one-electron atoms
a and b with corresponding electrons labeled (1) and (2). This is the hydrogen
molecule (H
2
) employing the valence-bond approach of Heitler and London [9].
The Hamiltonian (here representing a local Madelung energy) for this pair before
taking into account the electron–electron repulsion is therefore
H
0
D
„
2
2m
e
r
2
a
Cr
2
b
Z
a
e
2
r
a1
Z
b
e
2
r
b2
; (1.53)
where Z
a
and Z
b
are the respective nuclear charges. There are two possible solutions
to this equation, '
0
D '
a
.1/ '
b
(2) where the two electrons remain on their “home”
atoms, and '
ex
D '
a
.2/ '
b
(1) where the electrons are exchanged between the two
atoms.
To this point in the analysis, the electrons have been treated as “distinguishable.”
However, orthogonality requires that their eigenfunctions satisfy the relation
jh
'
0
j
'
0
ij
2
D
jh
'
ex
j
'
ex
ij
2
; (1.54)
which means that two solutions exist:
'
0
D˙'
ex
: (1.55)
Because neither of the basic wavefunctions '
0
nor '
ex
can fulfill the indistinguisha-
bility requirement of (1.55), the usual linear combinations are constructed,
'
sym
D
1
p
2
.'
0
C '
ex
/;
'
anti
D
1
p
2
.'
0
'
ex
/: (1.56)
To satisfy the indistinguishability, wavefunctions must always be antisymmetric,
meaning that exchange of two fermions must always involve a sign reversal [8].
However, only '
anti
meets this criterion. To render both eigenfunctions antisymmet-
ric, the orbital functions must be completed by attaching the spin matrix functions
sym
(for parallel spins) and
anti
(for antiparallel spins) to create the final set
""
D '
anti
sym
;
"#
D '
sym
anti
: (1.57)
For a two-electron molecule, the total spin for
sym
is S D 1 from parallel spin
vectors, thereby forming a spin angular momentum triplet (S
z
D 1; 0; and 1 in
units of „). Conversely,
anti
is the singlet S D 0 from antiparallel spins.
1.3 Spontaneous Magnetism 21
Fig. 1.10 Basic diagram of
magnetic exchange
interaction identifying the
various linkage distances
among positively charged
nuclei a and b and their
bonding electrons 1 and 2
The problem can now be solved by considering a perturbed Hamiltonian in the
form of H D H
0
C H
1
and by calculating the eigenvalues of the two states for the
various electrostatic interactions among the nuclei and electrons, according to
H
1
D
e
2
r
12
C
Z
a
Z
b
e
2
r
ab
Z
a
e
2
r
a2
C
Z
b
e
2
r
b1
;
repulsion attraction (1.58)
as depicted in Fig. 1.10. Computing the expectation values of the integrals
˝
"#
ˇ
ˇ
H
1
ˇ
ˇ
"#
˛
;
˝
"#
ˇ
ˇ
H
1
ˇ
ˇ
""
˛
; and
˝
""
ˇ
ˇ
H
1
ˇ
ˇ
""
˛
produces the energy eigen-
values given in a form that exposes the spin exchange operator as determined by the
procedure of second quantization using Fermi operators [10,11],
E D K J
1
2
C 2s
1
s
2
; (1.59)
where
K D
h
'
0
j
H
1
j
'
0
i
.Coulomb integral/;
J D
h
'
0
j
H
1
j
'
ex
i
.exchange integral/: (1.60)
To calculate the exact quantum mechanical values of the s
1
s
2
scalar product (in
units of „
2
), the standard relation for the angular momentum vector addition s
1
C
s
2
D S can be used:
2s
1
s
2
D S.SC 1/ s
1
.s
1
C 1/ s
2
.s
2
C 1/ : (1.61)
For the parallel and antiparallel values of S D 1 and 0, respectively, and the values
of s
1
D s
2
D 1=2,(1.59) reduces to
E D K J for S D 1.ferromagnetism/;
E D K C J for S D 0.antiferromagnetism/: (1.62)
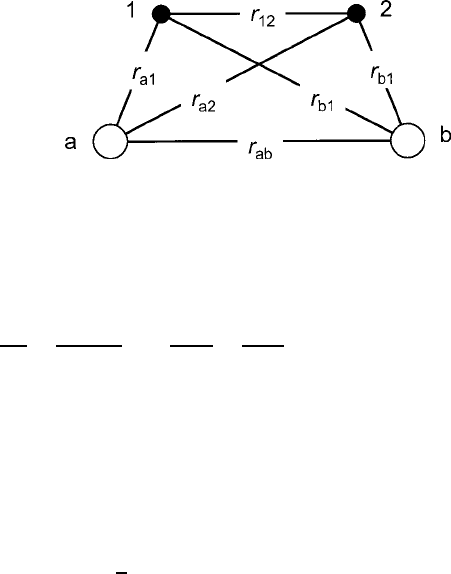
22 1 Introductory Magnetism
Fig. 1.11 Orbital interaction
diagram contrasting the
competing roles of mutual
electron repulsion and
electron-nuclear attraction as
nuclear separation decreases:
(a) ferromagnetism in an
antibonding state, and (b)
antiferromagnetism in a
bonding state. Note the
reversed locations of
electrons 1 and 2 relative to
the two nuclei
The choice of ground state in (1.62) is therefore determined by the sign of J ,which
follows the same rules that are stated below (1.52), that is, ferromagnetism for J>
0, antiferromagnetism for J<0.
3
Computation of J is not a trivial task, but some insight may be gained by in-
specting the relative sizes of the terms in (1.58) and comparing them with the
diagrams in Fig. 1.11. For overlapping orbital wave function lobes of the type shown
in Fig. 1.11a, the close proximity of the electron charge concentrations can make
the repulsive e
2
=r
12
term dominant and render J>0, thereby establishing the
ferromagnetic
""
as the ground state. If the overlap is more extreme because of
a smaller r
ab
distance, the situation of Fig. 1.11b would arise and the attractive
Z
a
e
2
=r
a2
and Z
b
e
2
=r
b2
terms could be large enough to make J<0and pro-
duce the antiferromagnetic
"#
ground state.
Overlapping wavefunction lobes are characteristic of d and f electron shells.
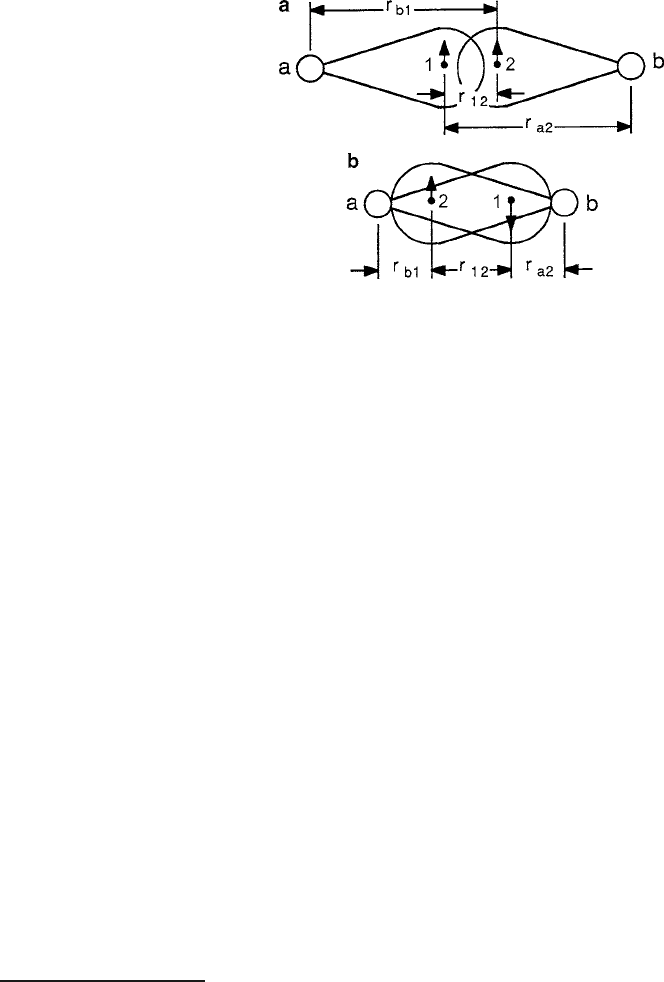
Ferromagnetism is more likely to occur in the upper half of the iron group elements
because of their larger populations of unpaired and itinerant electrons. The graph
in Fig. 1.12 illustrates the qualitative support for this model, where J values were
estimated from Curie and N´eel temperature measurements. Positive J values occur
for larger r
ab
distances in the familiar Fe, Co, and Ni of the 3d
n
transition group
and members of the 4f
n
rare-earth series.
An early phenomenological description of magnetic exchange was introduced by
Stoner [12]. Because of its success in interpreting magnetism in metals, it is known
as the theory of collective electron ferromagnetism. The result of this approach is
the band theory model that has helped to explain some features of the 3d
n
transition
series of elements. The theory predicts that ferromagnetic metals have moments that
are lower than anticipated based on the number of unpaired Bohr magnetons of the
3
Since the spins of an antiferromagnet are antisymmetric, it follows that the orbital functions must
be symmetric, and vice-versa for the ferromagnet. The former case is referred to as a “bonding”
state because of the orbital wavefunction overlaps, which is why most ionic compounds are in-
trinsically antiferromagnetic. The antibonding state then becomes a ferromagnet, as explained in
Chaps. 2 and 3.
1.3 Spontaneous Magnetism 23
Fig. 1.12 Chart of exchange constant data compiled as a function of the distance of closest ap-
proach of electron of neighboring atoms. Adapted from Lax and Button [14]
Table 1.3 Magnetic moments of ferromagnetic atoms
Transition
element
Free atom
configuration
Solid state
distribution
Net 3d or 4f Bohr
magnetons
Fe 3d
6
4s
2
3d
7:4
4s
0:6
C4:8 2:6 D 2:2
Co 3d
7
4s
2
3d
8:3
4s
0:7
C5 3:3 D 1:7
Ni 3d
8
4s
2
3d
9:4
4s
0:6
C5 4:4 D 0:6
Gd 4f
7
5d
1
6s
2
4f
7:1
5d
2
6s
0:9
7.10
free atom, as shown in Table 1.3. The reasons lie in the details of the overlapping
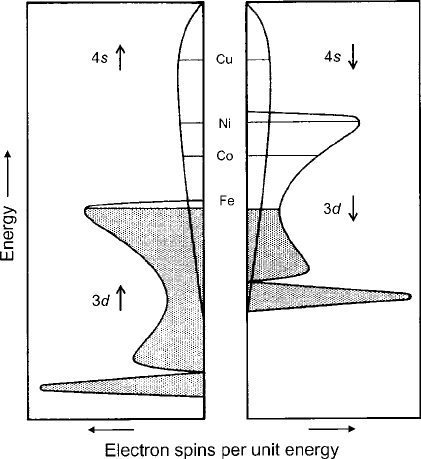
of 4s and 3d electronic bands in metals [13], as sketched in the density of states of
the split magnetic bands in Fig.1.13. Further support for the band concept is given
by the result for Gd
3C
, which has seven unpaired electrons that occupy the shielded
4f inner shell and are thus exempted from the distractions of the 6s bonding states.
An effective approximation for insulator compounds is based on single-electron
molecular-orbital theory of covalent bonding. In contrast to the valence bond
method described earlier, molecular orbital theory focuses specifically on the
electron–nuclear interaction terms of (1.58) and ignores the mutual repulsion
e
2
=r
12
, thereby favoring antiparallel spin alignment in the ground state. The
spin stabilization state that emerges is called indirect or superexchange because
the interactions between the positive magnetic ions (spin cations) occur through
the mediation of negative ligands (anions). It is particularly well suited for de-
scribing transition-metal insulating compounds where the basic exchange is
24 1 Introductory Magnetism
Fig. 1.13 Band model of
electron spin occupancy as a
function of energy for the
overlapping 3d and 4s bands
axis [12].Thebandsarefilled
to the levels indicated for iron
metal. The concept of
dividing the bands into
collective up and down spins
separated in energy by the
exchange stabilization [11]
resembles the application of
Hund’s rule in formulating
the ground-state spin
configuration of
transition-metal ions, as
diagrammed in Figs. 2.3 and
2.19. Adapted from Lax and
Button [14]
antiferromagnetic. However, there are varying conditions of superexchange (in-
cluding charge transfer or itinerance) that apply to different cation–anion–cation
situations.
This subject is placed into a broader context in Chap. 3, where collective and
localized spontaneous magnetism are contrasted. In a normally antiferromagnetic
structure, ferromagnetism requires electron delocalization in the form of Fermi gas
conductivity or large-polaron charge transfer between magnetic cations in mixed-
valence compounds.
1.3.4 The Ising Approximation
The exact solutions of the total spin scalar product S
j
S
j
eigenvalues require
the application of Fermi operators that lead to the mathematical and conceptual
complexities indicated by the stated result for the exchange energy E
ex
taken from
(1.59). An effective simplification called the Ising approximation has proven to be
accurate for most practical situations. If the exchange constant is the same for all
spin interactions, J
ij
of (1.52) may be replaced by J and the scalar product can be
expanded and summed over the nearest neighbors S
j
of spin S
i
to give
H
ex
D2J
X
i>j
S
xi
S
xj
C S
yi
S
yj
C S
zi
S
zj
: (1.63)

1.4 Gyromagnetism 25
If the spin components are replaced by their time averages, and a total of z identical
nearest neighbors are included in the sum, (1.63) may be expressed as
H
ex
D2zJ
N
S
xi
N
S
xj
C
N
S
yi
N
S
yj
C
N
S
zi
N
S
zj
: (1.64)
If the classical Larmor theory is used (see Sect. 1.5.1), the spin vectors will precess
about an effective molecular (or exchange) field directed along the z-axis of quan-
tization. As a consequence, the time averages of the x and y spin components are
assumed to be zero, and
H
ex
2zJ
N
S
zi
N
S
zj
: (1.65)
From this approximation a relation between the exchange integral J and the Weiss
molecular field coefficient N
W
may be derived. Since we consider only the z com-
ponent of the magnetization M
z
Dngm
B
S
zj
and (1.65) becomes
H
ex
2
z
n
JS
zi
M
z
g
i
m
B
: (1.66)
For H D 0, H
ex
can also be expressed in terms of the Weiss molecular field by
equating to E
m
from (1.33), according to
H
ex
g
i
S
zi
m
B
N
w
M
z
; (1.67)
where M
j
becomes S
zj
and the applied field H is replaced by the exchange field
N
W
M
z
.From(1.66)and(1.67)
N
W
D
2zJ
ng
i
2
m
B
2
; (1.68)
and we have for the Curie temperature from (1.48), after (1.68) is substituted,
T
C
D
2zJS .S C 1/
3k
; (1.69)
with S replacing the total angular momentum .J / for our discussion of spin
exchange. Equations (1.68)and(1.69) are important in the models for thermomag-
netism and magnetoresistance.
1.4 Gyromagnetism
To this point in the discussion, there has been little mention of the spectroscopic
splitting factor g that was introduced as part of the quantum mechanical definition
of the magnetic moment. Before leaving this introductory chapter, it is appropriate
to explain the classical origins of the magnetic resonance phenomenon that is not
only an important topic for later parts of this text, but also provides a direct means
of measuring g.

26 1 Introductory Magnetism
1.4.1 Larmor Precession and Resonance
Magnetic resonance is based on a theorem that the motion of an electron under a
central force, for example, Coulomb attraction to a nucleus, and a magnetic field H
in a fixed coordinate system is identical to that of an electron under the same central
force with H D 0 in a coordinate system that is rotating about the H axis with
angular frequency
!
0
D H D
ge
2m
e
c
H; (1.70)
where !
0
is the Larmor precession frequency and g equals 1 for an orbiting electron
and 2 for an electron spin;
D 1:76 10
7
rad=s=Oe
is usually referred to as the
gyromagnetic constant.
4
The derivation of this relation may be found in many stan-
dard textbooks [14,15]. For the diamagnetic case with g D 1, the sense of rotation
in the precession orbit is to establish a magnetic moment that is directed against H ,
thereby providing the basic mechanism for diamagnetism.
From the general case of a fixed magnetic moment m in a field H , the rotation
direction would be consistent with the moment and field in parallel. The basic theory
of magnetic resonance may be derived from the equation of motion
dm
dt
D
ge
2m
e
c
.m H /; (1.71)
where the vector m H is normal to the plane containing m and H . Consequently,
the resulting torque causes the clockwise precession of m about H viewed along
the direction chosen as the z-axis of the magnetic field. The solutions of (1.71)for
the individual components of m may be expressed as
m
x
D m sin cos !
L
t;
m
y
D m sin sin !
L
t; (1.72)
m
z
D m cos D constant:
where m forms a constant angle with the z axis in establishing a cone of precession
at the Larmor frequency. Recalling the earlier discussion of the quantum mechanical
constraints on the values of the projection of m on the axis of quantization, we
express m
J z
D gm
B
J
z
since Planck’s constant „ is absorbed in the Bohr magneton.
Note also that from a quantum energy standpoint, the Zeeman splitting
„!
0
D gm
B
HJ
z
D gm
B
H forJ
z
D 1: (1.73)
The equivalence of (1.70)and(1.73) can be determined by inspection.
4
Where the frequency is designated by the symbols or f expressed in cycles/s (Hz), an alterna-
tive constant
0
.
D2:78GHz=kOe
/
is defined. However, confusion can arise when the symbol !
is used for , usually in microwave engineering literature. In such instances,
0
must be employed
with H . Note also that the negative sign of the electron charge e has been absorbed in the defini-
tion of !
0
in (1.70), thereby allowing to be treated as positive wherever frequency and field or
magnetization are related.
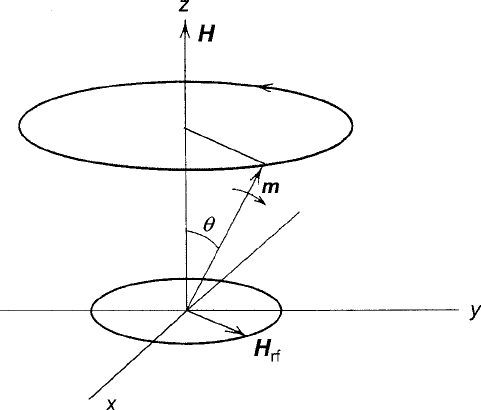
1.4 Gyromagnetism 27
Fig. 1.14 Standard classical diagram of magnetic resonance showing the precession of a magnetic
moment m about a field H at the Larmor frequency. When a circularly polarized oscillating field
H
rf
matches the frequency and sense of the precession, m is subjected to a torque that rotates it
away from the H axis, thereby producing a complex susceptibility
Magnetic resonance occurs where an alternating (usually radio frequency) mag-
netic field H
rf
is applied in a direction perpendicular to H. Because a linearly
polarized signal can be decomposed into two counterrotating circularly polarized
signals, the physical situation resembles that depicted in Fig. 1.14, where only the
component that rotates in the direction of the precessing moment is capable of con-
tinuously influencing the angle of m relative to the z axis by creating a second torque
m H
rf
normal to the m H direction. By setting the frequency of the alternating
field at ! D !
0
, H
rf
will synchronize with the precession and apply a constant
torque that will cause the cone half-angle to oscillate from full alignment with H
. D 0/ to its opposite limit . D /. The rotation of m away from H represents
the absorption of energy. Where a quantum mechanical model can be applied, the
two extreme values of represent two energy levels of a degeneracy that is split by
H (Zeeman effect). This view of magnetic resonance is explored in a discussion of
electron paramagnetic resonance in Chap. 6.
1.4.2 Phenomenological Relaxation Theory
An important feature of all resonating systems is the effect of damping. It
occurs most visibly in vibrating mechanical systems that reduce their amplitudes
exponentially because of air resistance or friction. The decay is a manifestation of
the loss of energy as the system relaxes back to equilibrium. For systems under
28 1 Introductory Magnetism
constant excitation, it is the energy that must be supplied to maintain the reso-
nance condition at a given intensity. In magnetic resonance, the relaxation arises
principally from two sources: (1) the transfer of magnetic energy imparted by the
alternating magnetic fields to the system of magnetic moments, commonly referred
to as the “spin system,” back to the environment, which is the lattice in the case of
solids and (2) the tendency of the precessing moments to lose phase coherence due
to perturbations of the external magnetic field by local dipolar fields. The first is loss
of signal energy directly to the lattice; the second is a loss of signal by decoherence
of the precessing spins. Eventually this energy will also be transformed into heat or
radiation.
In mathematical terms, following the approach developed by Bloch and
Bloembergen [16, 17], the relaxation of magnetization M .D˙m/ back toward
the z axis (longitudinal relaxation) following the removal of the H
rf
field is deter-
mined by the relaxation rate
1
z
of its M
z
component
dM
z
dt
D
M
z
M
z
D
M
z
M
1
; (1.74)
where
1
.D
z
/ is the spin–lattice relaxation time and M.DM
s
/ is the equilibrium
value of M
z
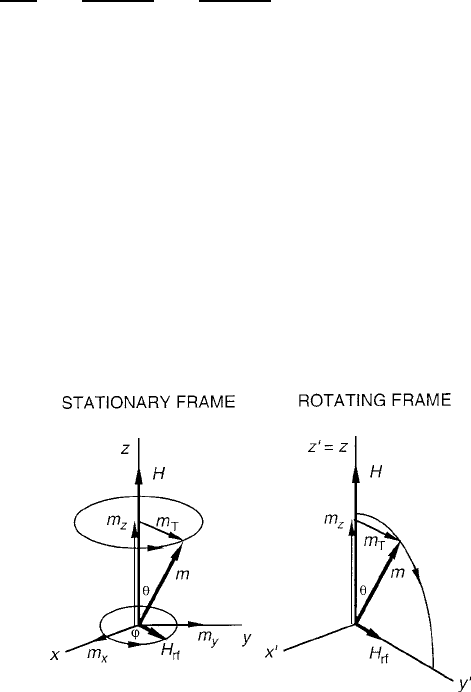
. In the stationary frame of reference of Fig. 1.15, the decay process of
the individual m vector can be visualized as following an inwardly precessing spiral.
Accordingly,
M M
z
.t/ D ŒM M
z
.t/ exp .t=
1
/: (1.75)
The second relaxation phenomenon of importance concerns the phase decoherence
of M
x
and M
y
among individual spin vectors as they precess about H (transverse
relaxation), which occurs in paramagnetic systems through dipole–dipole interac-
tions. In ferromagnetic systems, the decoherence represented by
2
is seen in the
form of spin waves, which occur under special conditions. In this case the vector
components of the spins that are perpendicular to H ,thatis,M
x
and M
y
lose their
collective coherence at a rate defined as
1
2
, once the alternating drive field is re-
Fig. 1.15 Two frames of
reference for magnetic
moment precession:
stationary and rotating at the
precession angular frequency
